Assessment of Stress in Dogs Under Cancer Therapy via Faecal Cortisol Metabolite Analysis: A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Dogs for the Study
2.2. Description of the Study Participants
2.3. Description of the Treatment Protocols
2.4. Faeces Sampling
2.5. Analysis of Faecal Cortisol Metabolites (FCMs)
2.6. Statistical Analysis
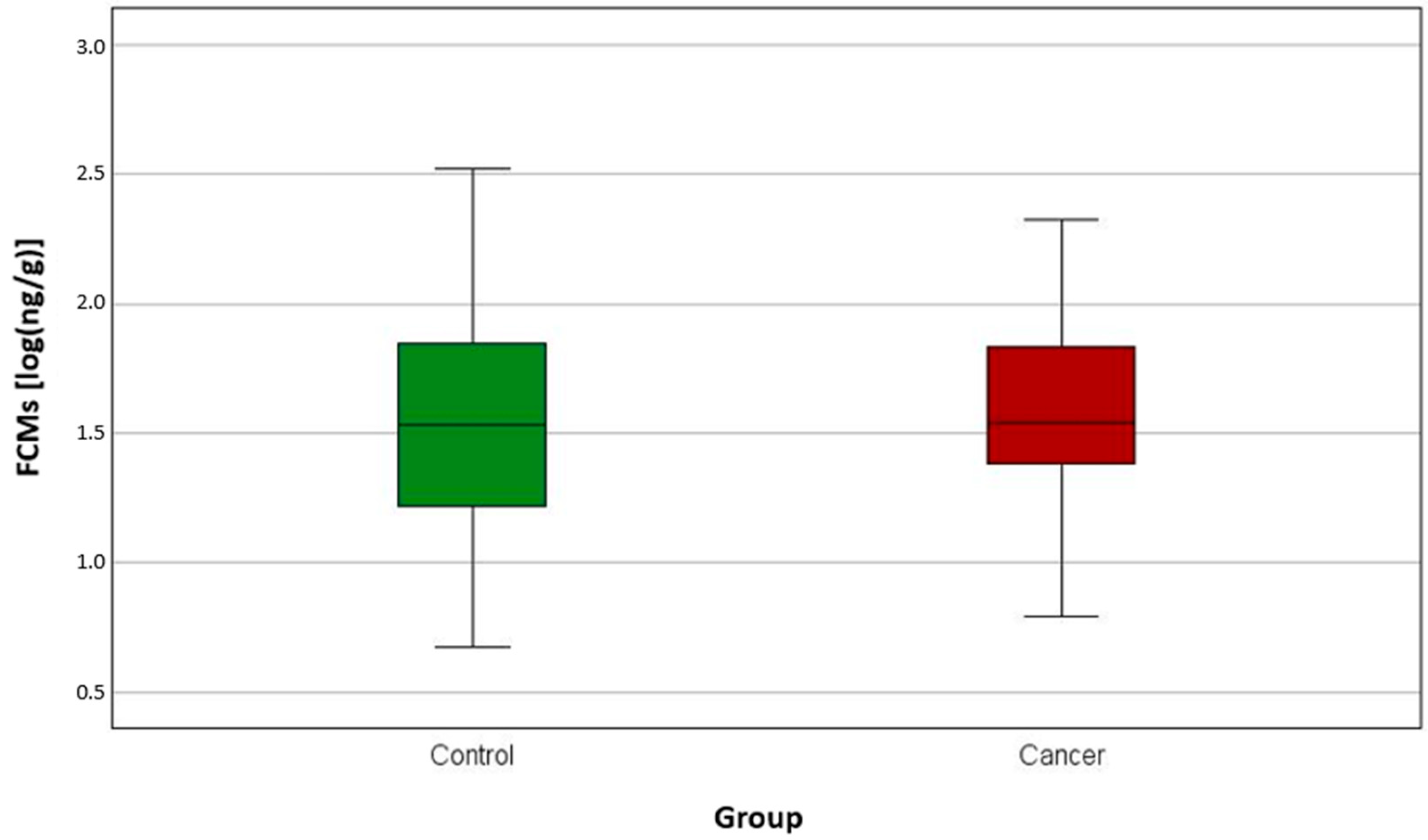
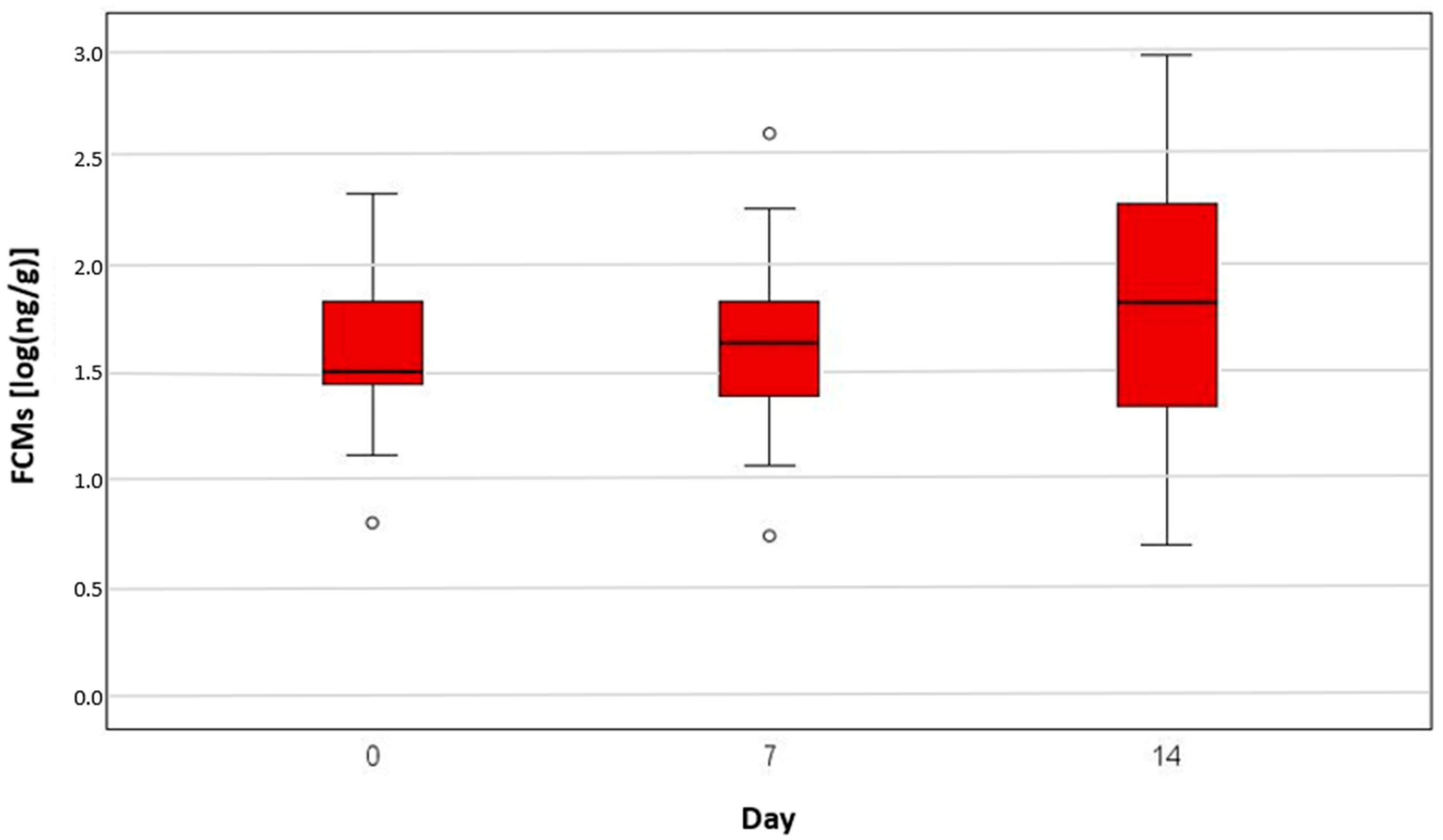
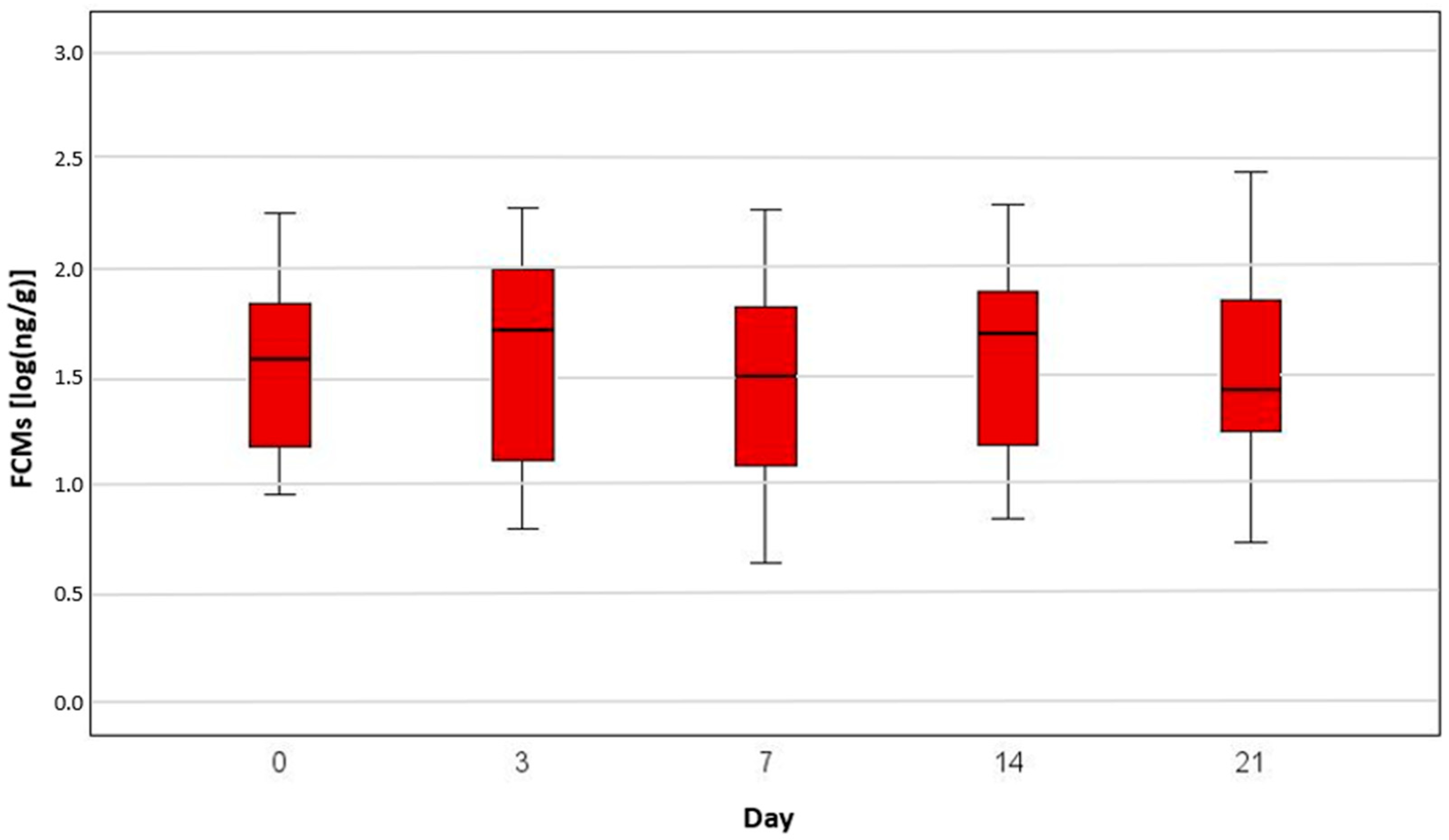
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EIA | enzyme immunoassay |
| FCMs | faecal cortisol metabolites |
References
- Wielebnowski, N. Stress and distress: Evaluating their impact for the well-being of zoo animals. J. Am. Vet. Med. Assoc. 2003, 223, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Kopin, I.J. Evolution of concepts of stress. Stress 2007, 10, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, N.; Ironson, G.; Siegel, S.D. Stress and health: Psychological, behavioral, and biological determinants. Annu. Rev. Clin. Psychol. 2005, 1, 607–628. [Google Scholar] [CrossRef]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef]
- Kessler, M.; Ammer, H. (Eds.) Vollständig überarbeitete und erweiterte Auflage. In Kleintieronkologie: Diagnose und Therapie von Tumorerkrankungen bei Hund und Katze, 3rd ed.; Enke: Stuttgart, Germany, 2013; pp. 98–134. ISBN 9783830411376. [Google Scholar]
- Taylor, W. The excretion of steroid hormone metabolites in bile and feces. Vitam. Horm. 1971, 29, 201–285. [Google Scholar] [CrossRef]
- Palme, R.; Rettenbacher, S.; Touma, C.; El-Bahr, S.M.; Möstl, E. Stress hormones in mammals and birds: Comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Ann. N. Y. Acad. Sci. 2005, 1040, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Palme, R. Monitoring stress hormone metabolites as a useful, non-invasive tool for welfare assessment in farm animals. Anim. Welf. 2012, 21, 331–337. [Google Scholar] [CrossRef]
- Ganswindt, A.; Brown, J.L.; Freeman, E.W.; Kouba, A.J.; Penfold, L.M.; Santymire, R.M.; Vick, M.M.; Wielebnowski, N.; Willis, E.L.; Milnes, M.R. International Society for Wildlife Endocrinology: The future of endocrine measures for reproductive science, animal welfare and conservation biology. Biol. Lett. 2012, 8, 695–697. [Google Scholar] [CrossRef]
- Schatz, S.; Palme, R. Measurement of faecal cortisol metabolites in cats and dogs: A non-invasive method for evaluating adrenocortical function. Vet. Res. Commun. 2001, 25, 271–287. [Google Scholar] [CrossRef]
- Lane, J. Can non-invasive glucocorticoid measures be used as reliable indicators of stress in animals? Anim. Welf. 2006, 15, 331–342. [Google Scholar] [CrossRef]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- Eggermann, J.; Theuerkauf, J.; Pirga, B.; Milanowski, A.; Gula, R. Stress-hormone levels of wolves in relation to breeding season, pack size, human activity, and prey density. Ann. Zool. Fenn. 2013, 50, 170–175. [Google Scholar] [CrossRef]
- Escobar-Ibarra, I.; Mayagoitia-Novales, L.; Alcántara-Barrera, A.; Cerda-Molina, A.L.; Mondragón-Ceballos, R.; Ramírez-Necoechea, R.; Alonso-Spilsbury, M. Long-term quantification of faecal glucocorticoid metabolite concentrations reveals that Mexican grey wolves may habituate to captivity. Eur. Zool. J. 2017, 84, 311–320. [Google Scholar] [CrossRef]
- Stevenson, E.T.; Gese, E.M.; Neuman-Lee, L.A.; French, S.S. Levels of plasma and fecal glucocorticoid metabolites following an ACTH challenge in male and female coyotes (Canis latrans). J. Comp. Physiol. B 2018, 188, 345–358. [Google Scholar] [CrossRef] [PubMed]
- van den Berghe, F.; Paris, M.C.J.; Sarnyai, Z.; Vlamings, B.; Millar, R.P.; Ganswindt, A.; Cozzi, A.; Pageat, P.; Paris, D.B.B.P. Dog appeasing pheromone prevents the androgen surge and may reduce contact dominance and active submission after stressful interventions in African wild dogs (Lycaon pictus). PLoS ONE 2019, 14, e0212551. [Google Scholar] [CrossRef]
- Hovland, A.L.; Rød, A.M.S.; Eriksen, M.S.; Palme, R.; Nordgreen, J.; Mason, G.J. Faecal cortisol metabolites as an indicator of adrenocortical activity in farmed silver foxes (Vulpes vulpes). Appl. Anim. Behav. Sci. 2017, 197, 75–80. [Google Scholar] [CrossRef]
- Kozlowski, C.P.; Clawitter, H.; Guglielmino, A.; Schamel, J.; Baker, S.; Franklin, A.D.; Powell, D.; Coonan, T.J.; Asa, C.S. Factors affecting glucocorticoid and thyroid hormone production of Island foxes. J. Wildl. Manag. 2020, 84, 505–514. [Google Scholar] [CrossRef]
- Palme, R.; Touma, C.; Arias, N.; Dominchin, M.F.; Lepschy, M. Steroid extraction: Get the best out of faecal samples. Wien. Tierärztl. Mschrift 2013, 100, 238–246. [Google Scholar]
- Palme, R.; Möstl, E. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Z. Saugetierkd. 1997, 62 (Suppl. SII), 192–197. [Google Scholar]
- Patel, K.V.; Hunt, A.B.G.; Castillo-Fernandez, J.; Abrams, C.; King, T.; Watson, P.; Amos, G.C.A. Impact of acute stress on the canine gut microbiota. Sci. Rep. 2024, 14, 18897. [Google Scholar] [CrossRef]
- Carlson, L.E.; Campbell, T.S.; Garland, S.N.; Grossman, P. Associations among salivary cortisol, melatonin, catecholamines, sleep quality and stress in women with breast cancer and healthy controls. J. Behav. Med. 2007, 30, 45–58. [Google Scholar] [CrossRef] [PubMed]
- van der Pompe, G.; Antoni, M.H.; Heijnen, C.J. Elevated basal cortisol levels and attenuated ACTH and cortisol responses to a behavioral challenge in women with metastatic breast cancer. Psychoneuroendocrinology 1996, 21, 361–374. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef] [PubMed]
- Irvine, C.H.; Alexander, S.L. Factors affecting the circadian rhythm in plasma cortisol concentrations in the horse. Domest. Anim. Endocrinol. 1994, 11, 227–238. [Google Scholar] [CrossRef]
- Giannetto, C.; Fazio, F.; Assenza, A.; Alberghina, D.; Panzera, M.; Piccione, G. Parallelism of circadian rhythmicity of salivary and serum cortisol concentration in normal dogs. J. Appl. Biomed. 2014, 12, 229–233. [Google Scholar] [CrossRef]
- Perego, R.; Proverbio, D.; Spada, E. Increases in heart rate and serum cortisol concentrations in healthy dogs are positively correlated with an indoor waiting-room environment. Vet. Clin. Pathol. 2014, 43, 67–71. [Google Scholar] [CrossRef]
- Young, K.M.; Walker, S.L.; Lanthier, C.; Waddell, W.T.; Monfort, S.L.; Brown, J.L. Noninvasive monitoring of adrenocortical activity in carnivores by fecal glucocorticoid analyses. Gen. Comp. Endocrinol. 2004, 137, 148–165. [Google Scholar] [CrossRef]
- Accorsi, P.A.; Carloni, E.; Valsecchi, P.; Viggiani, R.; Gamberoni, M.; Tamanini, C.; Seren, E. Cortisol determination in hair and faeces from domestic cats and dogs. Gen. Comp. Endocrinol. 2008, 155, 398–402. [Google Scholar] [CrossRef]
- Palme, R.; Schatz, S.; Möstl, E. Einfluss der Impfung auf die Konzentration von Kortisolmetaboliten im Kot von Fleischfressern. Dtsch. Tierarztl. Wochenschr. 2001, 108, 23–25. [Google Scholar]
- Farca, A.M.; Cavana, P.; Badino, P.; Barbero, R.; Odore, R.; Pollicino, P. Measurement of faecal corticoid metabolites in domestic dogs. Schweiz. Arch. Tierheilkd. 2006, 148, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Dreschel, N.A.; Granger, D.A. Methods of collection for salivary cortisol measurement in dogs. Horm. Behav. 2009, 55, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Merl, S.; Scherzer, S.; Palme, R.; Möstl, E. Pain causes increased concentrations of glucocorticoid metabolites in horse feces. J. Equine Vet. Sci. 2000, 20, 586–590. [Google Scholar] [CrossRef]
- Ganswindt, A.; Münscher, S.; Henley, M.; Palme, R.; Thompson, P.; Bertschinger, H. Concentrations of faecal glucocorticoid metabolites in physically injured free—Ranging African elephants Loxodonta africana. Wildl. Biol. 2010, 16, 323–332. [Google Scholar] [CrossRef]
- Carlson, L.E.; Speca, M.; Patel, K.D.; Goodey, E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology 2004, 29, 448–474. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Ha, K.S.; Hwang, I.C.; Ahn, H.Y.; Youn, C.H. Random serum cortisol as a predictor for survival of terminally ill patients with cancer: A preliminary study. Am. J. Hosp. Palliat. Care 2016, 33, 281–285. [Google Scholar] [CrossRef]
- Lengacher, C.A.; Kip, K.E.; Barta, M.; Post-White, J.; Jacobsen, P.B.; Groer, M.; Lehman, B.; Moscoso, M.S.; Kadel, R.; Le, N.; et al. A pilot study evaluating the effect of mindfulness-based stress reduction on psychological status, physical status, salivary cortisol, and interleukin-6 among advanced-stage cancer patients and their caregivers. J. Holist. Nurs. 2012, 30, 170–185. [Google Scholar] [CrossRef]
- Lissoni, P.; Messina, G.; Balestra, A.; Colciago, M.; Brivio, F.; Fumagalli, L.; Fumagalli, G.; Parolini, D. Efficacy of cancer chemotherapy in relation to synchronization of cortisol rhythm, immune status and psychospiritual profile in metastatic non-small cell lung cancer. In Vivo 2008, 22, 257–262. [Google Scholar]
- Sephton, S.E.; Sapolsky, R.M.; Kraemer, H.C.; Spiegel, D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Natl. Cancer Inst. 2000, 92, 994–1000. [Google Scholar] [CrossRef]
- Vadiraja, H.S.; Raghavendra, R.M.; Nagarathna, R.; Nagendra, H.R.; Rekha, M.; Vanitha, N.; Gopinath, K.S.; Srinath, B.S.; Vishweshwara, M.S.; Madhavi, Y.S.; et al. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: A randomized controlled trial. Integr. Cancer Ther. 2009, 8, 37–46. [Google Scholar] [CrossRef]
- Vedhara, K.; Tuinstra, J.; Miles, J.N.V.; Sanderman, R.; Ranchor, A.V. Psychosocial factors associated with indices of cortisol production in women with breast cancer and controls. Psychoneuroendocrinology 2006, 31, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Fredrikson, M.; Hursti, T.; Fürst, C.J.; Steineck, G.; Börjeson, S.; Wikblom, M.; Peterson, C. Nausea in cancer chemotherapy is inversely related to urinary cortisol excretion. Br. J. Cancer 1992, 65, 779–780. [Google Scholar] [CrossRef] [PubMed]
- Morrow, G.R.; Hickok, J.T.; Andrews, P.L.R.; Stern, R.M. Reduction in serum cortisol after platinum based chemotherapy for cancer: A role for the HPA axis in treatment-related nausea? Psychophysiology 2002, 39, 491–495. [Google Scholar] [CrossRef]
- Limberaki, E.; Eleftheriou, P.; Gasparis, G.; Karalekos, E.; Kostoglou, V.; Petrou, C. Cortisol levels and serum antioxidant status following chemotherapy. Health 2011, 3, 512–517. [Google Scholar] [CrossRef]
| Group | Day | Minimum | Maximum | Percentiles | ||||
|---|---|---|---|---|---|---|---|---|
| N | 25 | Median | 75 | |||||
| FCMs (ng/g) | Control | 0 | 53 | 4.7 | 334.0 | 16.4 | 33.9 | 72.2 |
| Chemotherapy | 0 | 15 | 8.7 | 174.5 | 14.5 | 37.0 | 68.2 | |
| 3 | 15 | 6.1 | 185.0 | 10.6 | 50.7 | 97.0 | ||
| 7 | 15 | 4.2 | 181.4 | 8.7 | 31.1 | 67.2 | ||
| 14 | 15 | 6.9 | 192.7 | 11.5 | 48.6 | 95.4 | ||
| 21 | 15 | 5.3 | 270.1 | 17.0 | 26.8 | 97.9 | ||
| Radiation | 0 | 25 | 6.2 | 211.2 | 27.4 | 31.2 | 95.8 | |
| 7 | 25 | 5.4 | 406.1 | 23.3 | 42.6 | 77.9 | ||
| 14 | 25 | 4.9 | 932.8 | 21.1 | 66.7 | 195.6 | ||
| FCMs [log(ng/g)] | Control | 0 | 53 | 0.67 | 2.52 | 1.21 | 1.53 | 1.86 |
| Chemotherapy | 0 | 15 | 0.94 | 2.24 | 1.16 | 1.57 | 1.83 | |
| 3 | 15 | 0.79 | 2.27 | 1.02 | 1.71 | 1.99 | ||
| 7 | 15 | 0.63 | 2.26 | 0.94 | 1.49 | 1.83 | ||
| 14 | 15 | 0.84 | 2.28 | 1.06 | 1.69 | 1.98 | ||
| 21 | 15 | 0.72 | 2.43 | 1.23 | 1.43 | 1.99 | ||
| Radiation | 0 | 25 | 0.79 | 2.32 | 1.44 | 1.49 | 1.96 | |
| 7 | 25 | 0.73 | 2.61 | 1.37 | 1.63 | 1.89 | ||
| 14 | 25 | 0.69 | 2.97 | 1.32 | 1.82 | 2.29 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziegerhofer, C.; Tichy, A.; Kleiter, M.; Wolfesberger, B.; Palme, R. Assessment of Stress in Dogs Under Cancer Therapy via Faecal Cortisol Metabolite Analysis: A Pilot Study. Animals 2025, 15, 1809. https://doi.org/10.3390/ani15121809
Ziegerhofer C, Tichy A, Kleiter M, Wolfesberger B, Palme R. Assessment of Stress in Dogs Under Cancer Therapy via Faecal Cortisol Metabolite Analysis: A Pilot Study. Animals. 2025; 15(12):1809. https://doi.org/10.3390/ani15121809
Chicago/Turabian StyleZiegerhofer, Christina, Alexander Tichy, Miriam Kleiter, Birgitt Wolfesberger, and Rupert Palme. 2025. "Assessment of Stress in Dogs Under Cancer Therapy via Faecal Cortisol Metabolite Analysis: A Pilot Study" Animals 15, no. 12: 1809. https://doi.org/10.3390/ani15121809
APA StyleZiegerhofer, C., Tichy, A., Kleiter, M., Wolfesberger, B., & Palme, R. (2025). Assessment of Stress in Dogs Under Cancer Therapy via Faecal Cortisol Metabolite Analysis: A Pilot Study. Animals, 15(12), 1809. https://doi.org/10.3390/ani15121809





