Effect of Adding Alkaline Metal Ions Complexes Rumen Microbiota and Metabolome of Hu Lambs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
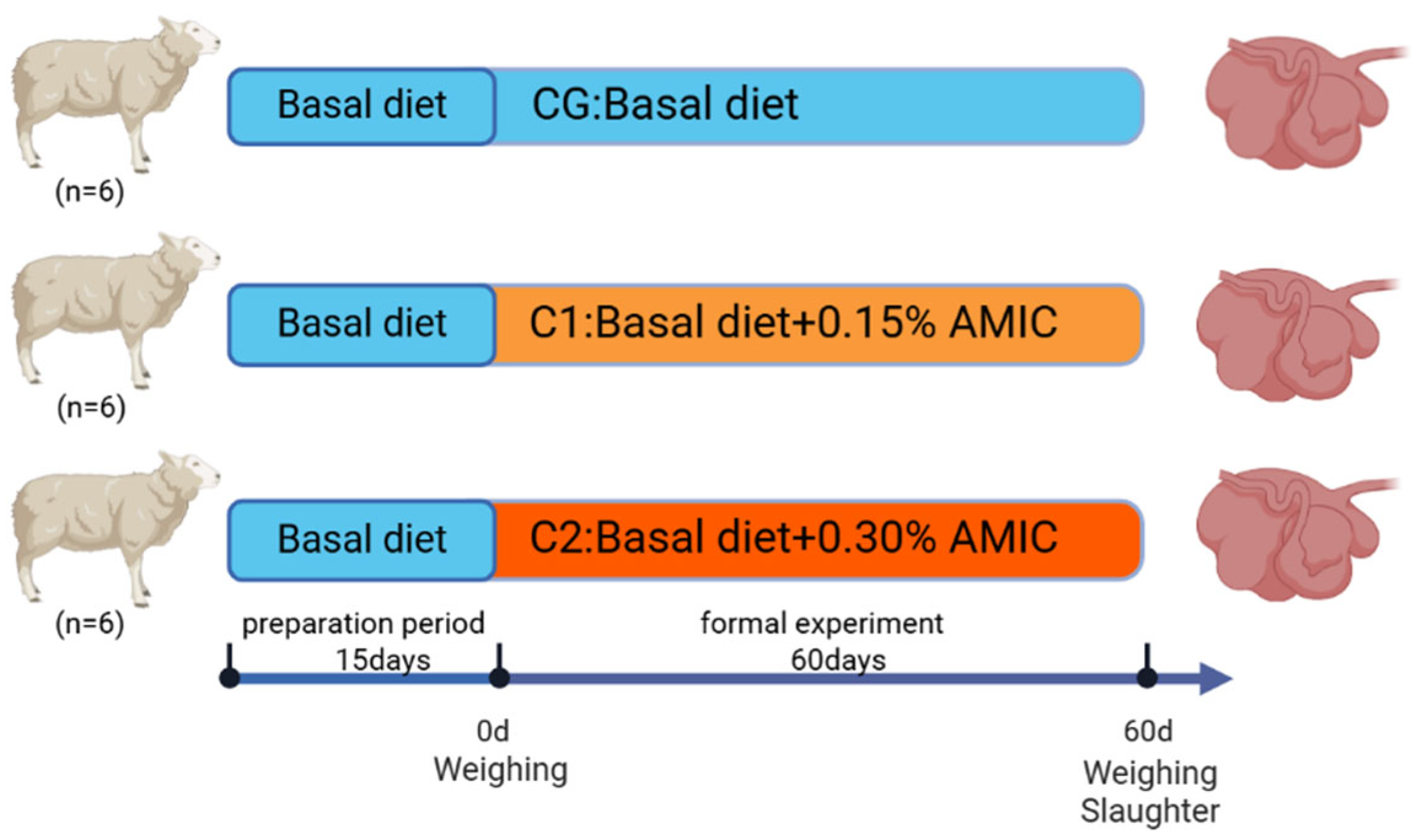
2.1. Experimental Animals, Design, and Management
2.2. Growth Performance Determination
2.3. Sample Collection
2.4. Rumen Fermentation Parameters
2.5. Rumen Tissue Morphology
2.6. Rumen Microbiota
2.7. Rumen Untargeted Metabolomics
2.8. Statistical Analysis
3. Results
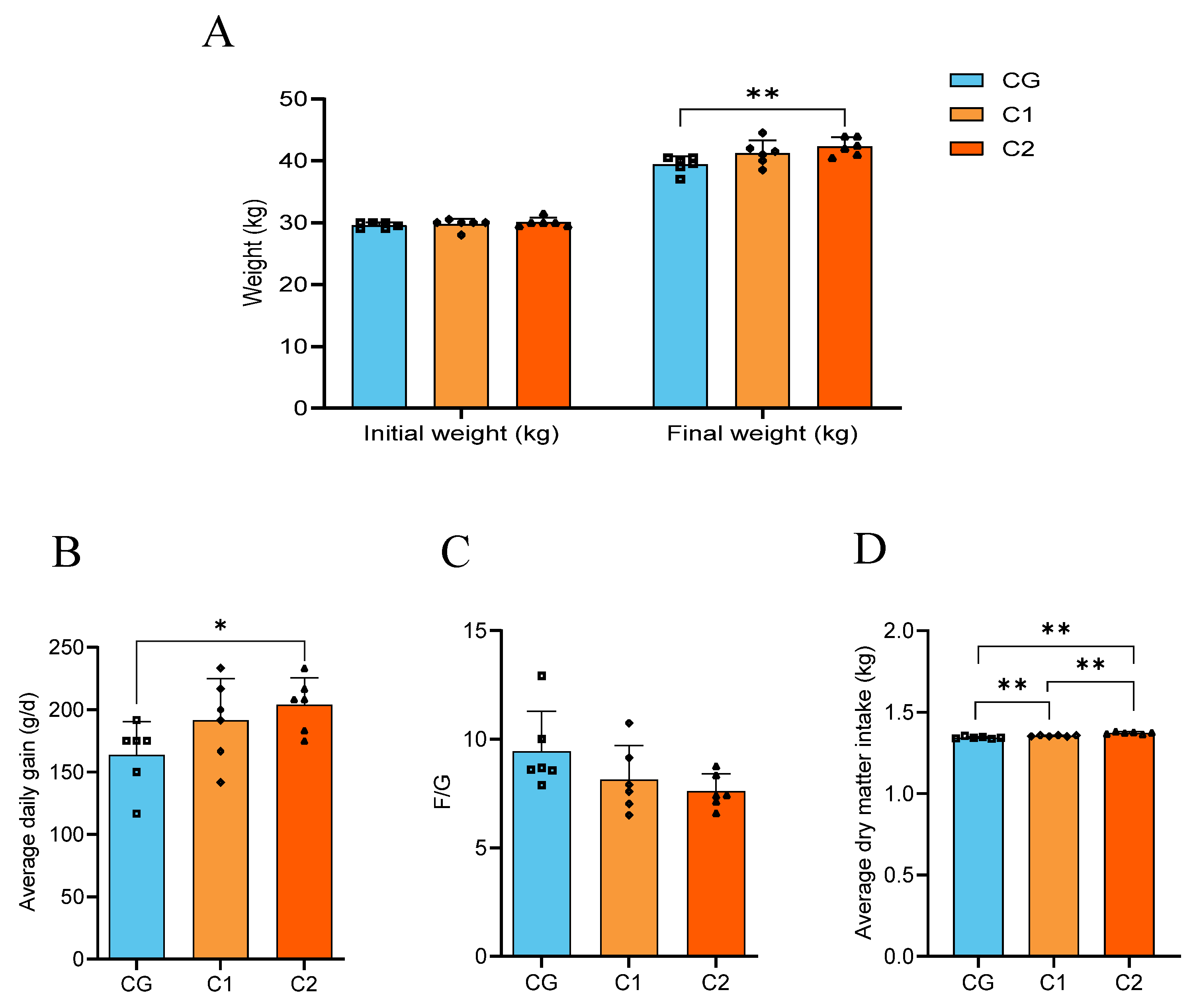
3.1. Growth Performance
3.2. Rumen Fermentation Parameters
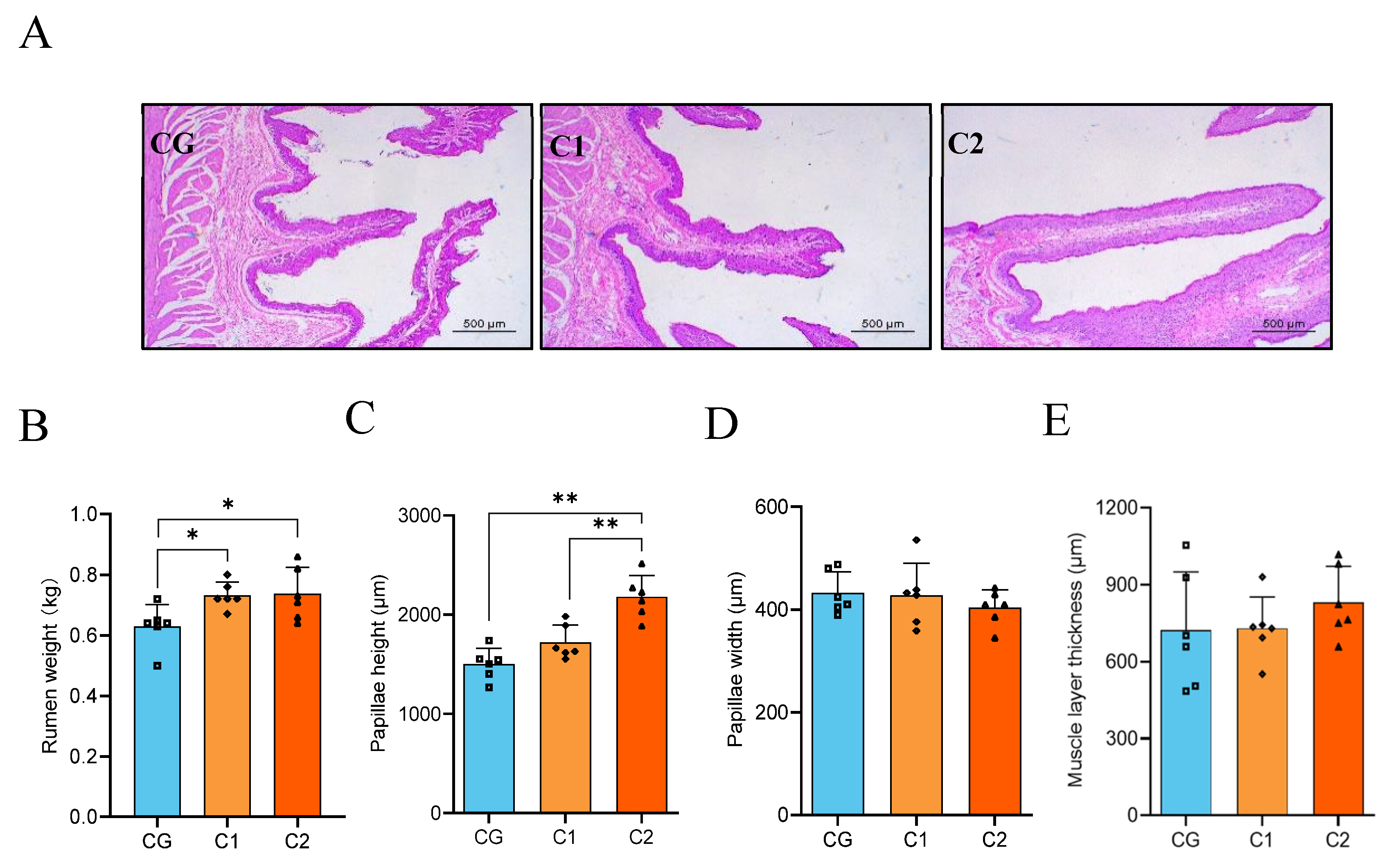
3.3. Rumen Slice
3.4. Rumen Microbiota
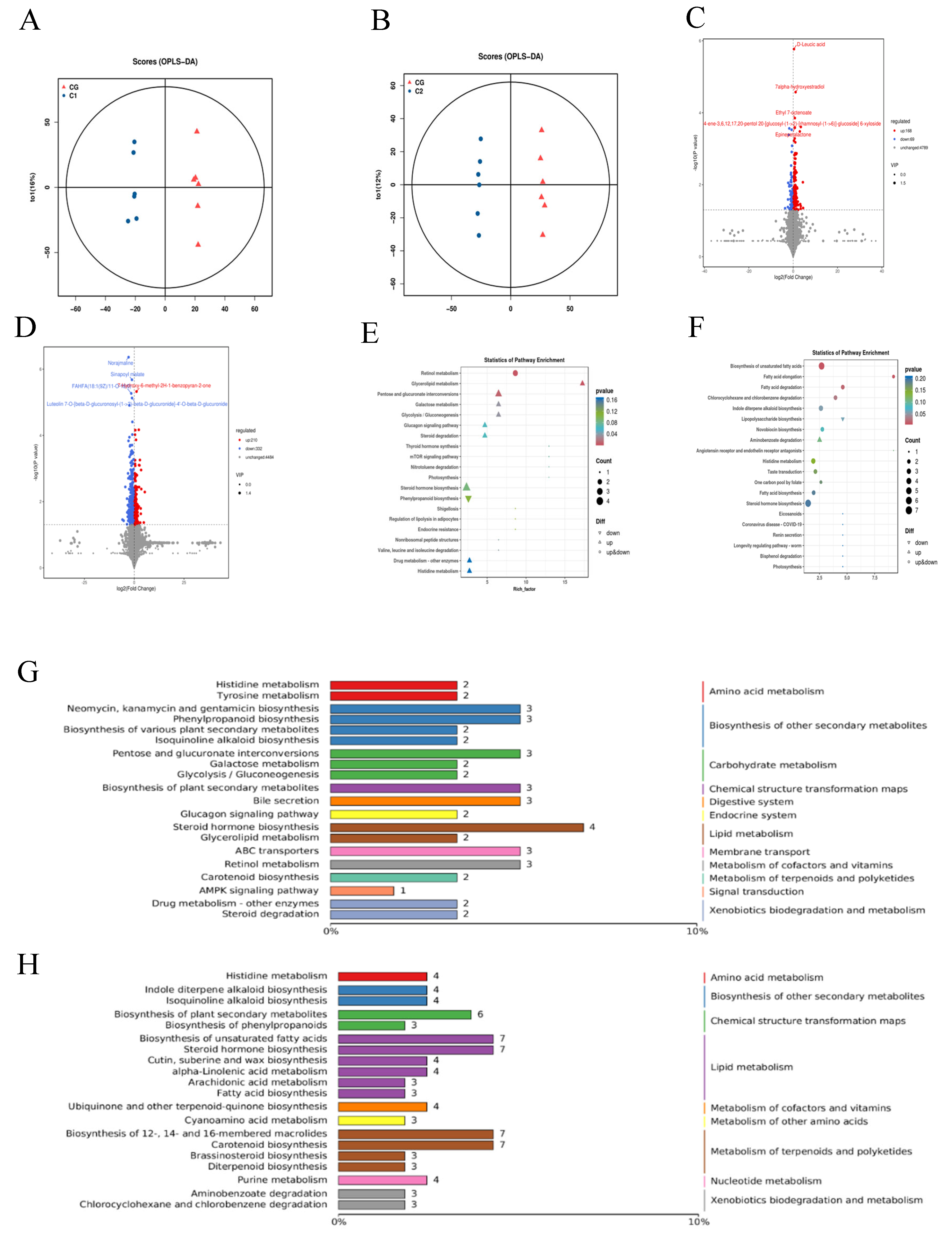
3.5. Rumen Metabolism
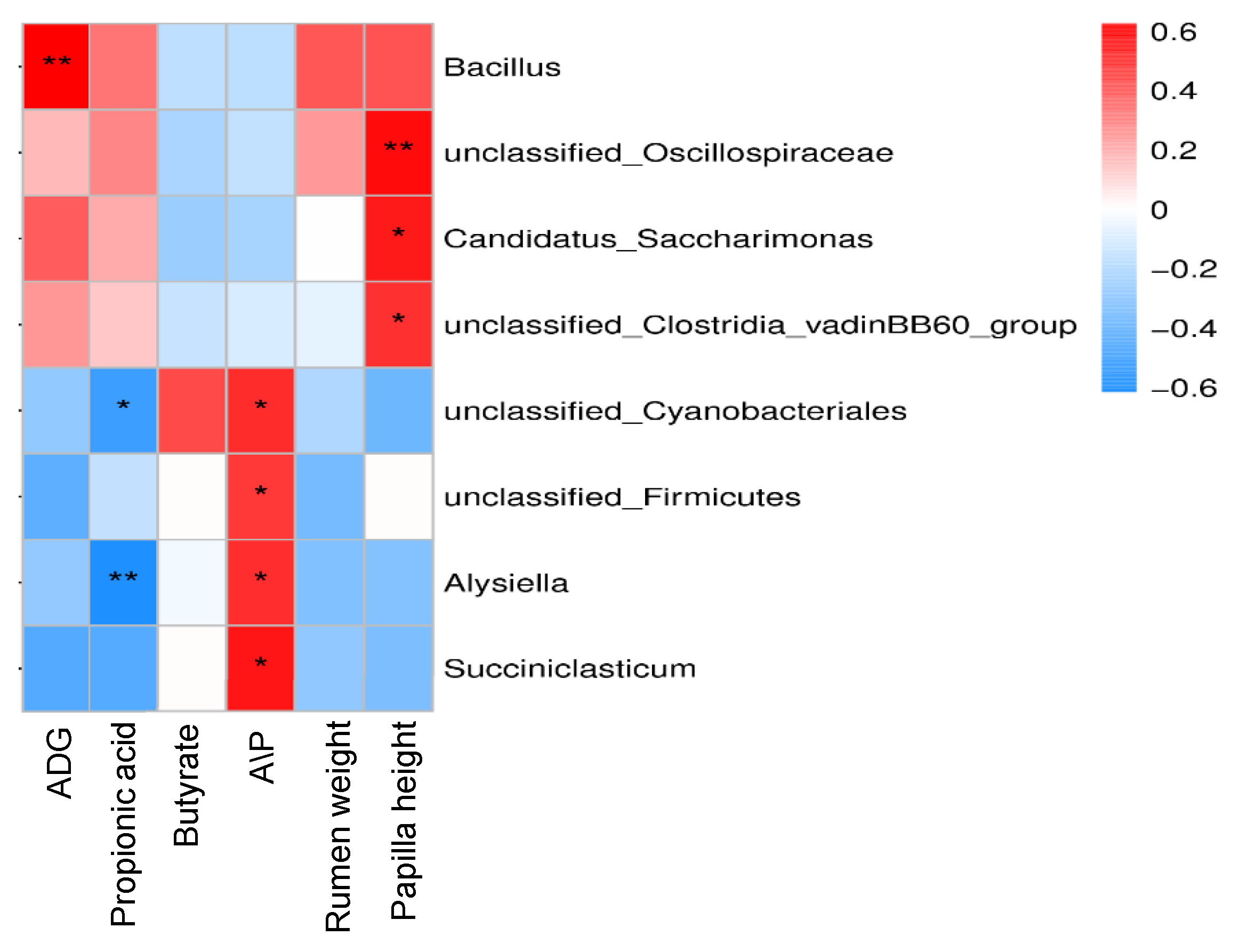
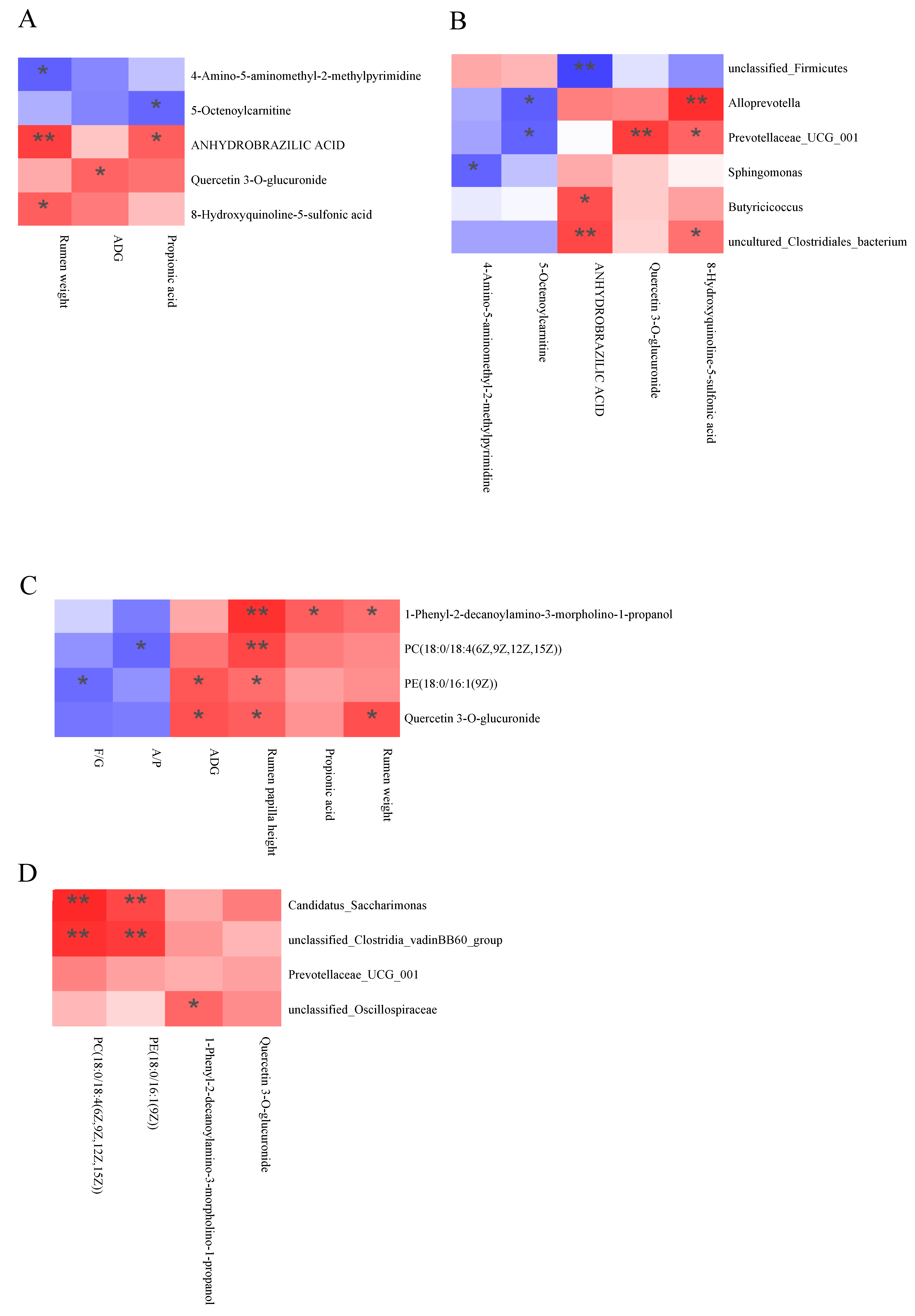
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belanche, A.; Yáñez-Ruiz, D.R.; Detheridge, A.P.; Griffith, G.W.; Kingston-Smith, A.H.; Newbold, C.J. Maternal versus artificial rearing shapes the rumen microbiome having minor long-term physiological implications. Environ. Microbiol. 2019, 21, 4360–4377. [Google Scholar] [CrossRef] [PubMed]
- Orpin, C.G. Invasion of plant tissue in the rumen by the flagellate Neocallimastix frontalis. J. Gen. Microbiol. 1977, 98, 30–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, H.; Browne, F.; Roehe, R.; Dewhurst, R.J.; Engel, F.; Hemmje, M.; Lu, X.; Walsh, P. Integrated metagenomic analysis of the rumen microbiome of cattle reveals key biological mechanisms associated with methane traits. Methods 2017, 124, 108–119. [Google Scholar] [CrossRef]
- Ishaq, S.L.; Wright, A.D. Insight into the bacterial gut microbiome of the North American moose (Alces alces). BMC Microbiol. 2012, 12, 212. [Google Scholar] [CrossRef]
- Tong, F.; Wang, T.; Gao, N.L.; Liu, Z.; Cui, K.; Duan, Y.; Wu, S.; Luo, Y.; Li, Z.; Yang, C.; et al. The microbiome of the buffalo digestive tract. Nat. Commun. 2022, 13, 823. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.P.; Teng, J.L.L.; Chiu, T.H.; Chan, E.; Tsang, A.K.L.; Panagiotou, G.; Zhai, S.L.; Woo, P.C.Y. Differential Microbial Communities of Omnivorous and Herbivorous Cattle in Southern China. Comput. Struct. Biotechnol. J. 2018, 16, 54–60. [Google Scholar] [CrossRef]
- Tang, Y. Effects of AMC on Intestinal Health in Chicks. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2022. [Google Scholar]
- Guo, C.; Kong, F.; Li, S.; Wang, X.; Sun, X.; Du, W.; Dai, D.; Wang, S.; Xie, B.; Xu, X. Effect of alkaline mineral complex buffer supplementation on milk performance, serum variables, rumen fermentation and rumen microbiota of transition dairy cows. Fermentation 2023, 9, 792. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.R.; Kang, J.X.; Zhao, B.C.; Dai, X.Y.; Qiu, B.H.; Li, J.L. Effects of alkaline mineral complex water supplementation on growth performance, inflammatory response, and intestinal barrier function in weaned piglets. J. Anim. Sci. 2022, 100, skac251. [Google Scholar] [CrossRef]
- Wang, F.; Cao, B.; Zhang, W.; Li, Q.; Wu, J. Effects of dietary Cilijian supplemetation on growth performance, feed digestibility and serum biochemical index in finishing crossbred steers. J. China Agric. Univ. 2009, 14, 81–86. (In Chinese) [Google Scholar]
- Kang, Z.; Wang, Z.; LI, Q.; Yang, Z.; Zhang, D.; Chen, G.; Chen, S.; Jia, X. Effects of an Ionic Feed Additives on Rabbit Reproductive Performance of Rabbit Under Heat Stress. Chin. J. Rabbit. Farming 2023, 6, 7–11. (In Chinese) [Google Scholar]
- Chaney, A.L.; Marbach, E.P. Modified Reagents for Determination of Urea and Ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Verdouw, H.; Van Echteld, C.J.A.; Dekkers, E.M.J. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 1978, 12, 399–402. [Google Scholar] [CrossRef]
- Rehemujiang, H.; Yusuf, H.A.; Ma, T.; Diao, Q.; Kong, L.; Kang, L.; Tu, Y. Fermented cottonseed and rapeseed meals outperform soybean meal in improving performance, rumen fermentation, and bacterial composition in Hu sheep. Front. Microbiol. 2023, 14, 1119887. [Google Scholar] [CrossRef]
- Gunun, P.; Wanapat, M.; Gunun, N.; Cherdthong, A.; Sirilaophaisan, S.; Kaewwongsa, W. Effects of Condensed Tannins in Mao (Antidesma thwaitesianum Muell. Arg.) Seed Meal on Rumen Fermentation Characteristics and Nitrogen Utilization in Goats. Asian-Australas. Assoc. Anim. Prod. Soc. (AAAP) Korean Soc. Anim. Sci. Technol. (KSAST) 2015, 29, 1111. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, Y.; Zeng, X.; Wang, X.; Wang, Y.; Dai, C.; Li, J.; Huang, P.; Huang, J.; Hussain, T.; et al. Effects of Dietary Energy Levels on Rumen Fermentation, Gastrointestinal Tract Histology, and Bacterial Community Diversity in Fattening Male Hu Lambs. Front. Microbiol. 2021, 12, 695445. [Google Scholar] [CrossRef]
- Nkosi, B.V.Z.; Padayachee, T.; Gront, D.; Nelson, D.R.; Syed, K. Contrasting Health Effects of Bacteroidetes and Firmicutes Lies in Their Genomes: Analysis of P450s, Ferredoxins, and Secondary Metabolite Clusters. Int. J. Mol. Sci. 2022, 23, 5057. [Google Scholar] [CrossRef]
- Bowen, J.M.; McCabe, M.S.; Lister, S.J.; Cormican, P.; Dewhurst, R.J. Evaluation of Microbial Communities Associated with the Liquid and Solid Phases of the Rumen of Cattle Offered a Diet of Perennial Ryegrass or White Clover. Front. Microbiol. 2018, 9, 2389. [Google Scholar] [CrossRef]
- Ma, J.; Shah, A.M.; Shao, Y.; Wang, Z.; Zou, H.; Kang, K. Dietary supplementation of yeast cell wall improves the gastrointestinal development of weaned calves. Anim. Nutr. 2020, 6, 507–512. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Hou, S.; Raza, S.H.A.; Gui, L.; Sun, S.; Wang, Z.; Yang, B.; Yuan, Z.; Simal-Gandara, J.; et al. Metabolomics approach reveals high energy diet improves the quality and enhances the flavor of black Tibetan sheep meat by altering the composition of rumen microbiota. Front. Nutr. 2022, 9, 915558. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Z.; Tan, Y.; Chang, S.; Zheng, H.; Wang, H.; Yan, T.; Guru, T.; Hou, F. Selenium Yeast Dietary Supplement Affects Rumen Bacterial Population Dynamics and Fermentation Parameters of Tibetan Sheep (Ovis aries) in Alpine Meadow. Front. Microbiol. 2021, 12, 663945. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Hao, Y.; Zhang, Y.; Liu, Y.; Pu, Z.; Tian, Y.; Xu, D.; Xia, X.; He, Z.; et al. QTL Mapping for Grain Zinc and Iron Concentrations in Bread Wheat. Front. Nutr. 2021, 8, 680391. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, S.; Lei, L.; Tao, S.; Xiong, Y.; Wu, G.; Hu, J.; Yuan, X.; Zhao, S.; Zuo, B.; et al. Intrauterine growth restriction alters nutrient metabolism in the intestine of porcine offspring. J. Anim. Sci. Biotechnol. 2021, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Dong, G. Effects of Different Forms of Linseed Oil on Meat and Mutton Quality, Muscle Fatty Acid, Serum Biochemical Index, Rumen Fermentation Index Rumen Microbial. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2020. [Google Scholar]
- Yang, L.; Min, G.; Honglian, H.; Yanyong, S. Ruminal Absorption Mechanism of Volatile Fatty Acids in Ruminants. Chin. J. Anim. Nutr. 2018, 30, 2070–2078. (In Chinese) [Google Scholar]
- Froetschel, M.A.; Martin, A.C.; Amos, H.E.; Evans, J.J. Effects of zinc sulfate concentration and feeding frequency on ruminal protozoal numbers, fermentation patterns and amino acid passage in steers. J. Anim. Sci. 1990, 68, 2874–2884. [Google Scholar] [CrossRef]
- O’Shea, E.; Waters, S.M.; Keogh, K.; Kelly, A.K.; Kenny, D.A. Examination of the molecular control of ruminal epithelial function in response to dietary restriction and subsequent compensatory growth in cattle. J. Anim. Sci. Biotechnol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Rong, T.; Wang, G.; Liu, Z.; Chen, W.; Li, J.; Li, J.; Ma, X. Different dietary starch sources alter the carcass traits, meat quality, and the profile of muscle amino acid and fatty acid in finishing pigs. J. Anim. Sci. Biotechnol. 2020, 11, 78. [Google Scholar] [CrossRef]
- Guo, C.; Wang, X.; Dai, D.; Kong, F.; Wang, S.; Sun, X.; Li, S.; Xu, X.; Zhang, L. Effects of alkaline mineral complex supplementation on production performance, serum variables, and liver transcriptome in calves. Front. Vet. Sci. 2023, 10, 1282055. [Google Scholar] [CrossRef]
- Anderson, C.J.; Koester, L.R.; Schmitz-Esser, S. Rumen Epithelial Communities Share a Core Bacterial Microbiota: A Meta-Analysis of 16S rRNA Gene Illumina MiSeq Sequencing Datasets. Front. Microbiol. 2021, 12, 625400. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Pan, X.; Shi, Z.; Feng, X.; Gong, B.; Li, J.; Wang, L. Enhanced Growth and Activities of the Dominant Functional Microbiota of Chicken Manure Composts in the Presence of Maize Straw. Front. Microbiol. 2018, 9, 1131. [Google Scholar] [CrossRef]
- Liu, Z.; Li, A.; Wang, Y.; Iqbal, M.; Zheng, A.; Zhao, M.; Li, Z.; Wang, N.; Wu, C.; Yu, D. Comparative analysis of microbial community structure between healthy and Aeromonas veronii-infected Yangtze finless porpoise. Microb. Cell Factories 2020, 19, 123. [Google Scholar] [CrossRef]
- Liu, L.; Zou, Z.; Yang, J.; Li, X.; Zhu, B.; Zhang, H.; Sun, Y.; Zhang, Y.; Zhang, Z.J.; Wang, W. Jianpi Jieyu Decoction, An Empirical Herbal Formula, Exerts Psychotropic Effects in Association with Modulation of Gut Microbial Diversity and GABA Activity. Front. Pharmacol. 2021, 12, 645638. [Google Scholar] [CrossRef] [PubMed]
- Said, E.; Mousa, S.; Fawzi, M.; Sabry, N.A.; Farid, S. Combined effect of high-dose vitamin A, vitamin E supplementation, and zinc on adult patients with diabetes: A randomized trial. J. Adv. Res. 2021, 28, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Jiang, J.; Yang, P.; Cai, J. Synthesis, Characterization and Antioxidant Activity of a Novel Organogermanium Sesquioxide with Resveratrol. Bull. Korean Chem. Soc. 2012, 33, 1121–1122. [Google Scholar] [CrossRef]
- Zhang, Z.; Shahzad, K.; Shen, S.; Dai, R.; Lu, Y.; Lu, Z.; Li, C.; Chen, Y.; Qi, R.; Gao, P.; et al. Altering Dietary Soluble Protein Levels with Decreasing Crude Protein May Be a Potential Strategy to Improve Nitrogen Efficiency in Hu Sheep Based on Rumen Microbiome and Metabolomics. Front. Nutr. 2021, 8, 815358. [Google Scholar] [CrossRef]
- Wang, B.; Ma, M.P.; Diao, Q.Y.; Tu, Y. Saponin-Induced Shifts in the Rumen Microbiome and Metabolome of Young Cattle. Front. Microbiol. 2019, 10, 356. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, T.; Alugongo, G.M.; Khan, M.Z.; Li, T.; Ma, J.; Liu, S.; Wang, W.; Wang, Y.; Li, S.; et al. Effect of the Length of Oat Hay on Growth Performance, Health Status, Behavior Parameters and Rumen Fermentation of Holstein Female Calves. Metabolites 2021, 11, 890. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Vázquez-Fresno, R.; Serra-Cayuela, A.; Dong, E.; Mandal, R.; Hennessy, D.; McAuliffe, S.; Dillon, P.; Wishart, D.S.; Stanton, C.; et al. Pasture Feeding Changes the Bovine Rumen and Milk Metabolome. Metabolites 2018, 8, 27. [Google Scholar] [CrossRef]
- Qiu, Q.; Gao, C.; Aziz Ur Rahman, M.; Cao, B.; Su, H. Digestive Ability, Physiological Characteristics, and Rumen Bacterial Community of Holstein Finishing Steers in Response to Three Nutrient Density Diets as Fattening Phases Advanced. Microorganisms 2020, 8, 335. [Google Scholar] [CrossRef]
- Tamura, K.; Sasaki, H.; Shiga, K.; Miyakawa, H.; Shibata, S. The Timing Effects of Soy Protein Intake on Mice Gut Microbiota. Nutrients 2020, 12, 87. [Google Scholar] [CrossRef]
- Chen, T.J.; Feng, Y.; Liu, T.; Wu, T.T.; Chen, Y.J.; Li, X.; Li, Q.; Wu, Y.C. Fisetin Regulates Gut Microbiota and Exerts Neuroprotective Effect on Mouse Model of Parkinson’s Disease. Front. Neurosci. 2020, 14, 549037. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, J.; Hu, C.; Ruan, B.; Zhu, B. Oral Microbiota Is Associated with Immune Recovery in Human Immunodeficiency Virus-Infected Individuals. Front. Microbiol. 2021, 12, 794746. [Google Scholar] [CrossRef] [PubMed]
- Falalyeyeva, T.; Chornenka, N.; Cherkasova, L.; Tsyryuk, O. Gut Microbiota Interactions with Obesity. In Comprehensive Gut Microbiota; Glibetic, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–219. [Google Scholar] [CrossRef]
- Watanabe, S.; Nishijima, N.; Hirai, K.; Shibata, K.; Hase, A.; Yamanaka, T.; Inazu, M. Anticancer Activity of Amb4269951, a Choline Transporter-Like Protein 1 Inhibitor, in Human Glioma Cells. Pharmaceuticals 2020, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Huo, J.; Zhan, K.; Zhao, G.; Wu, Y.; Ma, Y. Effects of quercetin on antioxidant and anti-inflammatory properties of goat rumen epithelial cells stimulated with lipopolysaccharide. Pratacultural Sci. 2021, 38, 1393–1401. (In Chinese) [Google Scholar]
- Domínguez Moré, G.P.; Cardona, M.I.; Sepúlveda, P.M.; Echeverry, S.M.; Oliveira Simões, C.M.; Aragón, D.M. Matrix Effects of the Hydroethanolic Extract of Calyces of Physalis peruviana L. on Rutin Pharmacokinetics in Wistar Rats Using Population Modeling. Pharmaceutics 2021, 13, 535. [Google Scholar] [CrossRef]

| Item | Ingredient, % | Nutrient Composition | Content, g/kg |
|---|---|---|---|
| Corn | 29.00 | DE (MJ/kg) | 11.18 |
| Melon seed shells | 16.00 | Dry matter (%) | 89.11 |
| Corn germ meaL-so1 | 14.50 | Crude ash (%) | 9.12 |
| Soyabean meal | 6.00 | Crude Protein (%) | 19.71 |
| Cottonseed meal | 5.50 | Crude fat (%) | 12.90 |
| Black flour | 5.00 | Neutral detergent fiber, NDF (%) | 32.68 |
| DDGS | 4.00 | Acid detergent fiber, ADF (%) | 13.77 |
| Feed material, sprayed corn bran | 3.00 | Acid detergent lignin, ADL (%) | 2.28 |
| Jujube powder | 3.00 | Calcium (%) | 1.93 |
| Phragmites australis | 3.00 | Total phosphorus (%) | 0.43 |
| Rice protein meal | 2.00 | ||
| Molasses | 2.00 | ||
| Bentonite | 1.50 | ||
| Stone dust | 1.30 | ||
| Soybean germ powder | 1.00 | ||
| Calcium sulfate | 1.00 | ||
| Salt | 0.70 | ||
| Premix | 1.50 | ||
| Total | 100 |
| Ions | Calculate Concentration (mg/kg) |
|---|---|
| Na+ | 27,482.00 |
| K+ | 25,103.00 |
| Zn2+ | 5.20 |
| Ge4+ | 0.13 |
| HCO3− | 36,218.00 |
| Item | CG | C1 | C2 | SEM | p-Value |
|---|---|---|---|---|---|
| pH | 6.01 | 6.07 | 6.12 | 0.04 | 0.1707 |
| NH3-N (mg/dL) | 12.32 | 12.94 | 12.80 | 1.32 | 0.9422 |
| BCP (mg/dL) | 28.78 a | 30.25 ab | 30.87 b | 0.63 | 0.0327 |
| Acetate (mmol/L) | 38.03 | 38.12 | 40.84 | 2.26 | 0.6143 |
| Propionate (mmol/L) | 21.01 a | 27.41 b | 26.89 b | 1.63 | 0.0248 |
| Butyrate (mmol/L) | 10.23 a | 7.97 b | 8.97 ab | 0.67 | 0.0303 |
| Acetate/Propionate | 1.90 a | 1.40 b | 1.52 b | 0.12 | 0.0231 |
| Total VFA (mmol/L) | 69.26 | 73.50 | 76.70 | 3.73 | 0.3920 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Ma, C.; Li, Y.; An, Z.; Yang, Y.; Gao, F.; Li, C.; Liu, Y. Effect of Adding Alkaline Metal Ions Complexes Rumen Microbiota and Metabolome of Hu Lambs. Animals 2025, 15, 1816. https://doi.org/10.3390/ani15121816
Li M, Ma C, Li Y, An Z, Yang Y, Gao F, Li C, Liu Y. Effect of Adding Alkaline Metal Ions Complexes Rumen Microbiota and Metabolome of Hu Lambs. Animals. 2025; 15(12):1816. https://doi.org/10.3390/ani15121816
Chicago/Turabian StyleLi, Mingyue, Chi Ma, Yalin Li, Ziyi An, Yilin Yang, Feng Gao, Changqing Li, and Yingchun Liu. 2025. "Effect of Adding Alkaline Metal Ions Complexes Rumen Microbiota and Metabolome of Hu Lambs" Animals 15, no. 12: 1816. https://doi.org/10.3390/ani15121816
APA StyleLi, M., Ma, C., Li, Y., An, Z., Yang, Y., Gao, F., Li, C., & Liu, Y. (2025). Effect of Adding Alkaline Metal Ions Complexes Rumen Microbiota and Metabolome of Hu Lambs. Animals, 15(12), 1816. https://doi.org/10.3390/ani15121816





