Feline Leishmaniosis: A Retrospective Study of Seroprevalence in Cats in the Campania Region, Southern Italy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Consent to Participate
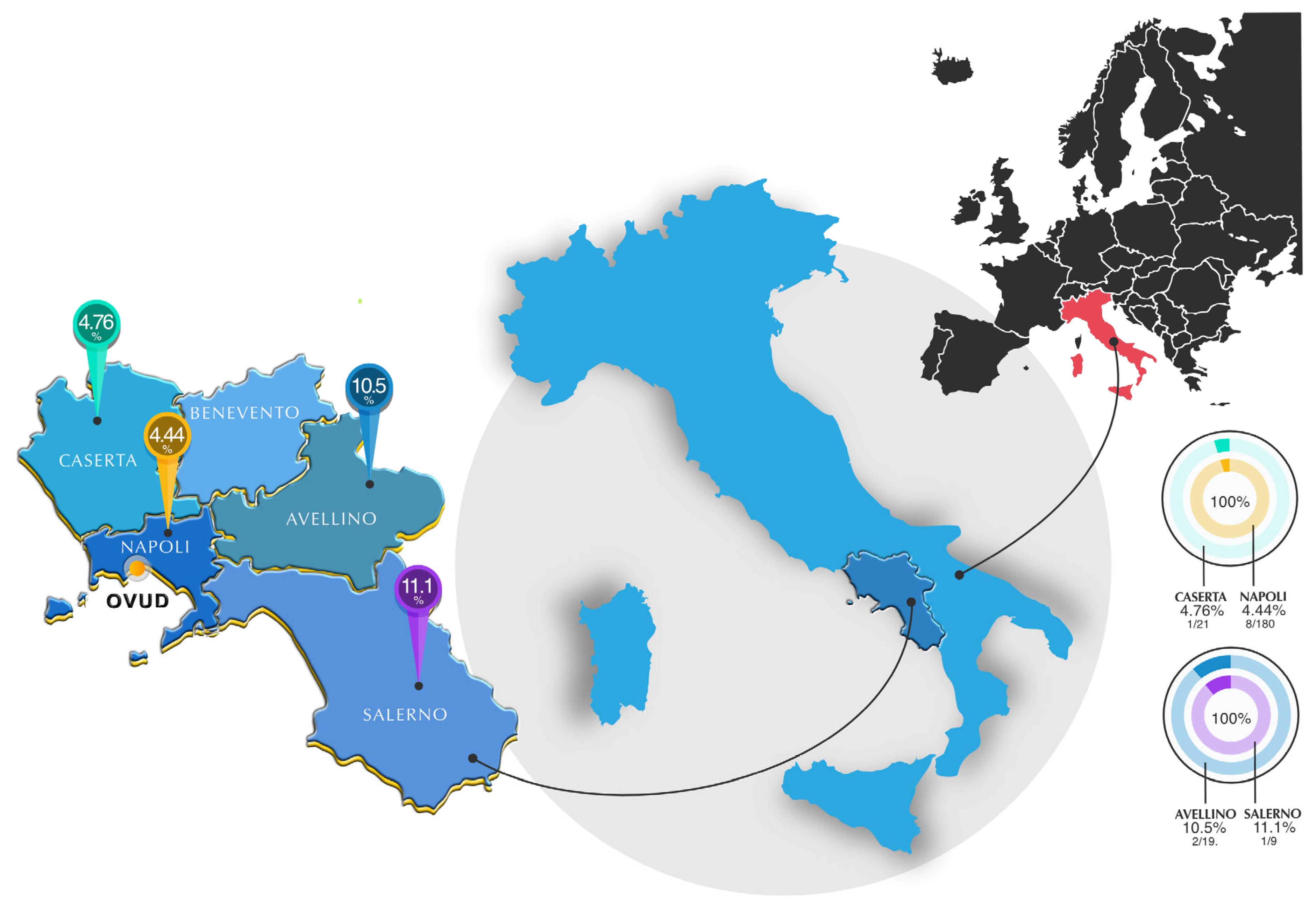
2.2. Study Area, Inclusion Criteria and Sample Size
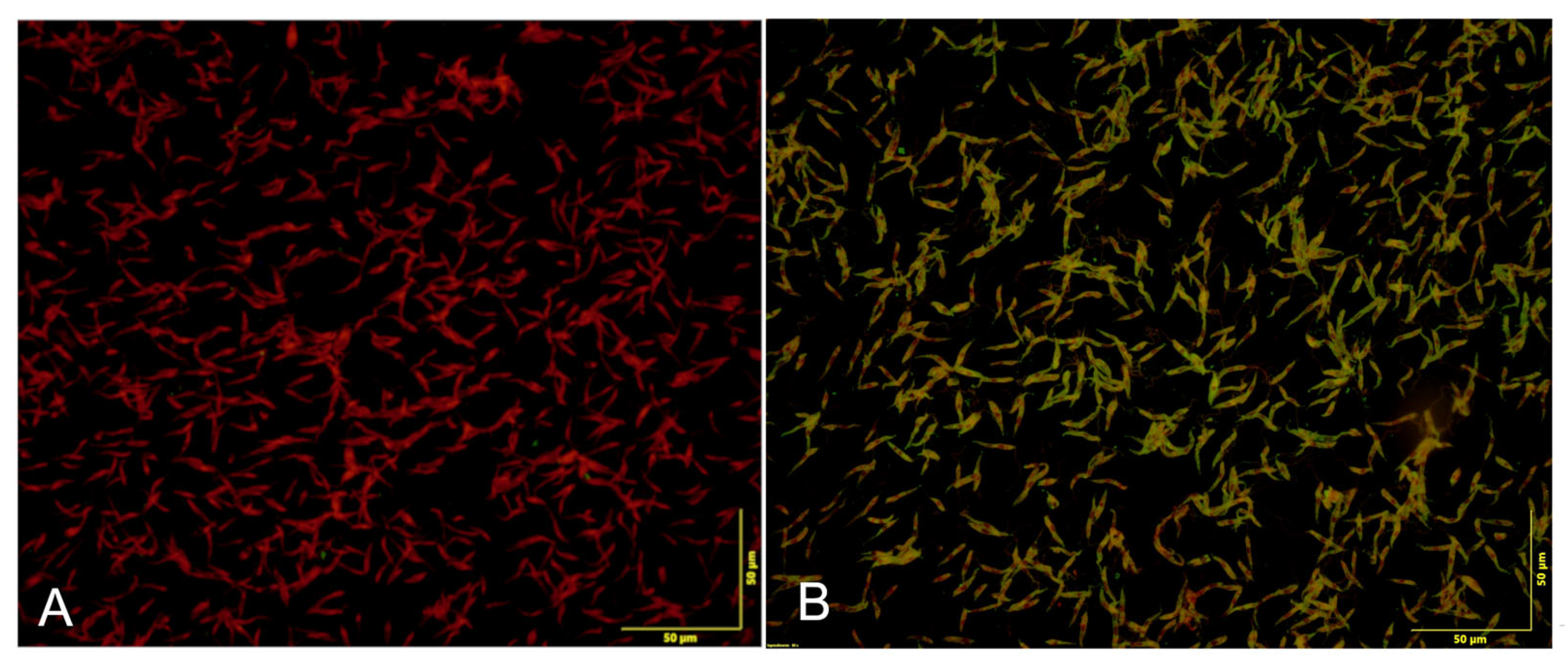
2.3. Sample Preparation and Serological Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FeL | Feline leishmaniasis |
| CanL | Canine leishmaniasis |
| IFAT | Immunofluorescence antibody test |
| FIV | Feline immunodeficiency virus |
| FeLV | Feline leukaemia virus |
| ELISA | Enzyme-linked immunosorbent assay |
| PBS | Phosphate buffer saline |
| CI | Confidence interval |
| OVUD | University Veterinary Teaching Hospital |
| WOAH | World Organisation for Animal Health |
| OR | Odds ratio |
References
- Gianchecchi, E.; Montomoli, E. The enemy at home: Leishmaniasis in the Mediterranean basin, Italy on the focus. Expert Rev. Anti-Infect. Ther. 2020, 18, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, M.; Iatta, R.; Miró, G.; Montoya, A.; Benelli, G.; Mendoza-Roldan, J.A.; Papadopoulos, E.; Lima, C.; Bouhsira, E.; Nachum-Biala, Y.; et al. Feline leishmaniosis in the Mediterranean Basin: A multicenter study. Parasit. Vectors 2024, 19, 346. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Colombo, M.; Dimzas, D.; Barlaam, A.; Traversa, D.; Di Cesare, A.; Russi, I.; Spoletini, R.; Paoletti, B.; Diakou, A. Leishmania infantum Seroprevalence in Cats from Touristic Areas of Italy and Greece. Front. Vet. Sci. 2020, 7, 616566. [Google Scholar] [CrossRef] [PubMed]
- Iatta, R.; Furlanello, T.; Colella, V.; Tarallo, V.D.; Latrofa, M.S.; Brianti, E.; Trerotoli, P.; Decaro, N.; Lorusso, E.; Schunack, B.; et al. A nationwide survey of Leishmania infantum infection in cats and associated risk factors in Italy. PLoS Negl. Trop. Dis. 2019, 13, e0007594. [Google Scholar] [CrossRef]
- Millán, J.; Ferroglio, E.; Solano-Gallego, L. Role of wildlife in the epidemiology of Leishmania infantum infection in Europe. Parasitol. Res. 2014, 113, 2005–2014. [Google Scholar] [CrossRef]
- Pennisi, M.G.; Persichetti, M.F. Feline leishmaniosis: Is the cat a small dog? Vet. Parasitol. 2018, 251, 131–137. [Google Scholar] [CrossRef]
- Pennisi, M.G.; Cardoso, L.; Baneth, G.; Bourdeau, P.; Koutinas, A.; Miró, G.; Oliva, G.; Solano-Gallego, L. LeishVet update and recommendations on feline leishmaniosis. Parasit. Vectors 2015, 8, 302. [Google Scholar] [CrossRef]
- Abramo, F.; Albanese, F.; Gattuso, S.; Randone, A.; Fileccia, I.; Dedola, C.; Ibba, F.; Ottaiano, P.; Brianti, E. Skin lesions in feline leishmaniosis: A systematic review. Pathogens 2021, 10, 472. [Google Scholar] [CrossRef]
- Baneth, G.; Solano-Gallego, L. Leishmaniasis. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 1359–1375. [Google Scholar] [CrossRef]
- Pereira, A.; Maia, C. Leishmania infection in cats and feline leishmaniosis: An updated review with a proposal of a diagnosis algorithm and prevention guidelines. Curr. Res. Parasitol. Vector Borne Dis. 2021, 1, 100035. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G.; The LeishVet Group. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit. Vectors 2011, 4, 86. [Google Scholar] [PubMed]
- Iatta, R.; Trerotoli, P.; Lucchese, L.; Natale, A.; Buonavoglia, C.; Nachum-Biala, Y.; Baneth, G.; Otranto, D. Validation of a new immunofluorescence antibody test for the detection of Leishmania infantum infection in cats. Parasitol. Res. 2020, 119, 1381–1386. [Google Scholar] [CrossRef]
- Persichetti, M.F.; Solano-Gallego, L.; Vullo, A.; Masucci, M.; Marty, P.; Delaunay, P.; Vitale, F.; Pennisi, M.G. Diagnostic performance of ELISA, IFAT and Western blot for the detection of anti-Leishmania infantum antibodies in cats using a Bayesian analysis without a gold standard. Parasit. Vectors 2017, 13, 119. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F. Canine and feline vector-borne diseases in Italy: Current situation and perspectives. Parasites Vectors 2010, 3, 2. [Google Scholar] [CrossRef]
- Petruccelli, A.; Ferrara, G.; Iovane, G.; Schettini, R.; Ciarcia, R.; Caputo, V.; Pompameo, M.; Pagnini, U.; Montagnaro, S. Seroprevalence of Ehrlichia spp., Anaplasma spp., Borreliaburgdorferi sensu lato, and Dirofilaria immitis in Stray Dogs, from 2016 to 2019, in Southern Italy. Animals 2020, 23, 9. [Google Scholar]
- Spada, E.; Perego, R.; Vitale, F.; Bruno, F.; Castelli, G.; Tarantola, G.; Baggiani, L.; Magistrelli, S.; Proverbio, D. Feline Leishmania spp. Infection in a Non-Endemic Area of Northern Italy. Animals 2020, 10, 817. [Google Scholar] [CrossRef]
- Bruno, F.; Vitale, F.; La Russa, F.; Reale, S.; Späth, G.F.; Oliveri, E.; Gargano, V.; Valenza, V.; Facciponte, F.; Giardina, S.; et al. Retrospective Analysis of Leishmaniasis in Sicily (Italy) from 2013 to 2021: One-Health Impact and Future Control Strategies. Microorganisms 2022, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Urbani, L.; Tirolo, A.; Salvatore, D.; Tumbarello, M.; Segatore, S.; Battilani, M.; Balboni, A.; Dondi, F. Serological, molecular and clinicopathological findings associated with Leishmania infantum infection in cats in Northern Italy. J. Feline. Med. Surg. 2020, 22, 935–943. [Google Scholar] [CrossRef]
- Elmahallawy, E.K.; Zanet, S.; Poggi, M.; Alsharif, K.F.; Agil, A.; Trisciuoglio, A.; Ferroglio, E. Feline Leishmaniosis in Northwestern Italy: Current Status and Zoonotic Implications. Vet. Sci. 2021, 8, 215. [Google Scholar] [CrossRef]
- Dedola, C.; Zobba, R.; Varcasia, A.; Visco, S.; Alberti, A.; Pipia, A.P.; Scala, A.; Pinna Parpaglia, M.L. Serological and molecular detection of Leishmania infantum in cats of Northern Sardinia, Italy. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 120–123. [Google Scholar] [CrossRef]
- Gusatoaia, O.; Perles, L.; Cavalera, M.A.; Uva, A.; Gernone, F.; Otranto, D.; Zatelli, A. Co-infections of rickettsiales in clinically healthy, Leishmania infantum seropositive and seronegative dogs: A systematic literature review and new findings from Southern Italy. Parasitol. Res. 2025, 124, 14. [Google Scholar] [CrossRef] [PubMed]
- Italian Ministry of Health. Leishmania. 3 January 2025. Available online: https://www.salute.gov.it/portale/sanitaAnimale/dettaglioContenutiSanitaAnimale.jsp?id=220&lingua=italiano&tab=2 (accessed on 28 February 2025).
- Montagnaro, S.; D’Ambrosi, F.; Petruccelli, A.; Ferrara, G.; D’Alessio, N.; Iovane, V.; Veneziano, V.; Fioretti, A.; Pagnini, U. A Serological Survey of Brucellosis in Eurasian Wild Boar (Sus scrofa) in Campania Region, Italy. J. Wildl. Dis. 2020, 56, 424–428. [Google Scholar] [CrossRef]
- Thrusfield, M.; Christley, R.; Brown, H.; Diggle, H.J.; French, N.; Howe, K.; Kelly, L.; O’Connor, A.; Sargeant, J.; Wood, H. Veterinary Epidemiology, 4th ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 275–276. [Google Scholar]
- Quimby, J.; Gowland, S.; Carney, H.C.; DePorter, T.; Plummer, P.; Westropp, J. 2021 AAHA/AAFP Feline Life Stage Guidelines. J. Feline Med. Surg. 2021, 23, 211–233. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (WOAH). Terrestrial Manual 2021. Chapter 3.1.11; Leishmaniosis. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.01.11_LEISHMANIOSIS.pdf (accessed on 11 March 2025).
- Paltrinieri, S.; Solano Gallego, L.; Fondati, A.; Lubas, G.; Gradoni, L.; Castagnaro, M.; Crotti, A.; Maroli, M.; Oliva, G.; Roura, X.; et al. Canine Leishmaniasis Working Group, Italian Society of Veterinarians of Companion Animals. Guidelines for diagnosis and clinical classification of leishmaniasis in dogs. J. Am. Vet. Med. Assoc. 2021, 236, 1184–1191. [Google Scholar] [CrossRef]
- Spada, E.; Canzi, I.; Baggiani, L.; Perego, R.; Vitale, F.; Migliazzo, A.; Proverbio, D. Prevalence of Leishmania infantum and co-infections in stray cats in northern Italy. Comp. Immunol. Microbiol. Infect. Dis. 2016, 45, 53–58. [Google Scholar] [CrossRef]
- Bezerra, J.A.B.; Oliveira, I.V.P.M.; Yamakawa, A.C.; Nilsson, M.G.; Tomaz, K.L.R.; Oliveira, K.D.S.; Rocha, C.S.D.; Calabuig, C.I.P.; Fornazari, F.; Langoni, H.; et al. Serological and molecular investigation of Leishmania spp. infection in cats from an area endemic for canine and human leishmaniasis in Northeast Brazil. Rev. Bras. Parasitol. Vet. 2019, 28, 790–796. [Google Scholar] [CrossRef]
- Fusco, G.; Marati, L.; Pugliese, A.; Levante, M.; Ferrara, G.; de Carlo, E.; Amoroso, M.G.; Montagnaro, S. Prevalence of feline leukemia virus and feline immunodeficiency virus in cats from southern Italy: A 10-year cross-sectional study. Front. Vet. Sci. 2023, 10, 1260081. [Google Scholar] [CrossRef]
- Aksulu, A.; Bilgiç, H.B.; Karagenç, T.; Bakırcı, S. Seroprevalence and molecular detection of Leishmania spp. in cats of West Aegean Region, Turkey. Vet. Parasitol. Reg. Stud. Rep. 2021, 24, 100573. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.V.V.O.; Moreno, B.F.S.; Cabral, A.D.; Louzeiro, N.M.; Miranda, L.M.; Santos, V.M.B.D.; Costa, F.B.; Nogueira, R.M.S.; Marcili, A.; Sperança, M.A.; et al. Diagnosis and epidemiology of Leishmania infantum in domestic cats in an endemic area of the Amazon region, Brazil. Vet. Parasitol. 2019, 273, 80–85. [Google Scholar] [CrossRef]
- Bezerra, J.A.B.; Haisi, A.; Rocha, G.D.S.; Lima, S.G.; Brasil, A.W.L.; Tomaz, K.L.R.; Fornazari, F.; Langoni, H.; Araújo Junior, J.P.; Antunes, J.M.A.P.; et al. Coinfection with Leishmania infantum and Toxoplasma gondii in Domestic Cats from a Region with a High Prevalence of Feline Immunodeficiency Virus. Microorganisms 2023, 12, 71. [Google Scholar] [CrossRef]
- Paiva, B.L.; Sousa-Paula, L.C.; Sales, K.G.D.S.; Costa, K.M.V.; Venuto, A.M.; Oriente, V.N.D.; Cavalcante, F.R.A.; Brito, R.L.L.; Santos, J.M.L.D.; Dantas-Torres, F. Prevalence of Leishmania infection in 205 cats from a referral hospital population in Brazil (2021–2022). Vet. Parasitol. Reg. Stud. Rep. 2024, 53, 101068. [Google Scholar] [CrossRef] [PubMed]
- Peris, M.P.; Planas, S.; Langa, J.; Laborda, A.; Castillo, J.A.; Gracia, M.J. Seroprevalence of zoonotic pathogens in stray cats in an urban area of northeast Spain. Vet. Parasitol. Reg. Stud. Rep. 2024, 53, 101052. [Google Scholar] [CrossRef] [PubMed]
- Priolo, V.; Masucci, M.; Donato, G.; Solano-Gallego, L.; Martínez-Orellana, P.; Persichetti, M.F.; Raya-Bermúdez, A.; Vitale, F.; Pennisi, M.G. Association between feline immunodeficiency virus and Leishmania infantum infections in cats: A retrospective matched case-control study. Parasit. Vectors 2022, 15, 107. [Google Scholar] [CrossRef]
- Marcondes, M.; Hirata, K.Y.; Vides, J.P.; Sobrinho, L.S.V.; Azevedo, J.S.; Vieira, T.S.W.J.; Vieira, R.F.C. Infection by Mycoplasma spp., feline immunodeficiency virus and feline leukemia virus in cats from an area endemic for visceral leishmaniasis. Parasit. Vectors 2018, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Melo, T.B.; Silva, T.R.M.; Almeida, T.L.A.C.; Tutija, J.F.; Silva, A.O.D.; Lira, M.D.S.; Amorim, D.; Giannelli, A.; Ramos, C.A.D.N.; Alves, L.C.; et al. Molecular detection of vector-borne pathogens in cats tested for FIV and FeLV. Vet. Parasitol. Reg. Stud. Rep. 2023, 40, 100857. [Google Scholar] [CrossRef]
- Nascimento, L.F.J.; Amado-Gomes, A.C.; Dantas-Torres, F.; Santos, F.L.N.; Neres, W.S.; Filho, P.E.S.; Santos, M.T.; Silva, J.R.S.; Resende, C.F.; Dos Reis, J.K.P.; et al. Feline leishmaniasis in an animal shelter in northeastern Brazil: Clinical aspects, coinfections, molecular detection, and serological study of a new recombinant protein. Res. Vet. Sci. 2024, 172, 105256. [Google Scholar] [CrossRef]
- Barbosa, W.Z.; Magalhães, K.A.; Pussi, K.F.; Lima Junior, M.S.D.C.; Neitzke-Abreu, H.C. Feline immunodeficiency virus, feline leukemia virus and Leishmania spp. prevalence in cats from shelters in Mato Grosso do Sul, Brazil. Rev. Bras. Parasitol. Vet. 2024, 33, e006324. [Google Scholar] [CrossRef]
- Akhtardanesh, B.; Moeini, E.; Sharifi, I.; Saberi, M.; Sadeghi, B.; Ebrahimi, M.; Otranto, D. Leishmania infection in cats positive for immunodeficiency virus and feline leukemia virus in an endemic region of Iran. Vet. Parasitol. Reg. Stud. Rep. 2020, 20, 100387. [Google Scholar] [CrossRef]
- Alcover, M.M.; Basurco, A.; Fernandez, A.; Riera, C.; Fisa, R.; Gonzalez, A.; Verde, M.; Garrido, A.M.; Ruíz, H.; Yzuel, A.; et al. A cross-sectional study of Leishmania infantum infection in stray cats in the city of Zaragoza (Spain) using serology and PCR. Parasit. Vectors 2021, 25, 178. [Google Scholar] [CrossRef]
- Gradoni, L. Recenti sviluppi nella terapia delle leishmaniosis. Ann. Ist. Super. Sanità 2001, 37, 255–263. [Google Scholar]
| Variable | n | Positive | % | SE % § | 95% CI ¥ | χ2 | p | OR # | 95% CI ¥ |
|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||
| Kitten ≤1 years old | 31 | 2 | 6.5 | 8.7 | 0.00–15.1 | Ref. * | |||
| Young adult >1–6 years old | 102 | 5 | 4.9 | 4.2 | 0.7–9.1 | 0.115 | 0.944 | 1.337 | 0.184–6.572 |
| Senior >7 years old | 96 | 5 | 5.2 | 4.4 | 0.8–9.7 | 1.255 | 0.173–6.170 | ||
| Gender | |||||||||
| Male | 140 | 8 | 5.7 | 3.8 | 1.9–9.6 | 0.010 | 0.920 | 1.287 | 0.376–4.409 |
| Female | 89 | 4 | 4.5 | 4.3 | 0.2–8.8 | ||||
| Lifestyle | |||||||||
| Outdoor | 47 | 5 | 10.6 | 8.8 | 1.8–19.5 | 2.237 | 0.134 | 2.976 | 0.900–9.842 |
| Indoor | 182 | 7 | 3.9 | 7.8 | 1.1–6.6 | ||||
| FIV/FeLV infections | |||||||||
| Yes | 27 | 2 | 7.4 | 9.8 | 0.00–17.3 | 0.017 | 0.897 | 1.2667 | 0.267–5.991 |
| No | 202 | 12 | 5.9 | 3.3 | 2.7–9.2 | ||||
| Location | |||||||||
| Napoli | 180 | 8 | 4.4 | 3.0 | 1.4–7.5 | 1.933 | 0.586 | Ref. * | |
| Caserta | 21 | 1 | 4.7 | 9.1 | 0.00–13.9 | 0.930 | 0.158–17.70 | ||
| Avellino | 19 | 2 | 10.5 | 13.8 | 0.00–24.3 | 0.395 | 0.08–2.75 | ||
| Salerno | 9 | 1 | 11.1 | 20.5 | 0.00–31.6 | 0.372 | 0.05–7.32 | ||
| Total | 229 | 12 | 5.2 | 2.9 | 2.4–8.1 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortese, L.; Abate, G.; Santoro, P.; Improda, E.; Ferrara, G.; Lucidi, V.; Sica, A.; Iovane, G.; Montagnaro, S. Feline Leishmaniosis: A Retrospective Study of Seroprevalence in Cats in the Campania Region, Southern Italy. Animals 2025, 15, 1801. https://doi.org/10.3390/ani15121801
Cortese L, Abate G, Santoro P, Improda E, Ferrara G, Lucidi V, Sica A, Iovane G, Montagnaro S. Feline Leishmaniosis: A Retrospective Study of Seroprevalence in Cats in the Campania Region, Southern Italy. Animals. 2025; 15(12):1801. https://doi.org/10.3390/ani15121801
Chicago/Turabian StyleCortese, Laura, Giulia Abate, Pasquale Santoro, Elvira Improda, Gianmarco Ferrara, Vincenzo Lucidi, Antonio Sica, Giuseppe Iovane, and Serena Montagnaro. 2025. "Feline Leishmaniosis: A Retrospective Study of Seroprevalence in Cats in the Campania Region, Southern Italy" Animals 15, no. 12: 1801. https://doi.org/10.3390/ani15121801
APA StyleCortese, L., Abate, G., Santoro, P., Improda, E., Ferrara, G., Lucidi, V., Sica, A., Iovane, G., & Montagnaro, S. (2025). Feline Leishmaniosis: A Retrospective Study of Seroprevalence in Cats in the Campania Region, Southern Italy. Animals, 15(12), 1801. https://doi.org/10.3390/ani15121801





