Monochromatic Light Impacts the Growth Performance, Intestinal Morphology, Barrier Function, Antioxidant Status, and Microflora of Yangzhou Geese
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Treatments
2.3. Growth Performance
2.4. Sample Collections
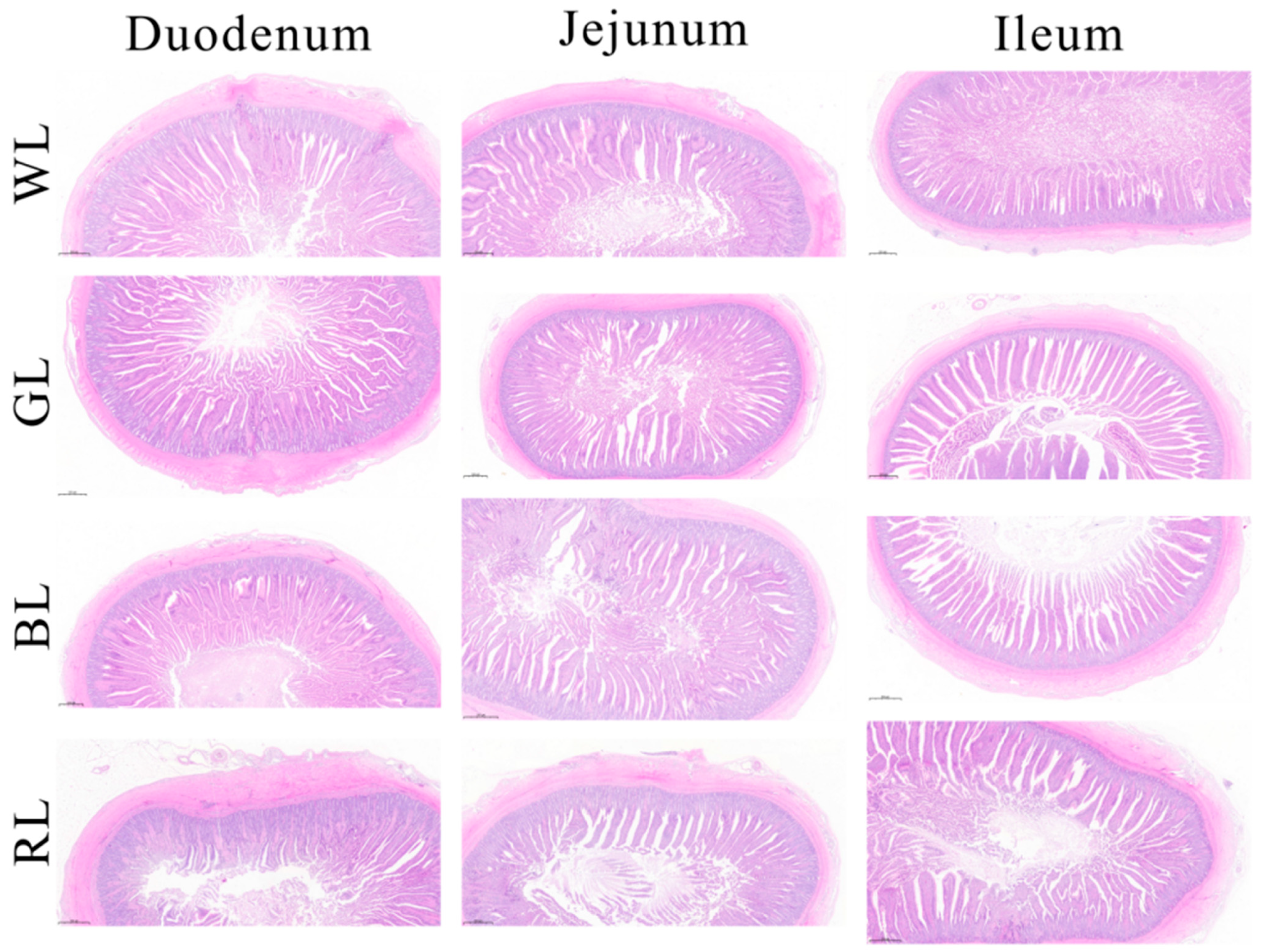
2.5. Intestinal Morphology
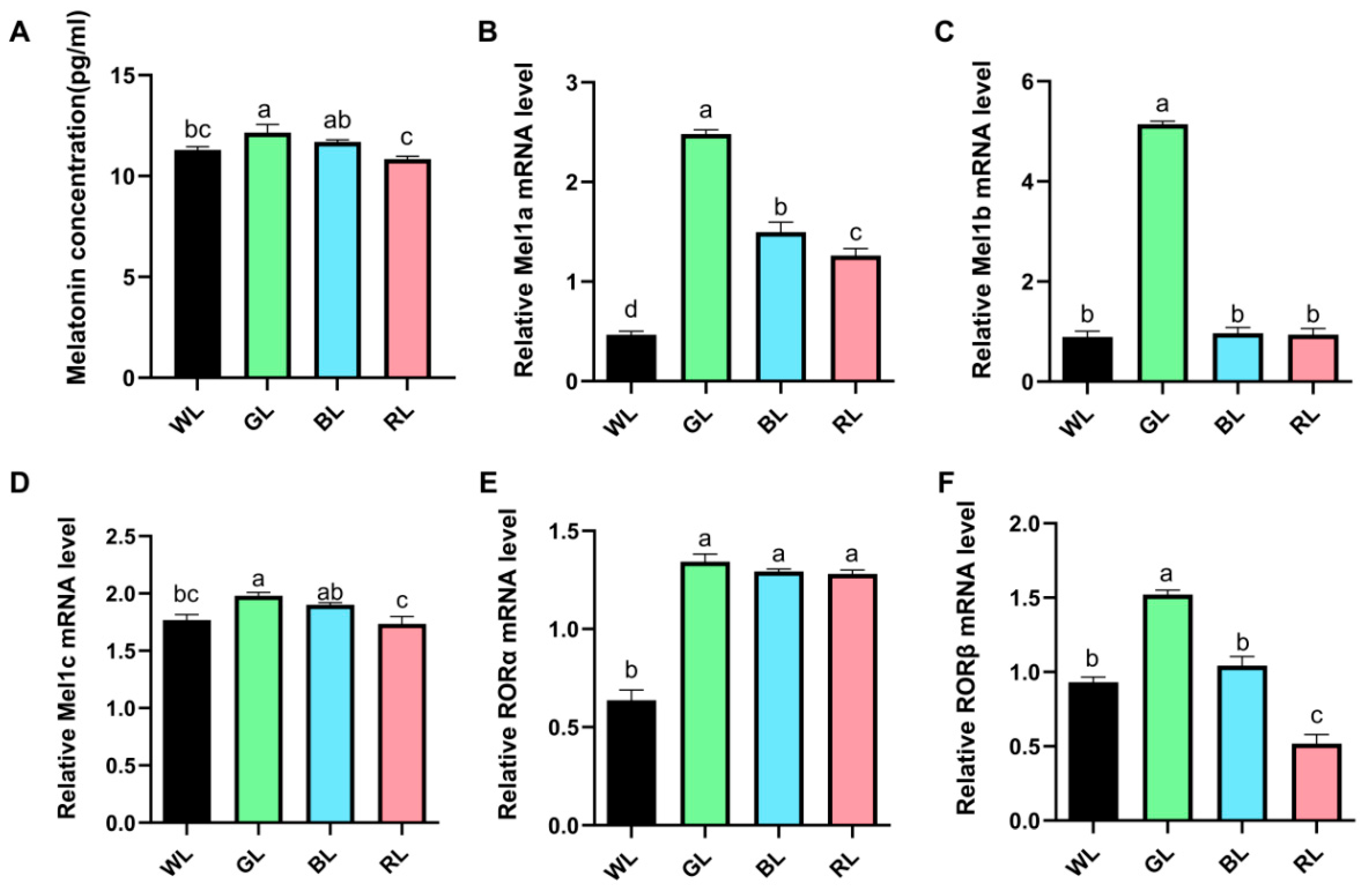
2.6. Detection of Serum Melatonin Level
2.7. Antioxidant Activity Assays
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Microbial Sequencing and Analysis
2.10. Statistical Analysis of Data
3. Results
3.1. Effect of Monochromatic Light on Growth Performance
3.2. Effect of Monochromatic Light on Intestinal Morphology
3.3. Serum Melatonin Levels and Intestine Melatonin Receptors Gene Expression
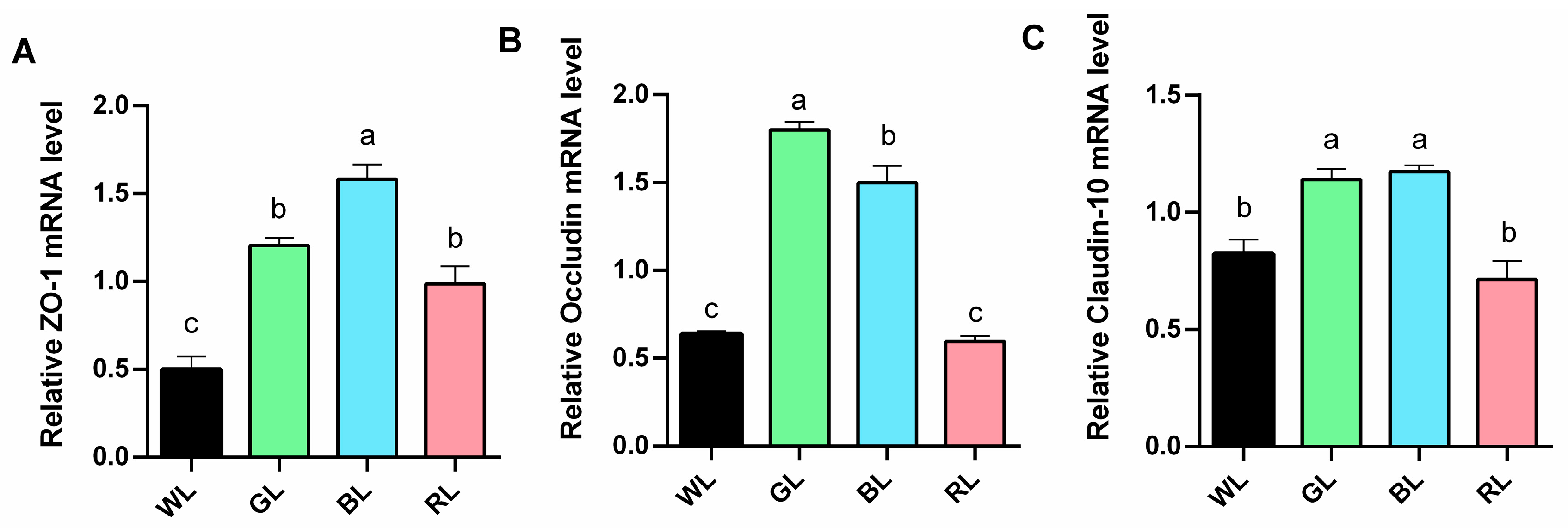
3.4. Mucosal Barrier Functions
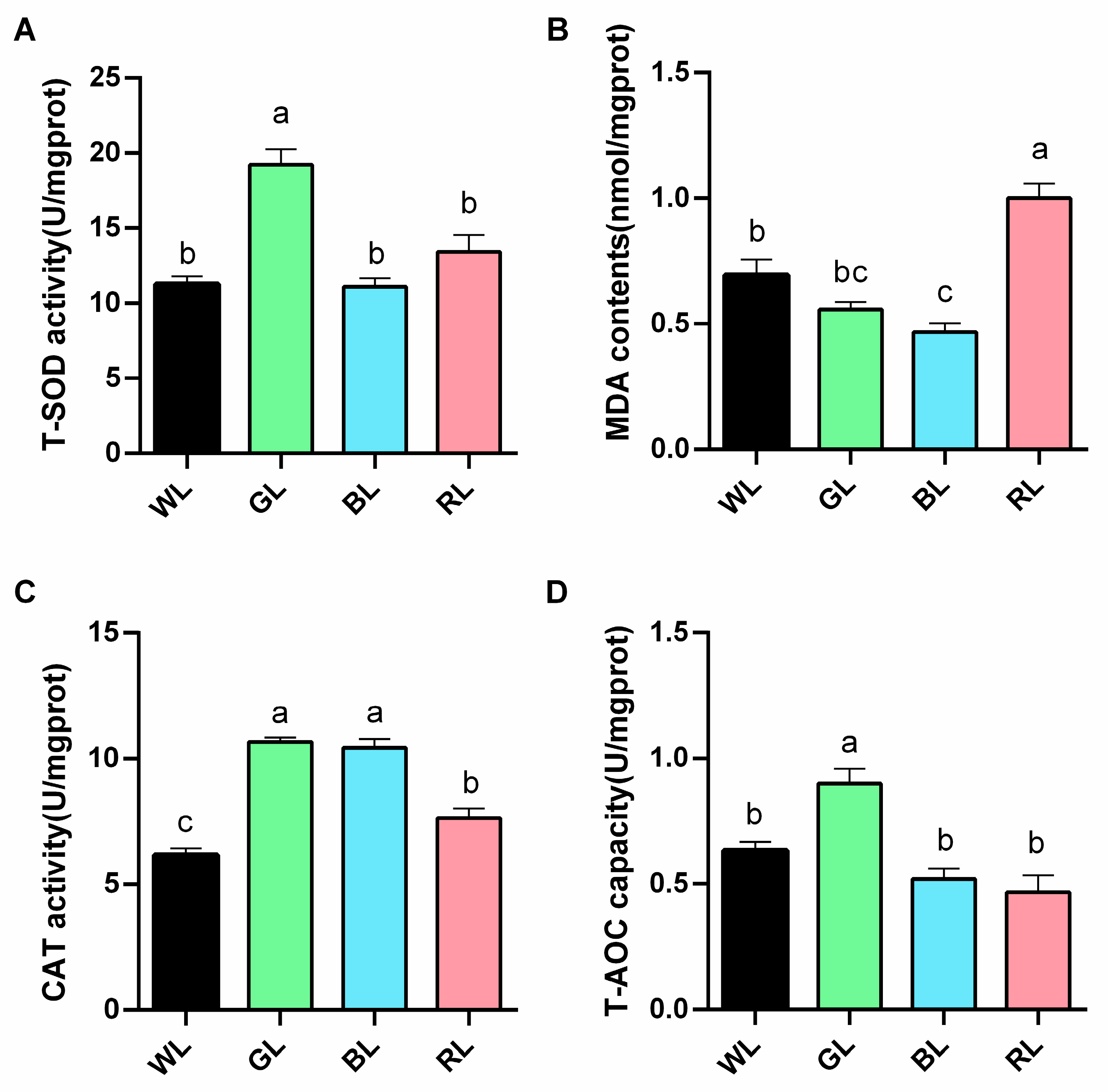
3.5. Antioxidant Status of the Jejunum of Meat Geese
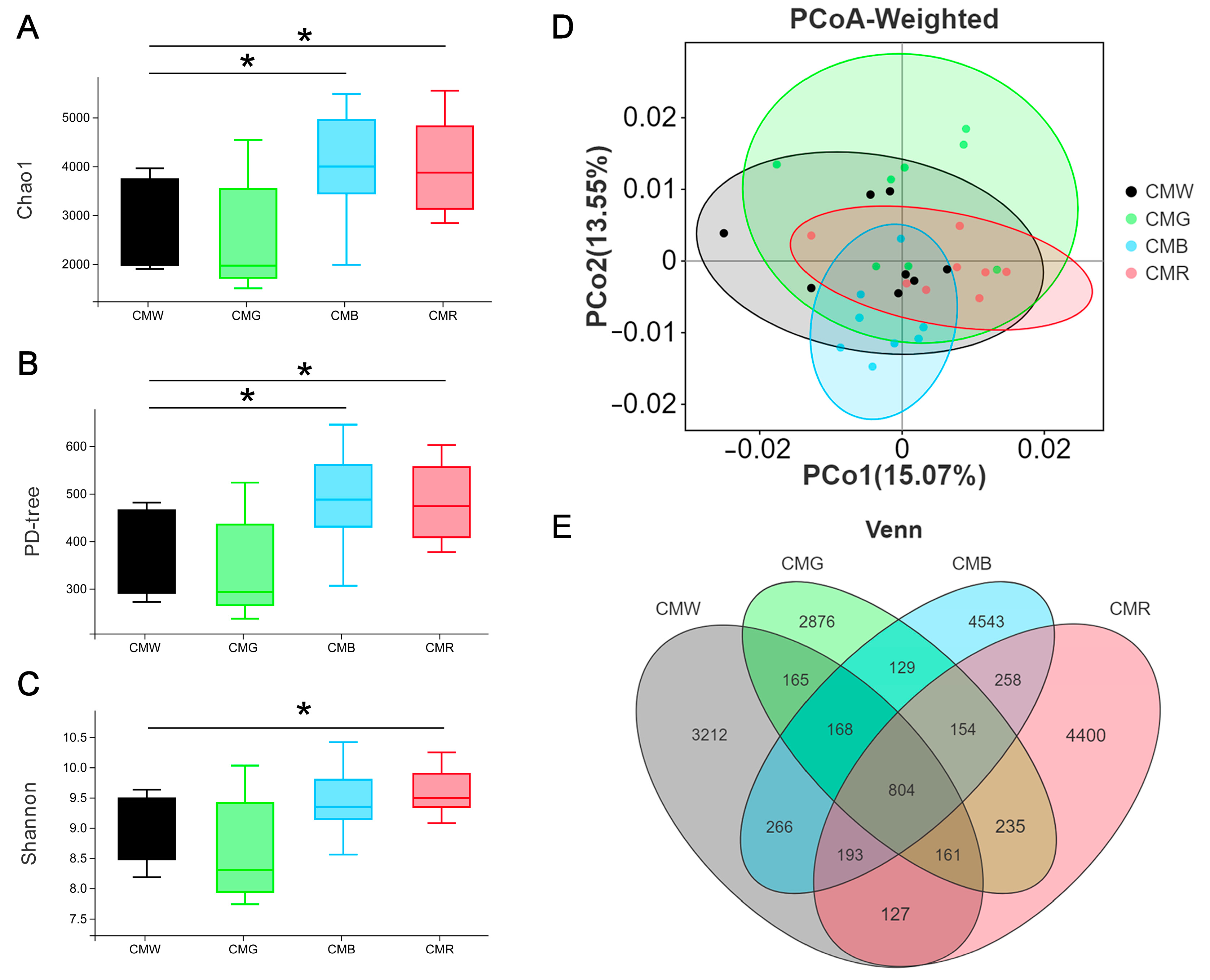
3.6. Effect of Different Monochromatic Light on the Diversity of Intestinal Microflora
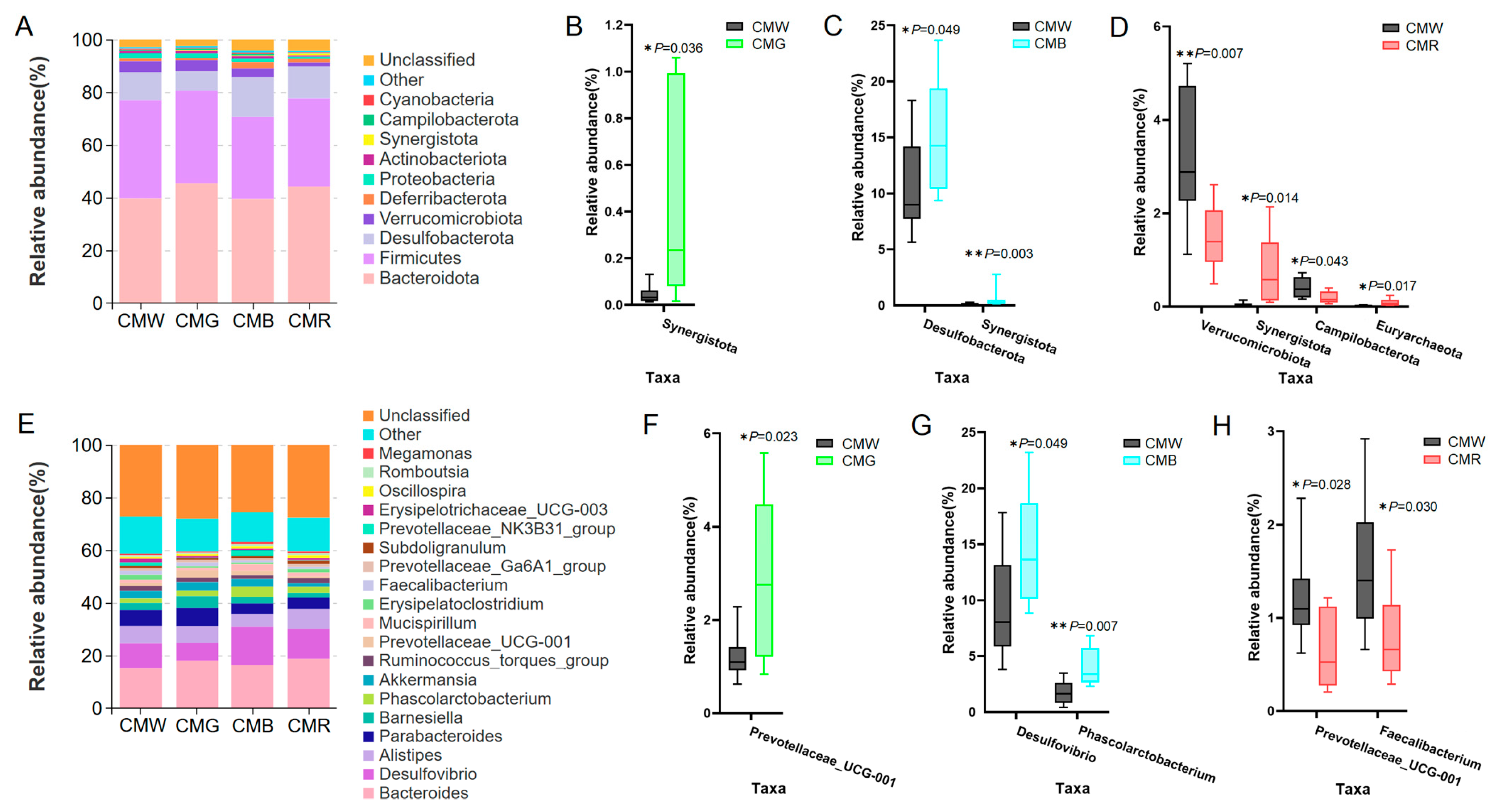
3.7. Changes in Gut Microbiota Composition at the Phylum and Genus Levels
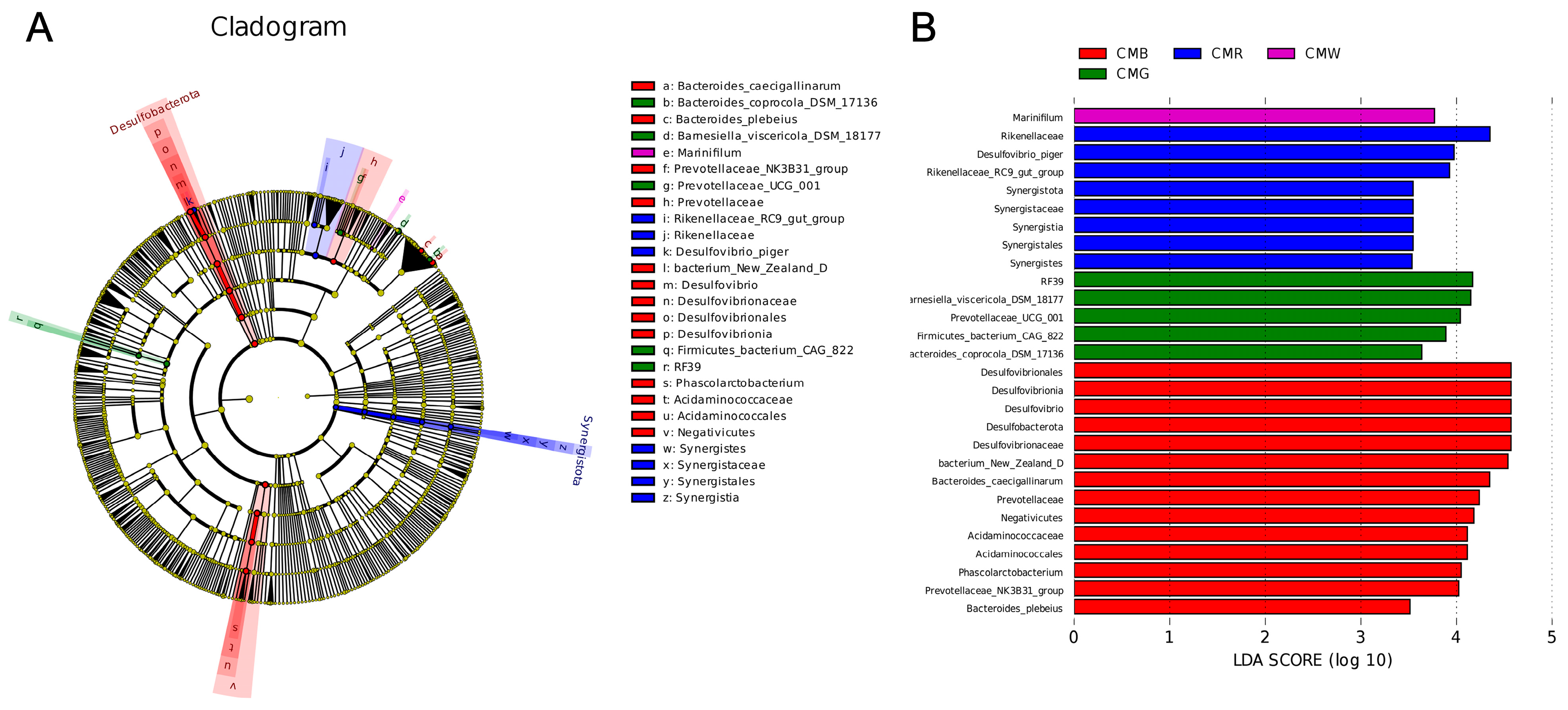
3.8. Linear Discriminant Analysis
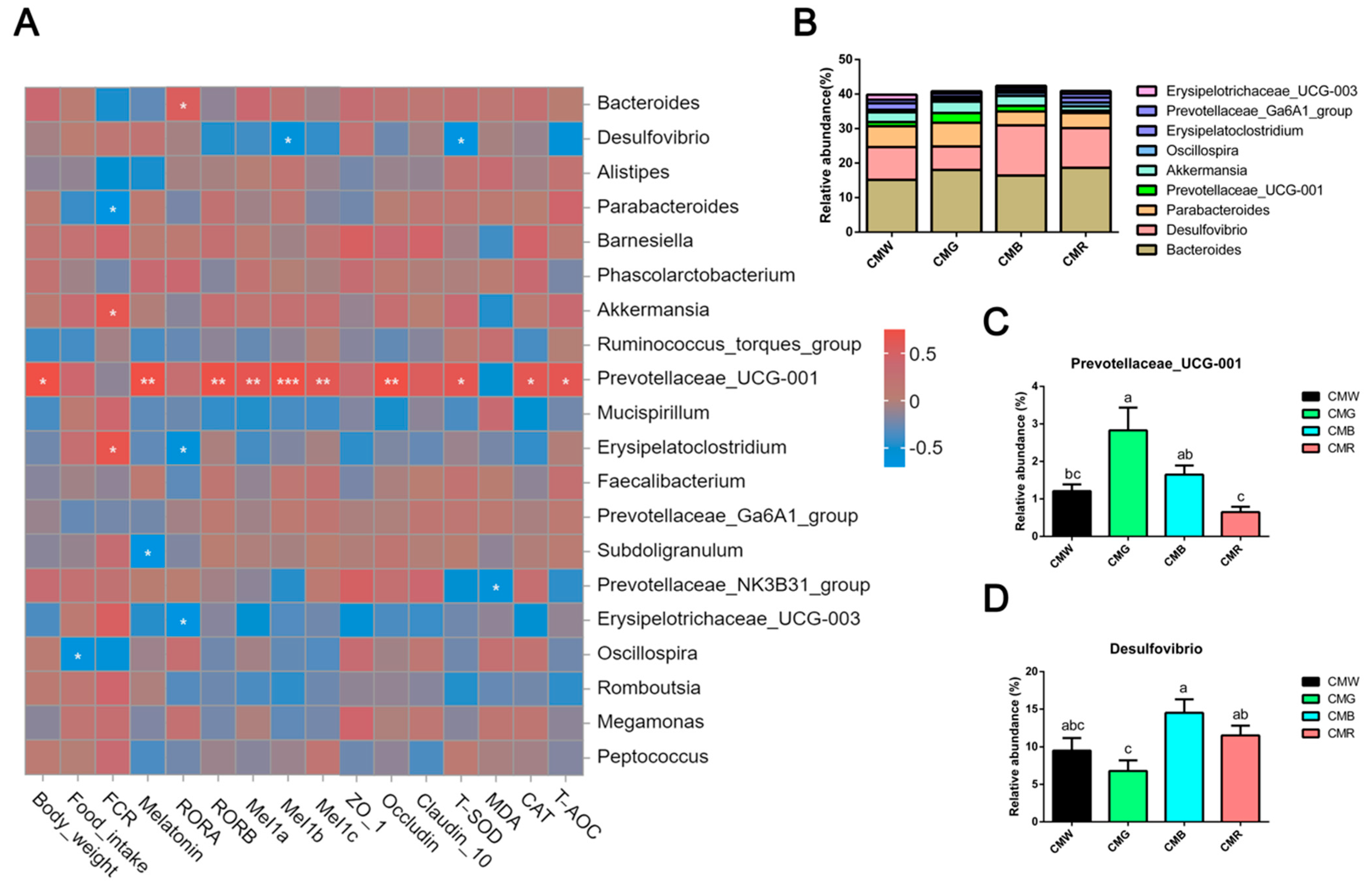
3.9. Correlation Analysis Between Growth Performance, Melatonin and Its Receptors, Gut Health, and Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADFI | average daily feed intake |
| BL | blue light |
| BW | body weight |
| CAT | catalase |
| CD | crypt depths |
| CMB | cecal microflora in the blue light group |
| CMG | cecal microflora in the green light group |
| CMR | cecal microflora in the red light group |
| CMW | cecal microflora in the white light group |
| FCR | feed conversion ratio |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GL | green light |
| H&E | hematoxylin and eosin staining |
| MDA | malondialdehyde |
| Mel | melatonin membrane receptor |
| LDA | linear discriminant analysis |
| LEfSe | linear discriminant analysis effect size |
| OTUs | operational taxonomic units |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| RL | red light |
| ROR | melatonin nuclear receptors |
| RORα | retinoic acid receptor-related orphan receptor α |
| RORβ | retinoic acid receptor-related orphan receptor β |
| T-AOC | total antioxidant capacity |
| T-SOD | total superoxide dismutase |
| VH | villus heights |
| WL | white light |
| ZO-1 | zonula occludens-1 |
References
- Prayitno, D.; Phillips, C.; Omed, H. The effects of color of lighting on the behavior and production of meat chickens. Poult. Sci. 1997, 76, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hu, M.; Guo, B.; Qu, X.; Lei, M.; Chen, R.; Chen, Z.; Shi, Z. Effect and molecular regulatory mechanism of monochromatic light colors on the egg-laying performance of Yangzhou geese. Anim. Reprod. Sci. 2019, 204, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Franco, B.R.; Shynkaruk, T.; Crowe, T.; Fancher, B.; French, N.; Gillingham, S.; Schwean-Lardner, K. Light color and the commercial broiler: Effect on behavior, fear, and stress. Poult. Sci. 2022, 101, 102052. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.M.; Carvalho, L.S.; Cowing, J.A.; Davies, W.L. Evolution and spectral tuning of visual pigments in birds and mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2941–2955. [Google Scholar] [CrossRef]
- Hart, N.S. The Visual Ecology of Avian Photoreceptors. Prog. Retin. Eye Res. 2001, 20, 675–703. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, C.; Zhong, R.; Pan, J. The quantitative models for broiler chicken response to monochromatic, combined, and mixed light-emitting diode light: A meta-analysis. Poult. Sci. 2018, 97, 1980–1989. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Y.; Li, Y.; Fan, J.; Zong, Y.; Isa, A.M.; Shi, L.; Wang, Y.; Ni, A.; Ge, P.; et al. Monochromatic green light stimulation during incubation shortened the hatching time via pineal function in White Leghorn eggs. J. Anim. Sci. Biotechnol. 2021, 12, 17. [Google Scholar] [CrossRef]
- Chen, Z.; Qu, X.; Feng, C.; Guo, B.; Zhu, H.; Yan, L. Monochromatic Green Light Stimulation during Incubation Alters Hepatic Glucose Metabolism That Improves Embryonic Development in Yangzhou Goose Eggs. Int. J. Mol. Sci. 2022, 24, 405. [Google Scholar] [CrossRef]
- Xu, X.; Fan, S.; Wu, H.; Li, H.; Shan, X.; Wang, M.; Zhang, Y.; Xu, Q.; Chen, G. A 16S RNA Analysis of Yangzhou Geese with Varying Body Weights: Gut Microbial Difference and Its Correlation with Body Weight Parameters. Animals 2024, 14, 2042. [Google Scholar] [CrossRef]
- Hao, C.; Wu, D.; Mo, L.; Xu, G. A review on gut microbial diversity and function of overwintering animals. Biodivers. Sci. 2024, 32, 23407. [Google Scholar] [CrossRef]
- Liu, G.; Luo, X.; Zhao, X.; Zhang, A.; Jiang, N.; Yang, L.; Huang, M.; Xu, L.; Ding, L.; Li, M.; et al. Gut microbiota correlates with fiber and apparent nutrients digestion in goose. Poult. Sci. 2018, 97, 3899–3909. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Ye, F.; He, F.; Song, Q.; Xiong, X.; Yang, W.; Gang, X.; Hu, J.; Hu, B.; Xu, H.; et al. Comparison of overfeeding effects on gut physiology and microbiota in two goose breeds. Poult. Sci. 2021, 100, 100960. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Liu, Y.; Wang, C.; Yang, Y.; Gong, S.; Zhu, L.; He, D.; Wang, H. Supplementation with honeysuckle extract improves growth performance, immune performance, gut morphology, and cecal microbes in geese. Front. Vet. Sci. 2022, 9, 1006318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Akhtar, M.; Chen, Y.; Ma, Z.; Liang, Y.; Shi, D.; Cheng, R.; Cui, L.; Hu, Y.; Nafady, A.A.; et al. Chicken jejunal microbiota improves growth performance by mitigating intestinal inflammation. Microbiome 2022, 10, 107. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Ansari, A.R.; Akhtar, M.; Chen, Y.; Cheng, R.; Cui, L.; Nafady, A.A.; Elokil, A.A.; Abdel-Kafy, E.M.; et al. Caecal microbiota could effectively increase chicken growth performance by regulating fat metabolism. Microb. Biotechnol. 2021, 15, 844–861. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Blue Light Alters the Composition of the Jejunal Microbiota and Promotes the Development of the Small Intestine by Reducing Oxidative Stress. Antioxidants 2022, 11, 274. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Álvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Kankova, Z.; Drozdova, A.; Hodova, V.; Zeman, M. Effect of blue and red monochromatic light during incubation on the early post-embryonic development of immune responses in broiler chicken. Br. Poult. Sci. 2022, 63, 541–547. [Google Scholar] [CrossRef]
- Nguyen, T.N.D.; Le, H.N.; Eva, P.; Alberto, F.; Le, T.H. Relationship between the ratio of villous height: Crypt depth and gut bacteria counts aswell production parameters in broiler chickens. J. Agric. Dev. 2021, 20, 1–10. [Google Scholar] [CrossRef]
- Barros, J.S.G.; Sartor, K.; Pedroso, T.F.; Vasconcelos, H.; Scopacasa, V.A.; Bottura, J.R.; Sena, R.G.; Salvador, M.J.; de Moura, D.J. Impact of light spectrum electromagnetic radiation variations on performance and hormonal profiles in laying hens. Sci. Rep. 2024, 14, 30250. [Google Scholar] [CrossRef]
- Pan, C.; Xiang, R.; Pan, J. Lighting quality evaluation on growth performance and feather pecking behavior of broilers. Poult. Sci. 2024, 104, 104656. [Google Scholar] [CrossRef] [PubMed]
- Oke, O.E.; Oni, A.I.; Adebambo, P.O.; Oso, O.M.; Adeoye, M.M.; Lawal, T.G.; Afolayan, T.R.; Ogunbajo, O.E.; Ojelade, D.I.; Bakre, O.A.; et al. Evaluation of light colour manipulation on physiological response and growth performance of broiler chickens. Trop. Anim. Health Prod. 2020, 53, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tang, Y.; Cheng, Y.; Yang, W.; Liu, J.; Guo, B.; Luo, G.; Zhu, H. Effects of different monochromatic light on growth performance and liver circadian rhythm of Yangzhou geese. Poult. Sci. 2025, 104, 104496. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Wang, Z.; Dong, Y.; Chen, Y.; Cao, J. Mel1b and Mel1c melatonin receptors mediate green light-induced secretion of growth hormone in chick adenohypophysis cells via the AC/PKA and ERK1/2 signalling pathways. J. Photochem. Photobiol. B 2021, 225, 112322. [Google Scholar] [CrossRef]
- Bao, Q.; Gu, W.; Song, L.; Weng, K.; Cao, Z.; Zhang, Y.; Zhang, Y.; Ji, T.; Xu, Q.; Chen, G. The Photoperiod-Driven Cyclical Secretion of Pineal Melatonin Regulates Seasonal Reproduction in Geese (Anser cygnoides). Int. J. Mol. Sci. 2023, 24, 11998. [Google Scholar] [CrossRef]
- Jin, E.; Jia, L.; Li, J.; Yang, G.; Wang, Z.; Cao, J.; Chen, Y. Effect of monochromatic light on melatonin secretion and arylalkylamine N-acetyltransferase mRNA expression in the retina and pineal gland of broilers. Anat. Rec. 2011, 294, 1233–1241. [Google Scholar] [CrossRef]
- Franco, B.R.; Shynkaruk, T.; Crowe, T.; Fancher, B.; French, N.; Gillingham, S.; Schwean-Lardner, K. Light wavelength and its impact on broiler health. Poult. Sci. 2022, 101, 102178. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. The signal pathway of melatonin mediates the monochromatic light-induced T-lymphocyte apoptosis in chicken thymus. Poult. Sci. 2024, 103, 103331. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Melatonin nuclear receptors mediate monochromatic light-induced T-lymphocyte proliferation of thymus through the AKT/GSK3β/β-catenin pathway in chick. Poult. Sci. 2024, 103, 104507. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Leo, M.D.M.; Subramani, J.; Anish, D.; Sudhagar, M.; Ahmed, K.A.; Saxena, M.; Tyagi, J.S.; Sastry, K.V.H.; Saxena, V.K. Expression analysis of melatonin receptor subtypes in the ovary of domestic chicken. Vet. Res. Commun. 2009, 33, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Alsiddig, M.A.; Yu, S.G.; Pan, Z.X.; Widaa, H.; Badri, T.M.; Chen, J.; Liu, H.L. Association of single nucleotide polymorphism in melatonin receptor 1A gene with egg production traits in Yangzhou geese. Anim. Genet. 2017, 48, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Dong, Y.; Cao, J.; Wang, Z.; Zhang, Z.; Chen, Y. Developmental changes of melatonin receptor expression in the spleen of the chicken, Gallus domesticus. Acta Histochem. 2015, 117, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin mediates monochromatic light–induced proliferation of T/B lymphocytes in the spleen via the membrane receptor or nuclear receptor. Poult. Sci. 2020, 99, 4294–4302. [Google Scholar] [CrossRef]
- Yu, Y. Effects of Different Light Wavelengths on Development of Small Intestine and Bursa in Chick Embryos during the Late Period and Its Modulation Mechanisms; China Agricultural University: Beijing, China, 2014. [Google Scholar]
- Xie, D.; Li, J.; Wang, Z.X.; Cao, J.; Li, T.T.; Chen, J.L.; Chen, Y.X. Effects of monochromatic light on mucosal mechanical and immunological barriers in the small intestine of broilers. Poult. Sci. 2011, 90, 2697–2704. [Google Scholar] [CrossRef]
- Li, J.; Cao, J.; Wang, Z.; Dong, Y.; Chen, Y. Melatonin plays a critical role in inducing B lymphocyte proliferation of the bursa of Fabricius in broilers via monochromatic lights. J. Photochem. Photobiol. B Biol. 2015, 142, 29–34. [Google Scholar] [CrossRef]
- Cui, Y.-M.; Wang, J.; Zhang, H.-J.; Qi, G.-H.; Qiao, H.-Z.; Gan, L.-P.; Wu, S.-G. Effect of Changes in Photoperiods on Melatonin Expression and Gut Health Parameters in Laying Ducks. Front. Microbiol. 2022, 13, 819427. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Effects of Different Monochromatic Light Combinations on Cecal Microbiota Composition and Cecal Tonsil T Lymphocyte Proliferation. Front. Immunol. 2022, 13, 849780. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Fan, S.; Gong, X.; Zhou, Y.; Jian, Y.; Ouyang, J.; Jiang, Q.; Zhang, P. Effects of LED Light Colors on the Growth Performance, Intestinal Morphology, Cecal Short-Chain Fatty Acid Concentrations and Microbiota in Broilers. Animals 2023, 13, 3731. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, Y.; Zhang, D.; Ma, K.; Gong, Y.; Luo, X.; Liu, J.; Cui, S. Intestinal melatonin levels and gut microbiota homeostasis are independent of the pineal gland in pigs. Front. Microbiol. 2024, 15, 1352586. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Z.; Pan, J.; Jiang, D.; Tian, Y.; Fang, L.; Huang, Y. Impacts of colored light-emitting diode illumination on the growth performance and fecal microbiota in goose. Poult. Sci. 2020, 99, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Morrison, M.; Yu, Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013, 92, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Leng, X.; Zhao, Y.; Zhao, Y.; Wang, Q. Effects of dietary Artemisia annua supplementation on growth performance, antioxidant capacity, immune function, and gut microbiota of geese. Poult. Sci. 2024, 103, 103594. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Pearston, F.; Pritchard, T.; Wall, E.; Coffey, M.; Nani, J.; Mucha, S.; McLaren, A.; Mrode, R.; Conington, J.; et al. Effects of supplementing sow diets during late gestation with Pennisetum purpureum on antioxidant indices, immune parameters and faecal microbiota. Vet. Med. Sci. 2021, 7, 1347–1358. [Google Scholar]
- Qi, Z.; Shi, S.; Tu, J.; Li, S. Comparative metagenomic sequencing analysis of cecum microbiotal diversity and function in broilers and layers. 3 Biotech 2019, 9, 316. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Cong, X.; Ren, J.; Li, J.; Zhu, J.; Dai, M.; Hrabchenko, N.; Du, Y.; Qi, J. The functional role of fecal microbiota transplantation on Salmonella Enteritidis infection in chicks. Vet. Microbiol. 2022, 269, 109449. [Google Scholar] [CrossRef]
| Genes | Primer Sequence (5′ to 3′) | Accession Number |
|---|---|---|
| Mella-F | GTTCCGCAGCTTCTTGTTG | XM_066996266.1 |
| Mella-R | CTGGGTCACCTCCACCTTG | |
| Mel1b-F | TGTGGTAATTCATTTCATCGTCCC | U30609 |
| Mel1b-R | TTGGTGCCATTTCTGAAGGATTGAT | |
| Mel1c-F | CAGATAAGTGGGTTCCTGATGGG | U31821 |
| Mel1c-R | ACCGAAGGCTGTGGCAGATGTAG | |
| RORα-F | GGCAGTTATGCGCAGTCAAA | XM_067003930.1 |
| RORα-R | TTCTGGGAGTCAAAGGCACG | |
| RORβ-F | GCAATGGCTTGAGCAACCTG | XM_048073568.1 |
| RORβ-R | GCTGGGCAGAATCCACATTG | |
| GAPDH-F | GCCATCACAGCCACACAGA | XM_067004670.1 |
| GAPDH-R | TTTTCCCACAGCCTTAGCA | |
| ZO-1-F | TACGCTGTTGAATGTCCC | XM_013177403.1 |
| ZO-1-R | ATGGTCTGAAGGCTCTGA | |
| occludin-F | TCCCGCCGCTTCTACCT | XM_013199669.1 |
| occludin-R | CACCTGGCTGCACATGG | |
| claudin-10F | ATGACTGGTTGTTCCCTGTA | XM_013201623.1 |
| claudin-10R | AGCCCATCCAATGAATAAAG |
| Items | Treatments 1 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| White Light | Green Light | Blue Light | Red Light | |||
| 1d BW(g) | 98.47 | 97.86 | 97.76 | 97.35 | 1.82 | 0.9386 |
| 70d BW(g) | 4336 bc | 4537 a | 4425 abc | 4254 c | 0.35 | 0.0013 |
| ADFI(g) | 200.46 a | 199.68 a | 198.93 a | 187.69 b | 4.54 | 0.0486 |
| FCR | 3.05 | 3.01 | 3.08 | 2.98 | 0.09 | 0.6616 |
| Items 1 | Treatments 1 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| White Light | Green Light | Blue Light | Red Light | |||
| Duodenum | ||||||
| VH, μm | 3339.20 c | 4645.45 a | 4299.88 a | 3371.95 c | 134.81 | <0.0001 |
| CD, μm | 1401.03 b | 1007.49 c | 973.92 c | 1527.66 a | 52.47 | |
| VH/CD | 2.39 b | 4.62 a | 4.45 a | 2.22 b | 0.17 | |
| Jejunum | ||||||
| VH, μm | 3869.17 c | 4822.63 a | 4263.07 b | 3271.82 d | 82.48 | <0.0001 |
| CD, μm | 1250.97 b | 852.91 d | 1098.46 c | 1438.09 a | 52.79 | |
| VH/CD | 3.11 c | 5.68 a | 3.9 b | 2.29 d | 0.19 | |
| Ileum | ||||||
| VH, μm | 3329.6 b | 4092 a | 3931.18 a | 2972.76 c | 120.31 | <0.0001 |
| CD, μm | 1000.18 b | 773.44 c | 819.52 c | 1297.44 a | 34.41 | |
| VH/CD | 3.36 b | 5.3 a | 4.83 a | 2.29 c | 0.24 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, G.; Cheng, Y.; Xu, Y.; Liu, J.; Yang, W.; Liu, J.; Guo, B.; Zhu, H. Monochromatic Light Impacts the Growth Performance, Intestinal Morphology, Barrier Function, Antioxidant Status, and Microflora of Yangzhou Geese. Animals 2025, 15, 1815. https://doi.org/10.3390/ani15121815
Luo G, Cheng Y, Xu Y, Liu J, Yang W, Liu J, Guo B, Zhu H. Monochromatic Light Impacts the Growth Performance, Intestinal Morphology, Barrier Function, Antioxidant Status, and Microflora of Yangzhou Geese. Animals. 2025; 15(12):1815. https://doi.org/10.3390/ani15121815
Chicago/Turabian StyleLuo, Gang, Yiyi Cheng, Yingqing Xu, Jie Liu, Wen Yang, Jiying Liu, Binbin Guo, and Huanxi Zhu. 2025. "Monochromatic Light Impacts the Growth Performance, Intestinal Morphology, Barrier Function, Antioxidant Status, and Microflora of Yangzhou Geese" Animals 15, no. 12: 1815. https://doi.org/10.3390/ani15121815
APA StyleLuo, G., Cheng, Y., Xu, Y., Liu, J., Yang, W., Liu, J., Guo, B., & Zhu, H. (2025). Monochromatic Light Impacts the Growth Performance, Intestinal Morphology, Barrier Function, Antioxidant Status, and Microflora of Yangzhou Geese. Animals, 15(12), 1815. https://doi.org/10.3390/ani15121815







