Higher Dietary Fibre Increases the Faecal Microbiome Diversity of Golden Lion Tamarins (Leontopithecus rosalia)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group and Ethics Statement
2.2. Diet Composition
2.3. Sample Collection
2.4. DNA Extraction
2.5. High-Throughput Sequencing of the 16S rRNA Gene V3–V4 Hypervariable Regions Using the Illumina MiSeq Platform
2.6. Microbiota Profiling
2.7. Statistical Analysis
3. Results
3.1. Sequenced Faecal Samples from GLTs
3.2. GLT Microbiota
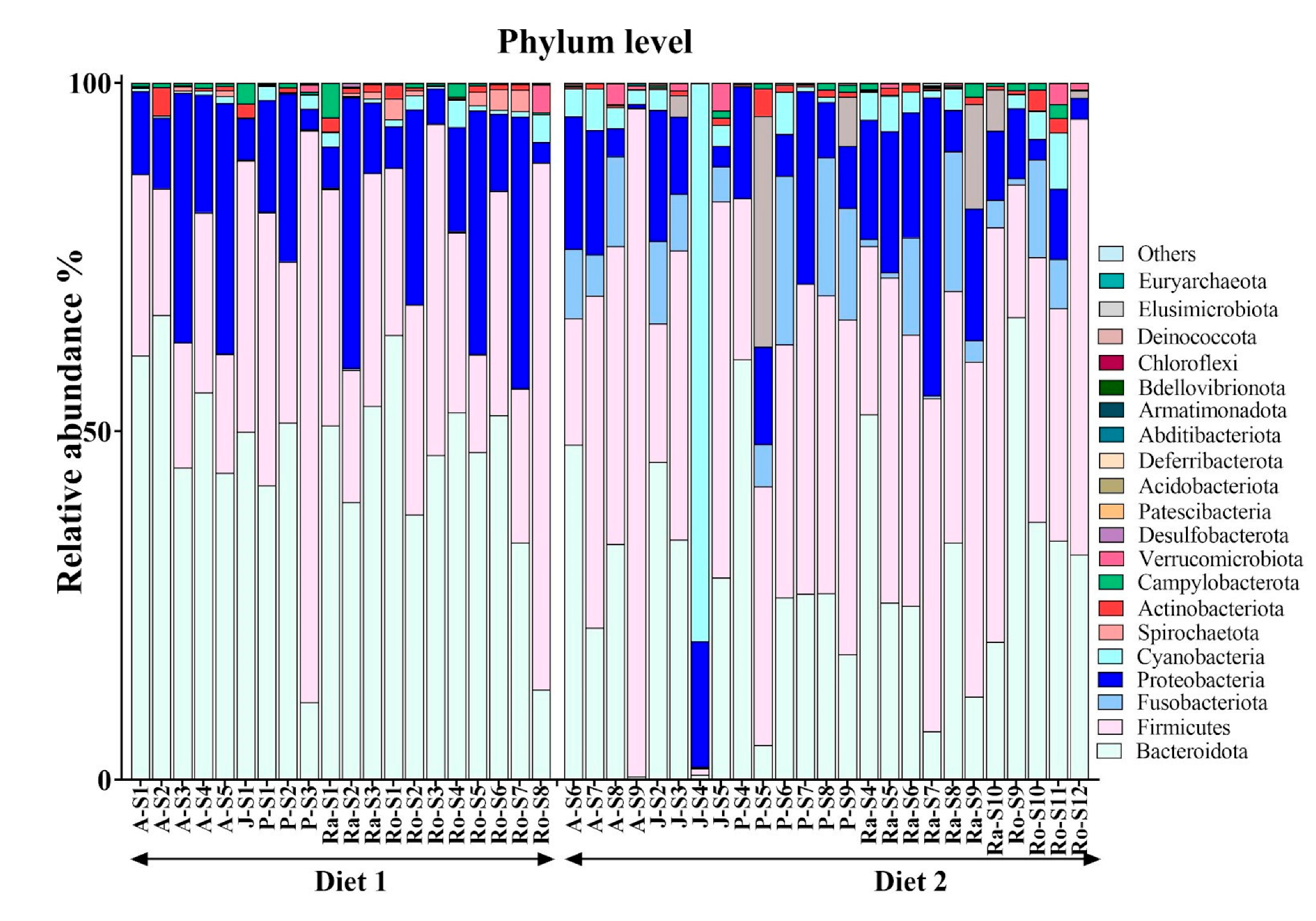
3.3. Phylum-Level Microbial Shifts in Response to High-Fibre Diet
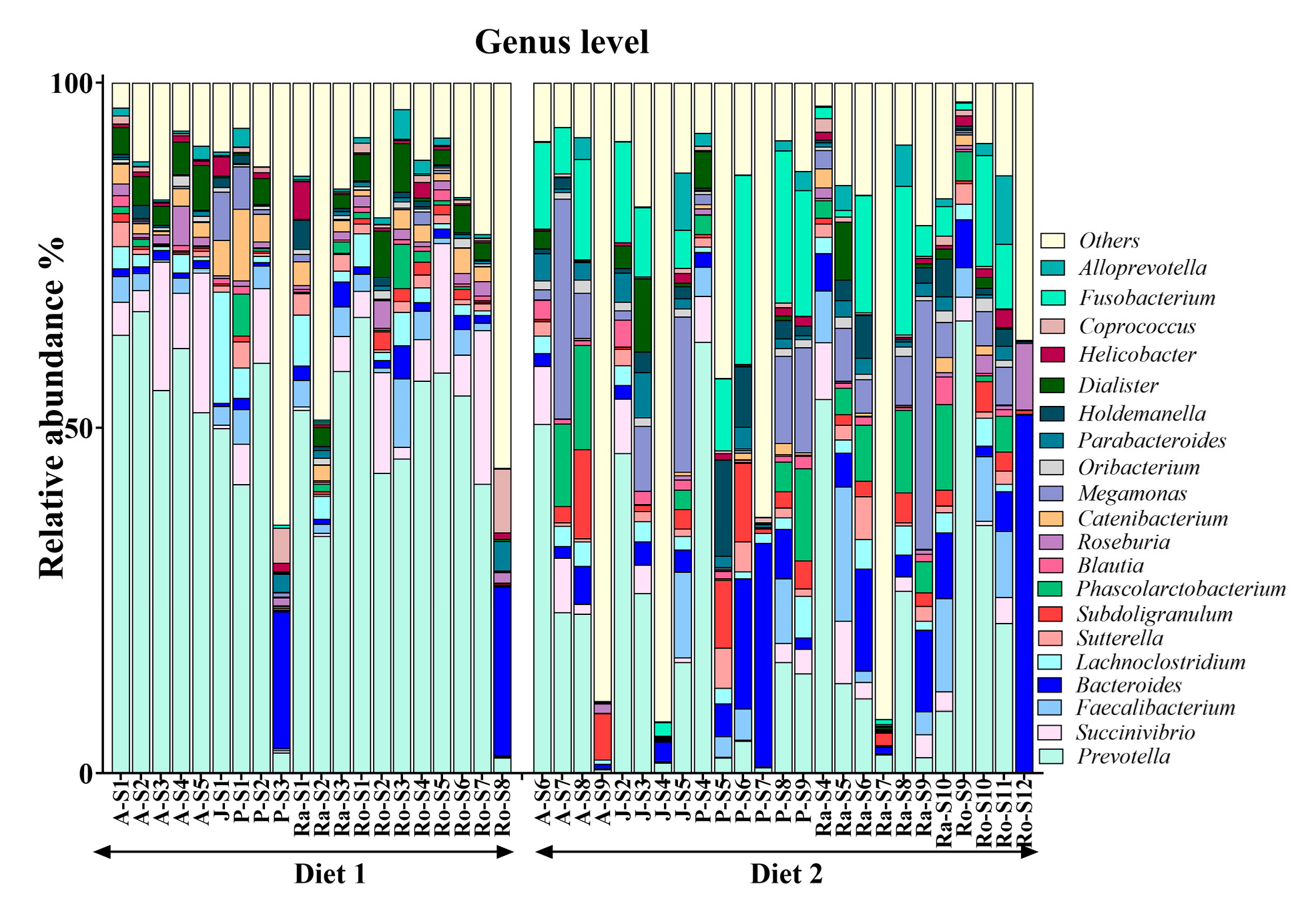
3.4. Alterations in Genus-Level Gut Microbiota After Higher-Fibre Dietary Intervention
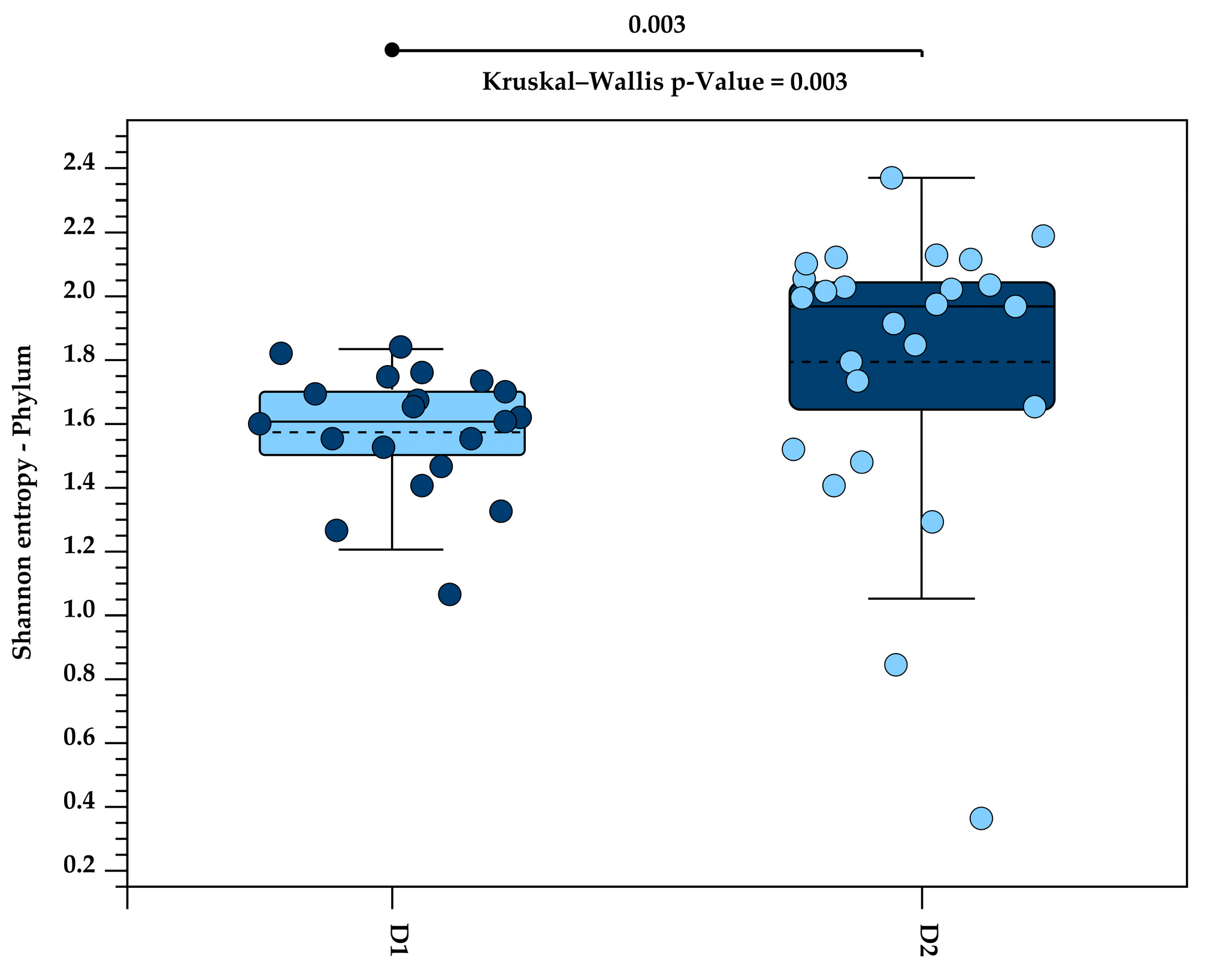
3.5. High-Fibre Diet Increased Alpha Diversity in Tamarin Gut Microbiota
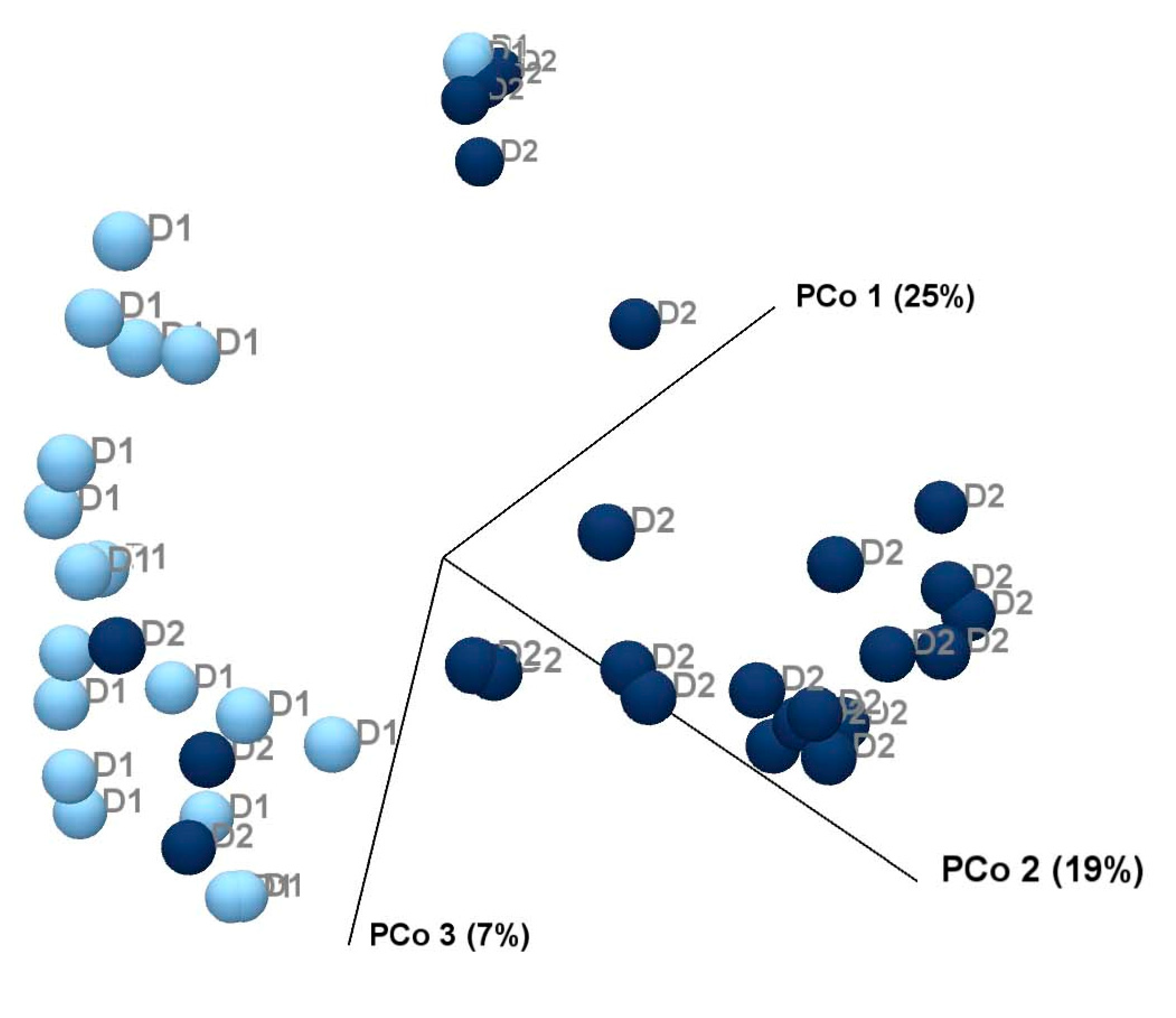
3.6. Beta Diversity Enhanced Following Increased Dietary Fibre Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabana, F.; Maguire, R.; Hsu, C.D.; Plowman, A. Identification of possible nutritional and stress risk factors in the development of marmoset wasting syndrome. Zoo Biol. 2018, 37, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Chalifoux, L.V.; Bronson, R.T. Colonic adenocarcinoma associated with chronic colitis in cotton top marmosets, Saguinus oedipus. Gastroenterology 1981, 80, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Chalifoux, L.; Bronson, R.; Escajadillo, A.; McKenna, S. An analysis of the association of gastroenteric lesions with chronic wasting syndrome of marmosets. Vet. Pathol. 1982, 19, 141–162. [Google Scholar] [CrossRef]
- Plowman, A.; Cabana, F. Transforming the Nutrition of Zoo Primates (or How We Became Known as Loris Man and That Evil Banana Woman). In Scientific Foundations of Zoos and Aquariums: Their Role in Conservation and Research; Kaufman, A.B., Bashaw, M.J., Maple, T.L., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 274–303. [Google Scholar]
- Schwitzer, C.; Polowinsky, S.; Solman, C. Fruits as foods–common misconceptions about frugivory. Zoo Anim. Nutr. IV 2009, 131–168. [Google Scholar]
- Britt, S.; Cowlard, K.; Baker, K.; Plowman, A. Aggression and self-directed behaviour of captive lemurs (Lemur catta, Varecia variegata, V. rubra and Eulemur coronatus) is reduced by feeding fruit-free diets. J. Zoo Aquar. Res. 2015, 3, 52–58. [Google Scholar]
- Plowman, A. Diet review and change for monkeys at Paignton Zoo Environmental Park. J. Zoo Aquar. Res. 2013, 1, 73–77. [Google Scholar]
- Power, M.; Toddes, B.; Koutsos, L. Nutrient Requirements and Dietary Husbandry Principles for Captive Nonhuman Primates. In Nonhuman Primates in Biomedical Research: Biology and Management; Abee, C.R., Mansfield, K., Tardif, S.D., Morris, T., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 1. [Google Scholar]
- Richards-Rios, P.; Wigley, P.; López, J.; Wormell, D.; Barbón, A. Changes in the faecal microbiome of pied tamarins (Saguinus bicolor) associated with chronic, recurrent diarrhoea and weight loss. Anim. Microb. 2021, 3, 1–15. [Google Scholar] [CrossRef]
- Amato, K.R.; Leigh, S.R.; Kent, A.; Mackie, R.I.; Yeoman, C.J.; Stumpf, R.M.; Wilson, B.A.; Nelson, K.E.; White, B.A.; Garber, P.A. The gut microbiota appears to compensate for seasonal diet variation in the wild black howler monkey (Alouatta pigra). Microb. Ecol. 2015, 69, 434–443. [Google Scholar] [CrossRef]
- The National Academies Press. Nutrient Requirements of Nonhuman Primates, 2nd Revised ed.; The National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Yip, S.; Dehcheshmeh, M.M.; McLelland, D.J.; Boardman, W.S.; Saputra, S.; Ebrahimie, E.; Weyrich, L.S.; Bird, P.S.; Trott, D.J. Porphyromonas spp., Fusobacterium spp., and Bacteroides spp. dominate microbiota in the course of macropod progressive periodontal disease. Sci. Rep. 2021, 11, 17775. [Google Scholar] [CrossRef]
- Xie, S.; Trott, D.J.; Saputra, S.; Ebrahimie, E.; Dehcheshmeh, M.M.; Page, C.; Woodward, N.; Griffiths, N.; Kimble, B.; Govendir, M. Pharmacokinetic profile and effect on the faecal microbiome of a single dose of pradofloxacin oral suspension in the rabbit (Oryctolagus cuniculus). J. Vet. Pharmacol. Ther. 2022, 45, 203–212. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Glöckner, F.O.; Yilmaz, P.; Quast, C.; Gerken, J.; Beccati, A.; Ciuprina, A.; Bruns, G.; Yarza, P.; Peplies, J.; Westram, R. 25 years of serving the community with ribosomal RNA gene reference databases and tools. J. Biotechnol. 2017, 261, 169–176. [Google Scholar] [CrossRef]
- Lin, H.; Peddada, S.D. Analysis of microbial compositions: A review of normalization and differential abundance analysis. NPJ Biofilms Microbiomes 2020, 6, 60. [Google Scholar] [CrossRef]
- Ferdous, T.; Jiang, L.; Dinu, I.; Groizeleau, J.; Kozyrskyj, A.L.; Greenwood, C.M.; Arrieta, M.-C. The rise to power of the microbiome: Power and sample size calculation for microbiome studies. Mucosal Immunol. 2022, 15, 1060–1070. [Google Scholar] [CrossRef]
- Nearing, J.T.; Douglas, G.M.; Hayes, M.G.; MacDonald, J.; Desai, D.K.; Allward, N.; Jones, C.M.; Wright, R.J.; Dhanani, A.S.; Comeau, A.M. Microbiome differential abundance methods produce different results across 38 datasets. Nat. Commun. 2022, 13, 342. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Singh, B. Psyllium as therapeutic and drug delivery agent. Int. J. Pharm. 2007, 334, 1–14. [Google Scholar] [CrossRef]
- De Godoy, M.R.; Kerr, K.R.; Fahey Jr, G.C. Alternative dietary fiber sources in companion animal nutrition. Nutrients 2013, 5, 3099–3117. [Google Scholar] [CrossRef]
- Di Caro, V.; Alcamo, A.M.; Cummings, J.L.; Clark, R.S.; Novak, E.A.; Mollen, K.P.; Morowitz, M.J.; Aneja, R.K. Effect of dietary cellulose supplementation on gut barrier function and apoptosis in a murine model of endotoxemia. PLoS ONE 2019, 14, e0224838. [Google Scholar] [CrossRef]
- Bornbusch, S.L.; Muletz-Wolz, C.R.; Lopez-Bondarchuk, E.; Maslanka, M.T.; Kendrick, E.L. Gut microbiomes of captive primates show phylosymbiosis, respond to dietary sugar reduction, and select for host-specific dietary microbes. FEMS Microbiol. Ecol. 2023, 99, fiad069. [Google Scholar] [CrossRef]
- Greene, L.K.; Clarke, T.A.; Southworth, C.A.; Bornbusch, S.L.; Ehmke, E.E. Daily lettuce supplements promote foraging behavior and modify the gut microbiota in captive frugivores. Zoo Biol. 2020, 39, 334–344. [Google Scholar] [CrossRef]
- Koliada, A.; Moseiko, V.; Romanenko, M.; Piven, L.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Vaiserman, A. Seasonal variation in gut microbiota composition: Cross-sectional evidence from Ukrainian population. BMC Microbiol. 2020, 20, 100. [Google Scholar] [CrossRef]
- Malukiewicz, J.; Cartwright, R.A.; Dergam, J.A.; Igayara, C.S.; Kessler, S.E.; Moreira, S.B.; Nash, L.T.; Nicola, P.A.; Pereira, L.C.; Pissinatti, A. The gut microbiome of exudivorous marmosets in the wild and captivity. Sci. Rep. 2022, 12, 5049. [Google Scholar] [CrossRef]
- Clayton, J.B.; Vangay, P.; Huang, H.; Ward, T.; Hillmann, B.M.; Al-Ghalith, G.A.; Travis, D.A.; Long, H.T.; Tuan, B.V.; Minh, V.V. Captivity humanizes the primate microbiome. Proc. Natl. Acad. Sci. USA 2016, 113, 10376–10381. [Google Scholar] [CrossRef]
- Malukiewicz, J.; D’arc, M.; Dias, C.A.; Cartwright, R.A.; Grativol, A.D.; Moreira, S.B.; Souza, A.R.; Tavares, M.C.H.; Pissinatti, A.; Ruiz-Miranda, C.R. Bifidobacteria define gut microbiome profiles of golden lion tamarin (Leontopithecus rosalia) and marmoset (Callithrix sp.) metagenomic shotgun pools. Sci. Rep. 2023, 13, 15679. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Wang, Z.; Zhang, M.; Wang, S.; Xiang, Z.; Pan, H.; Li, M. The relationship between gut microbiome and bile acids in primates with diverse diets. Front. Microbiol. 2022, 13, 899102. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Potrykus, J.; Mahaney, B.; White, R.L.; Bearne, S.L. Proteomic investigation of glucose metabolism in the butyrate-producing gut anaerobe Fusobacterium varium. Proteomics 2007, 7, 1839–1853. [Google Scholar] [CrossRef]
- Jiao, P.; Wang, Z.; Zhang, X.; Lu, X.; Sun, Q.; Zhao, H.; Xin, H.; Yang, W.; Lv, X.; Xie, X. Dietary supplementation of clostridium butyricum and rumen protected fat alters immune responses, rumen fermentation, and bacterial communities of goats. Anim. Feed Sci. Technol. 2024, 314, 116014. [Google Scholar] [CrossRef]
- Lindsay, E.C.; Metcalfe, N.B.; Llewellyn, M.S. The potential role of the gut microbiota in shaping host energetics and metabolic rate. J. Anim. Ecol. 2020, 89, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Adamberg, K.; Adamberg, S. Selection of fast and slow growing bacteria from fecal microbiota using continuous culture with changing dilution rate. Microb. Ecol. Health Dis. 2018, 29, 1549922. [Google Scholar] [CrossRef] [PubMed]
- Amir, I.; Bouvet, P.; Legeay, C.; Gophna, U.; Weinberger, A. Eisenbergiella tayi gen. nov., sp. nov., isolated from human blood. Int. J. Syst. Evol. Microbiol. 2014, 64, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Coccia, C.; Bonomi, F.; Lo Cricchio, A.; Russo, E.; Peretti, S.; Bandini, G.; Lepri, G.; Bartoli, F.; Moggi-Pignone, A.; Guiducci, S. The potential role of butyrate in the pathogenesis and treatment of autoimmune rheumatic diseases. Biomedicines 2024, 12, 1760. [Google Scholar] [CrossRef]
- Oakley, B.B.; Vasconcelos, E.J.; Diniz, P.P.; Calloway, K.N.; Richardson, E.; Meinersmann, R.J.; Cox, N.A.; Berrang, M.E. The cecal microbiome of commercial broiler chickens varies significantly by season. Poult. Sci. 2018, 97, 3635–3644. [Google Scholar] [CrossRef]
- Grant, L.; Mohammadi Dehcheshmeh, M.; Ebrahimie, E.; Khabiri, A.; Veltman, T.; Shipstone, M.; Trott, D.J. The effect of daily oral probiotic and postbiotic supplementation on the canine skin microbiota: Insights from culture-dependent and long-read 16S rRNA gene sequencing methods. Vet. Dermatol. 2025; Online ahead of print. [Google Scholar]

| Diet Item | Amount (g) |
|---|---|
| ”Primate Cake” a | 100 |
| Group A (leafy green) vegetables | 11 |
| Group B (other non-starchy) vegetables | 38 |
| Group C (starchy) vegetables | 38 |
| High protein supplement powder b | 5 |
| Dry dog food c | 3 |
| Discretionary item d | x1 serve |
| Primate Meal | Primate Cake | Total Diet | NRC (2003) [11] | ||||
|---|---|---|---|---|---|---|---|
| Diet 1 | Diet 2 | Diet 1 | Diet 2 | Diet 1 | Diet 2 | ||
| Dry Matter (%) | 94.0 | 94.1 | 39.2 | 47.1 | 29.9 | 33.3 | – |
| Energy (Kcal/ 100 g) ^ | 356 | 299 | 380 | 333 | 368 | 342 | – |
| Protein (%) ^ | 21.7 | 18.3 | 20.5 | 17.2 | 24.3 | 21.6 | 15–22 |
| Fat (%) ^ | 4.1 | 3.5 | 9.4 | 9.8 | 9.7 | 9.9 | – |
| Ash (%) ^ | 8.9 | 7.5 | 8.6 | 7.3 | 7.8 | 7.0 | – |
| NDF (%) | 14.9 | 26.9 | 13.6 1 | 24.4 3 | 9.2 5 | 17.3 5 | >10.0 |
| ADF (%) | 0.0 | 11.8 | 0.0 1 | 10.7 3 | 0.1 6 | 7.6 6 | >5.0 |
| TDF (%) ^ | – | 0.5 | 0.8 2 | 1.2 4 | 4.8 7 | 4.6 8 | – |
| NFC (%) * | 50.4 | 43.8 | 47.9 | 41.3 | 49.0 | 44.2 | – |
| Animal | Sex | Age (Years) | Samples with Good Quality DNA | |
|---|---|---|---|---|
| Diet 1 | Diet 2 | |||
| Roscoe | M | 7 | 8 | 4 |
| Juniper | F | 1 | 1 | 5 |
| Arella | F | 7 | 5 | 3 |
| Poko | M | 2 | 3 | 7 |
| Rafael | M | 2 | 3 | 6 |
| Total | 20 | 25 | ||
| Phylum | Median of Relative Abundance | Direction in Higher-Fibre Diet (Diet 2) | p-Value | FDR-Corrected p-Value | |
|---|---|---|---|---|---|
| Diet 1 | Diet 2 | ||||
| Fusobacteriota | 0.000357 | 0.06 | Increased | 0.0000 | 0.0000 |
| Spirochaetota | 0.001765 | 0.0000521 | Decreased | 0.0000 | 0.0000 |
| Desulfobacterota | 0.0003985 | 0.000194 | Decreased | 0.0000 | 0.0003 |
| Acidobacteriota | 0 | 0.00000843 | Increased | 0.0002 | 0.0011 |
| Cyanobacteria | 0.006805 | 0.03 | Increased | 0.0003 | 0.0012 |
| Deferribacterota | 0 | 0.00000815 | Increased | 0.0015 | 0.0048 |
| Bacteroidota | 0.485 | 0.27 | Decreased | 0.0017 | 0.0048 |
| Campylobacterota | 0.00327 | 0.0025 | Decreased | 0.0028 | 0.0071 |
| Patescibacteria | 0.000007145 | 0.0000218 | Increased | 0.0107 | 0.0245 |
| Proteobacteria | 0.135 | 0.11 | Decreased | 0.0263 | 0.0550 |
| Genus | Median of Relative Abundance | Direction in Higher-Fibre Diet (Diet 2) | p-Value | FDR-Corrected p-Value | |
|---|---|---|---|---|---|
| Diet 1 | Diet 2 | ||||
| Prevotella | 0.53620032 | 0.160230548 | Decreased | 0.0000 | 0.0000 |
| Succinivibrio | 0.054766508 | 0.027215864 | Decreased | 0.0000 | 0.0000 |
| Dialister | 0.02799577 | 0.001178487 | Decreased | 0.0059 | 0.0242 |
| Catenibacterium | 0.022250012 | 0.001121756 | Decreased | 0.0000 | 0.0000 |
| Megasphaera | 0.004360297 | 0.002163387 | Decreased | 0.0000 | 0.0000 |
| Anaerovibrio | 0.002077639 | 0.000183251 | Decreased | 0.0000 | 0.0001 |
| Treponema | 0.001095639 | 0.0000478 | Decreased | 0.0000 | 0.0001 |
| Coprococcus | 0.003996526 | 0.002971945 | Decreased | 0.0004 | 0.0021 |
| Butyricicoccus | 0.00227945 | 0.001463972 | Decreased | 0.0012 | 0.0056 |
| Allisonella | 0.000600753 | 0.000172341 | Decreased | 0.0000 | 0.0002 |
| Brachyspira | 0.000220978 | 0 | Decreased | 0.0075 | 0.0286 |
| Acidaminococcus | 0.000120786 | 0 | Decreased | 0.0000 | 0.0000 |
| Mitsuokella | 0.000098648 | 0.00000822 | Decreased | 0.0000 | 0.0000 |
| Asteroleplasma | 0.00006855 | 0.00000822 | Decreased | 0.0096 | 0.0341 |
| Moryella | 0.000009275 | 0 | Decreased | 0.0042 | 0.0182 |
| Victivallis | 0.00000445 | 0 | Decreased | 0.0048 | 0.0199 |
| Faecalitalea | 0.00000389 | 0 | Decreased | 0.0101 | 0.0348 |
| Stenotrophomonas | 0.00000389 | 0.0000109 | Increased | 0.0135 | 0.0446 |
| Psychrobacter | 0 | 0.00000917 | Increased | 0.0000 | 0.0000 |
| Variovorax | 0 | 0.00000956 | Increased | 0.0000 | 0.0001 |
| Comamonas | 0 | 0.00000956 | Increased | 0.0006 | 0.0031 |
| Mucispirillum | 0 | 0.0000108 | Increased | 0.0002 | 0.0013 |
| Duganella | 0 | 0.0000131 | Increased | 0.0017 | 0.0077 |
| Hymenobacter | 0 | 0.0000164 | Increased | 0.0000 | 0.0001 |
| Leuconostoc | 0.00000389 | 0.0000215 | Increased | 0.0134 | 0.0446 |
| Odoribacter | 0 | 0.0000265 | Increased | 0.0000 | 0.0000 |
| Chryseobacterium | 0 | 0.000028 | Increased | 0.0005 | 0.0027 |
| Eisenbergiella | 0 | 0.000052 | Increased | 0.0000 | 0.0000 |
| Cardiobacterium | 0 | 0.0000544 | Increased | 0.0000 | 0.0002 |
| Fusicatenibacter | 0.000082 | 0.000137358 | Increased | 0.0000 | 0.0000 |
| Peptococcus | 0.00001795 | 0.0000813 | Increased | 0.0000 | 0.0002 |
| Intestinimonas | 0.00001079 | 0.0000861 | Increased | 0.0002 | 0.0014 |
| Senegalimassilia | 0.00001685 | 0.000108213 | Increased | 0.0002 | 0.0009 |
| Phocea | 0 | 0.0000934 | Increased | 0.0064 | 0.0255 |
| Conchiformibius | 0.00002235 | 0.000118051 | Increased | 0.0021 | 0.0095 |
| Erysipelatoclostridium | 0 | 0.000115167 | Increased | 0.0000 | 0.0000 |
| Anaerotruncus | 0.000009015 | 0.000143451 | Increased | 0.0000 | 0.0002 |
| Acinetobacter | 0.0000562 | 0.000205443 | Increased | 0.0002 | 0.0014 |
| Hungatella | 0 | 0.00017126 | Increased | 0.0000 | 0.0000 |
| Erwinia | 0.00001395 | 0.000199622 | Increased | 0.0000 | 0.0000 |
| Oscillibacter | 0.0000462 | 0.000247741 | Increased | 0.0000 | 0.0000 |
| Paraprevotella | 0.00003325 | 0.000311881 | Increased | 0.0000 | 0.0000 |
| Alistipes | 0.000326094 | 0.00097874 | Increased | 0.0001 | 0.0005 |
| Pseudomonas | 0.000324257 | 0.001701902 | Increased | 0.0000 | 0.0000 |
| Streptococcus | 0.000388367 | 0.00390552 | Increased | 0.0003 | 0.0017 |
| Tyzzerella | 0.0000317 | 0.005430396 | Increased | 0.0000 | 0.0000 |
| Solobacterium | 0.000758143 | 0.017759871 | Increased | 0.0000 | 0.0000 |
| Subdoligranulum | 0.003984826 | 0.022146933 | Increased | 0.0000 | 0.0000 |
| Phascolarctobacterium | 0.00381844 | 0.028108821 | Increased | 0.0022 | 0.0097 |
| Bacteroides | 0.011833503 | 0.046910755 | Increased | 0.0000 | 0.0000 |
| Fusobacterium | 0.000449843 | 0.066997124 | Increased | 0.0000 | 0.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawless, C.; Kovacs, K.; Mohammadi Dehcheshmeh, M.; Ebrahimie, E.; Messele, Y.E.; Snowball, M.; Trott, D.J.; McLelland, D.J. Higher Dietary Fibre Increases the Faecal Microbiome Diversity of Golden Lion Tamarins (Leontopithecus rosalia). Animals 2025, 15, 1831. https://doi.org/10.3390/ani15131831
Lawless C, Kovacs K, Mohammadi Dehcheshmeh M, Ebrahimie E, Messele YE, Snowball M, Trott DJ, McLelland DJ. Higher Dietary Fibre Increases the Faecal Microbiome Diversity of Golden Lion Tamarins (Leontopithecus rosalia). Animals. 2025; 15(13):1831. https://doi.org/10.3390/ani15131831
Chicago/Turabian StyleLawless, Caitlin, Katrina Kovacs, Manijeh Mohammadi Dehcheshmeh, Esmaeil Ebrahimie, Yohannes E. Messele, Mark Snowball, Darren J. Trott, and David J. McLelland. 2025. "Higher Dietary Fibre Increases the Faecal Microbiome Diversity of Golden Lion Tamarins (Leontopithecus rosalia)" Animals 15, no. 13: 1831. https://doi.org/10.3390/ani15131831
APA StyleLawless, C., Kovacs, K., Mohammadi Dehcheshmeh, M., Ebrahimie, E., Messele, Y. E., Snowball, M., Trott, D. J., & McLelland, D. J. (2025). Higher Dietary Fibre Increases the Faecal Microbiome Diversity of Golden Lion Tamarins (Leontopithecus rosalia). Animals, 15(13), 1831. https://doi.org/10.3390/ani15131831







