Status of Selenium and Other Essential and Toxic Elements in Oregon Grazing Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Animal Use
- Have at least 10 ewes on-site.
- Have been feeding a local pasture diet with or without supplementation for the past three consecutive months, and the diet must omit any grain or forages grown outside of a 10-mile radius.
- Be able to provide a mineral tag if they are feeding a mineral supplement.
- Open and not lactating.
- Not have received an injection of Se in the past three months (e.g., BO-SE, MU-SE).
- Have lambed once before and are not known to be currently experiencing infertility.
- Healthy and not recently been ill.
- Between two and seven years of age.
- Body condition score between 2 and 5 on a five-point scale.
2.2. Definition of Terms
- Terms were adapted from Mineral Levels in Animal Health (Puls, 1988) [25].
- Deficient: Levels at which clinical or pathological signs should be apparent as described in the literature.
- Normal: Used where deficiencies are unknown; indicates normal background levels.
- Adequate: Levels sufficient for optimum functioning of all body mechanisms with a small margin of reserve to counteract commonly antagonistic conditions.
- Toxic: Levels at which subclinical, clinical, or pathological signs of toxicity would be expected to occur.
2.3. Blood Collection
2.4. Whole Blood and Plasma Digests
2.5. ICP/MS Analytical Method and Quality Assurance
2.6. Collection of Information from Producers
2.7. Data Processing and Statistical Analysis
3. Results
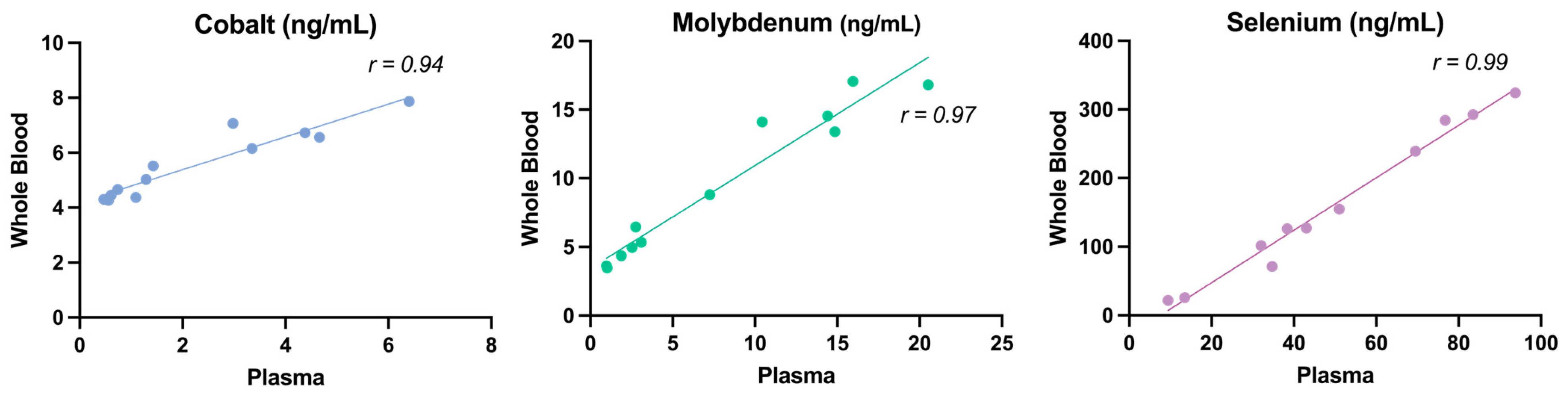
3.1. Validation of the Whole Blood Micromineral Analysis Method
3.2. Sample Summaries
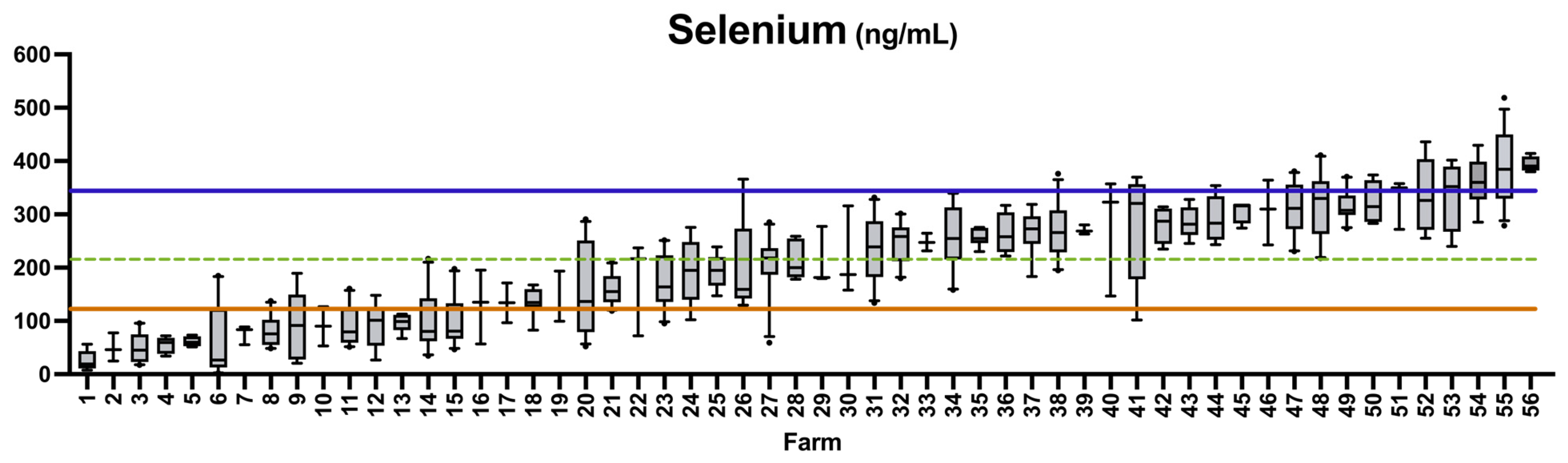
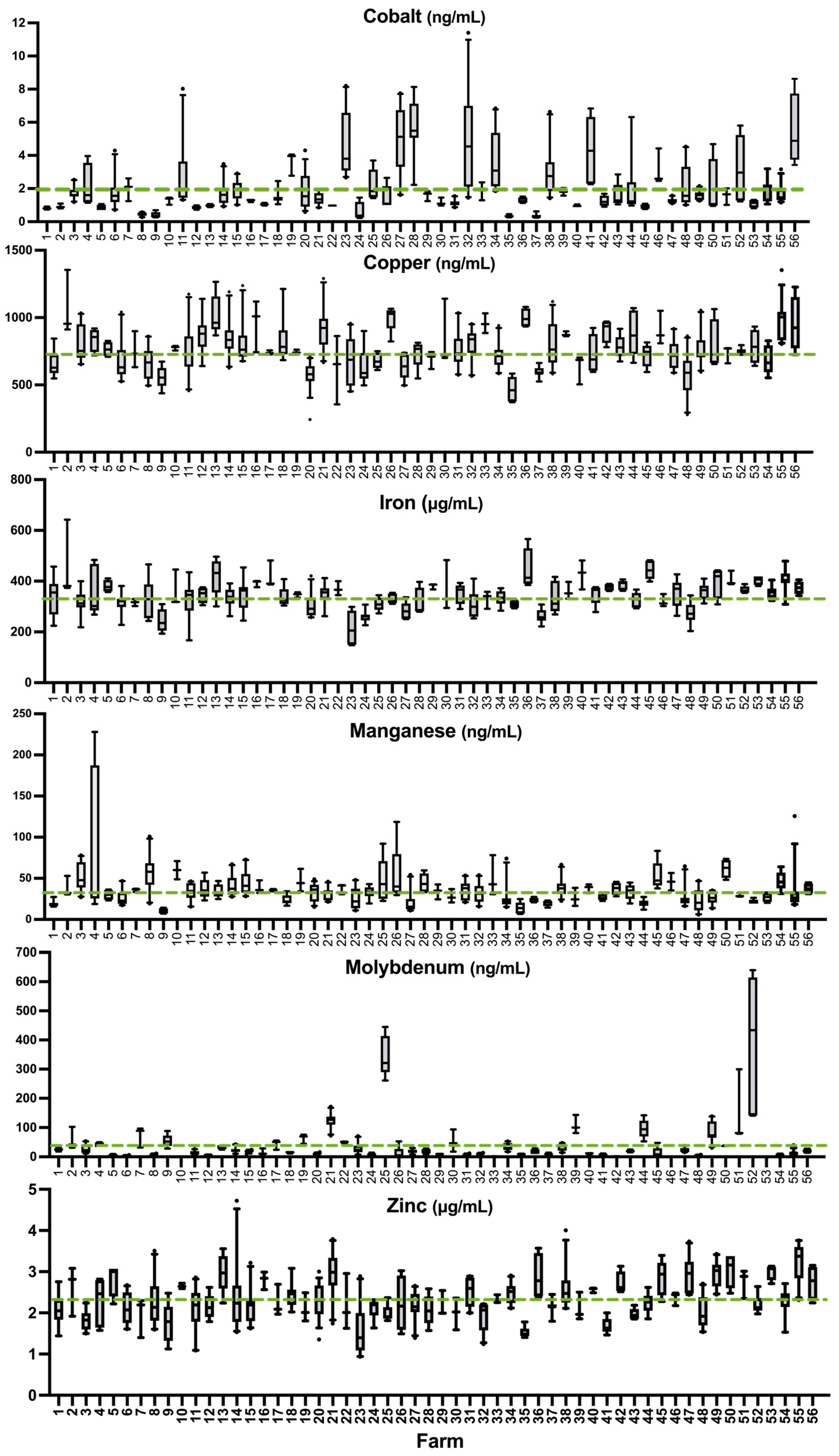
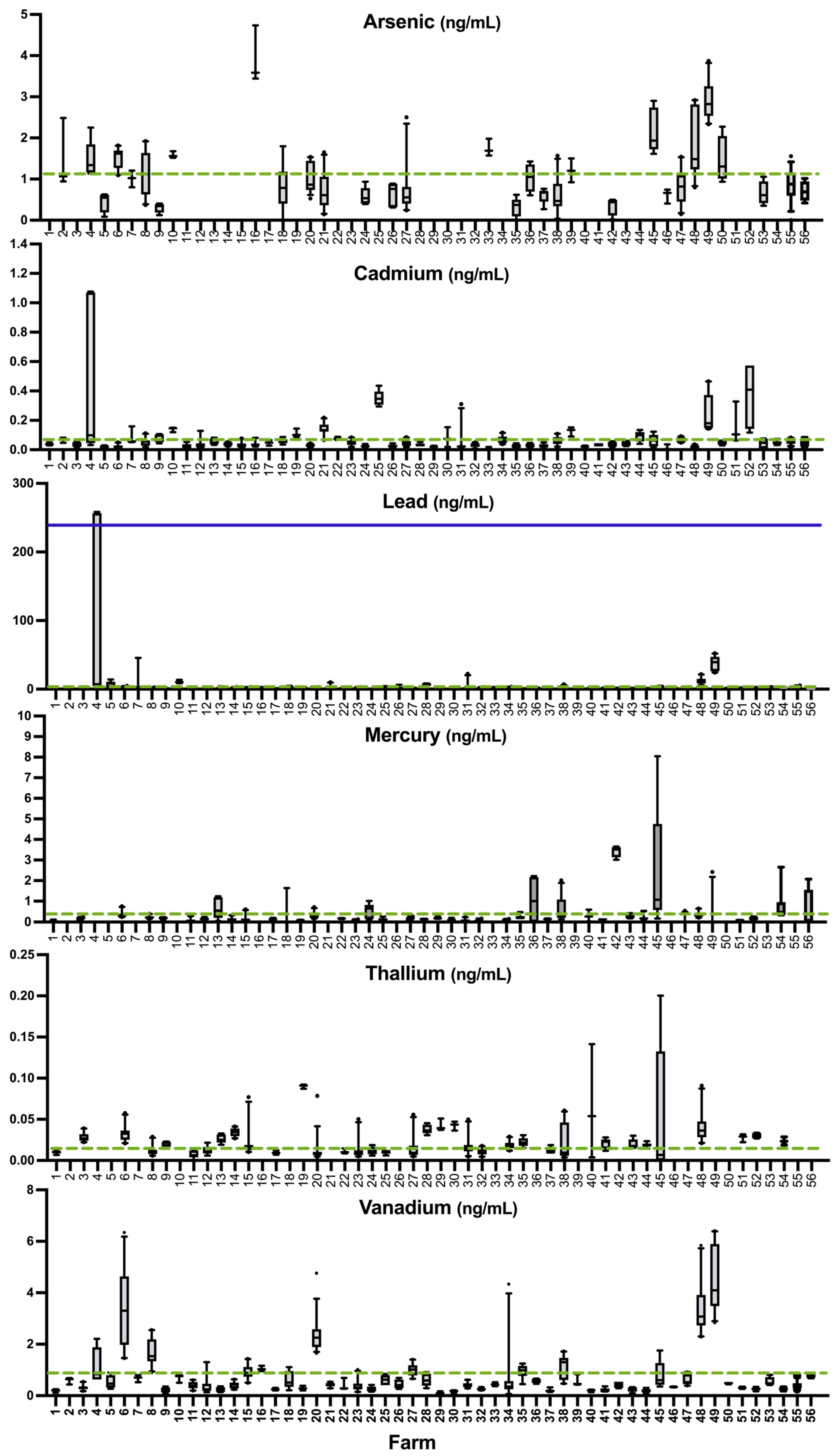
3.3. Prevalence of Deficiency or Excess According to Literature Values
3.4. Comparison of Sheep Whole Blood Mineral Values to Established Whole Blood Reference Values
3.5. Correlation Between Whole Blood Minerals, Age, and Body Condition Score
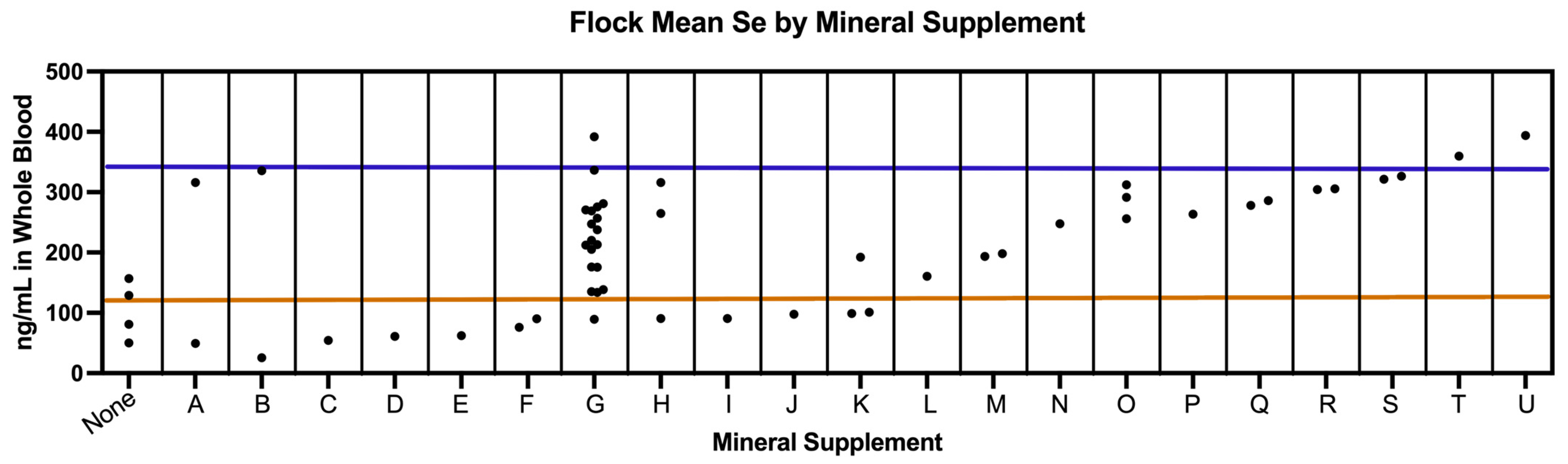
3.6. Survey Results
3.7. Comparison Between Mineral Concentrations in Blood Collected into Standard vs. Mineral-Free EDTA Tubes
3.8. Establishment of Whole Blood Reference Values for 18 Minerals in Oregon Sheep
4. Discussion
4.1. Method Validation
4.2. Comparison of Whole Blood Values to Known Plasma Reference Values in Sheep and Whole Blood Reference Values in Humans
4.3. Trace Mineral-Free Blood Tubes
4.4. Prevalence of Selenium Deficiency
4.5. Prevalence of Deficiency of Other Essential Trace Elements
4.5.1. Cobalt
4.5.2. Copper
4.5.3. Molybdenum
4.5.4. Iron
4.5.5. Manganese
4.5.6. Zinc
4.6. Other Potentially Essential or Toxic Elements in Sheep Whole Blood
4.7. Proposed Whole Blood Reference Values for 18 Minerals
4.8. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alimohamady, R.; Aliarabi, H.; Bahari, A.; Dezfoulian, A.H. Influence of different amounts and sources of selenium supplementation on performance, some blood parameters, and nutrient digestibility in lambs. Biol. Trace Elem. Res. 2013, 154, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Malevu, T.D.; Sochor, J.; Baron, M.; Melcova, M.; Zidkova, J.; et al. A Summary of New Findings on the Biological Effects of Selenium in Selected Animal Species—A Critical Review. Int. J. Mol. Sci. 2017, 18, 2209. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, J.E. Selenium in animal nutrition: The Oregon and San Joaquin Valley (California) experiences—Examples of correctable deficiencies in livestock. Biol. Trace Elem. Res. 1989, 20, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Oropeza-Moe, M.; Wisløff, H.; Bernhoft, A. Selenium deficiency associated porcine and human cardiomyopathies. J. Trace Elem. Med. Biol. 2015, 31, 148–156. [Google Scholar] [CrossRef]
- Pecoraro, B.M.; Leal, D.F.; Frias-De-Diego, A.; Browning, M.; Odle, J.; Crisci, E. The health benefits of selenium in food animals: A review. J. Anim. Sci. Biotechnol. 2022, 13, 58. [Google Scholar] [CrossRef]
- Asín, J.; Ramírez, G.A.; Navarro, M.A.; Nyaoke, A.C.; Henderson, E.E.; Mendonça, F.S.; Molín, J.; Uzal, F.A. Nutritional Wasting Disorders in Sheep. Animals 2021, 11, 501. [Google Scholar] [CrossRef]
- Oldfield, J.E.; Muth, O.H.; Schubert, J.R. Selenium and Vit. E as Related to Growth and White Muscle Disease in Lambs. Proc. Soc. Exp. Biol. Med. 1960, 103, 799–800. [Google Scholar] [CrossRef]
- Filley, S.; White, H.; Pirelli, G.J.; Hall, J.A. Selenium Supplementation Strategies for Livestock in Oregon. 2014. Available online: https://extension.oregonstate.edu/catalog/em-9094-selenium-supplementation-strategies-livestock-oregon?reference=catalog (accessed on 3 March 2025).
- National Research Council. Selenium in Nutrition; Revised Edition; National Academies Press: Washington, DC, USA, 1983; ISBN 978-0-309-03375-6. [Google Scholar]
- Oldfield, J.E. Selenium Deficiency in Soils and Its Effect on Animal Health. GSA Bull. 1972, 83, 173–180. [Google Scholar] [CrossRef]
- Beck, M.A.; Nelson, H.K.; Shi, Q.; Van Dael, P.; Schiffrin, E.J.; Blum, S.; Barclay, D.; Levander, O.A. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2001, 15, 1481–1483. [Google Scholar] [CrossRef]
- Berggren, M.M.; Mangin, J.F.; Gasdaska, J.R.; Powis, G. Effect of selenium on rat thioredoxin reductase activity: Increase by supranutritional selenium and decrease by selenium deficiency. Biochem. Pharmacol. 1999, 57, 187–193. [Google Scholar] [CrossRef]
- Chen, J. An original discovery: Selenium deficiency and Keshan disease (an endemic heart disease). Asia Pac. J. Clin. Nutr. 2012, 21, 320–326. [Google Scholar] [PubMed]
- Koller, L.D.; Exon, J.H. The two faces of selenium-deficiency and toxicity—Are similar in animals and man. Can. J. Vet. Res. 1986, 50, 297–306. [Google Scholar] [PubMed]
- McDonald, J.W. Selenium-responsive unthriftiness of young merino sheep in Central Victoria. Aust. Vet. J. 1975, 51, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Shreenath, A.P.; Hashmi, M.F.; Dooley, J. Selenium Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Jin, X.; Meng, L.; Zhang, R.; Tong, M.; Qi, Z.; Mi, L. Effects of essential mineral elements deficiency and supplementation on serum mineral elements concentration and biochemical parameters in grazing Mongolian sheep. Front. Vet. Sci. 2023, 10, 1214346. [Google Scholar] [CrossRef]
- Fechete, F.-I.; Popescu, M.; Mârza, S.-M.; Olar, L.-E.; Papuc, I.; Beteg, F.-I.; Purdoiu, R.-C.; Codea, A.R.; Lăcătuș, C.-M.; Matei, I.-R.; et al. Spatial and Bioaccumulation of Heavy Metals in a Sheep-Based Food System: Implications for Human Health. Toxics 2024, 12, 752. [Google Scholar] [CrossRef]
- Cherian, G. XVI. Microminerals. 2019. Available online: https://open.oregonstate.education/animalnutrition/chapter/chapter-16/ (accessed on 11 June 2024).
- Alhidary, I.A.; Shini, S.; Al Jassim, R.A.M.; Abudabos, A.M.; Gaughan, J.B. Effects of selenium and vitamin E on performance, physiological response, and selenium balance in heat-stressed sheep. J. Anim. Sci. 2015, 93, 576–588. [Google Scholar] [CrossRef]
- Hall, J.A.; Bailey, D.P.; Thonstad, K.N.; Van Saun, R.J. Effect of parenteral selenium administration to sheep on prevalence and recovery from footrot. J. Vet. Intern. Med. 2009, 23, 352–358. [Google Scholar] [CrossRef]
- Huo, B.; Wu, T.; Song, C.; Shen, X. Studies of Selenium Deficiency in the Wumeng Semi-Fine Wool Sheep. Biol. Trace Elem. Res. 2020, 194, 152–158. [Google Scholar] [CrossRef]
- Herdt, T.H.; Hoff, B. The Use of Blood Analysis to Evaluate Trace Mineral Status in Ruminant Livestock. Vet. Clin. Food Anim. Pract. 2011, 27, 255–283. [Google Scholar] [CrossRef]
- Kincaid, R.L.; Gay, C.C.; Krieger, R.I. Relationship of serum and plasma copper and ceruloplasmin concentrations of cattle and the effects of whole blood sample storage. Am. J. Vet. Res. 1986, 47, 1157–1159. Available online: https://avmajournals.avma.org/view/journals/ajvr/47/5/ajvr.1986.47.05.1157.xml (accessed on 13 February 2025). [CrossRef]
- Puls, R. Mineral Levels in Animal Health: Diagnostic Data; Sherpa International: British Columbia, Canada, 1988; ISBN 0-9693429-0-X. [Google Scholar]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Thomson Brooks/Cole Publishing Co.: Belmont, CA, USA, 1996; p. xxii, 600. ISBN 978-0-534-23100-2. [Google Scholar]
- Ademi, A.; Bernhoft, A.; Govasmark, E.; Bytyqi, H.; Sivertsen, T.; Singh, B.R. Selenium and other mineral concentrations in feed and sheep’s blood in Kosovo. Transl. Anim. Sci. 2017, 1, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Georgievskii, V.I.; Annenkov, B.N.; Samokhin, V.T. Mineral Nutrition of Animals: Studies in the Agricultural and Food Sciences; Elsevier: Amsterdam, The Netherlands, 1982; ISBN 978-1-4831-6272-0. [Google Scholar]
- 007245: Arsenic, Whole Blood. Available online: https://www.labcorp.com/tests/007245/arsenic-whole-blood (accessed on 16 May 2025).
- World Health Organization. Trace elements in human nutrition and health. Nutr. Health 1996, 11, 133–134. [Google Scholar] [CrossRef]
- 811562: Barium, Whole Blood. Available online: https://www.labcorp.com/tests/811562/barium-whole-blood (accessed on 16 May 2025).
- 811752: Beryllium, Whole Blood. Available online: https://www.labcorp.com/tests/811752/beryllium-whole-blood (accessed on 16 May 2025).
- 085340: Cadmium, Whole Blood. Available online: https://www.labcorp.com/tests/085340/cadmium-whole-blood (accessed on 16 May 2025).
- Cobalt, Blood | Test Detail | Quest Diagnostics. Available online: https://testdirectory.questdiagnostics.com/test/test-detail/35417/cobalt-blood?cc=MASTER (accessed on 16 May 2025).
- Chromium (Cr) Toxicity: Clinical Assessment—Laboratory Tests | Environmental Medicine | ATSDR. Available online: https://archive.cdc.gov/www_atsdr_cdc_gov/csem/chromium/laboratory_tests.html (accessed on 13 February 2025).
- 085324: Mercury, Whole Blood. Available online: https://www.labcorp.com/tests/085324/mercury-whole-blood (accessed on 16 May 2025).
- 007625: Lead, Whole Blood (Adult). Available online: https://www.labcorp.com/tests/007625/lead-whole-blood-adult (accessed on 16 May 2025).
- Moore, D.; House, I.; Dixon, A. Thallium poisoning. Diagnosis may be elusive but alopecia is the clue. Br. Med. J. 1993, 306, 1527–1529. [Google Scholar] [CrossRef]
- Friedrichs, K.R.; Harr, K.E.; Freeman, K.P.; Szladovits, B.; Walton, R.M.; Barnhart, K.F.; Blanco-Chavez, J. ASVCP reference interval guidelines: Determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 2012, 41, 441–453. [Google Scholar] [CrossRef]
- Waechter, S.R.; Vecchia, P.D.; Barin, J.S.; Flores, E.M.M.; Duarte, F.A. Microwave-based strategies for sample preparation and halogen determination in blood using ICP-MS. Talanta 2021, 226, 122157. [Google Scholar] [CrossRef]
- Wilschefski, S.C.; Baxter, M.R. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef]
- Kutscher, D.; Cui, J.; Cojocariu, C. Key Steps to Create a Sample Preparation Strategy for Inductively Coupled Plasma (ICP) or ICP–Mass Spectrometry (ICP-MS) Analysis. Spectroscopy 2022, 37, 38–42. [Google Scholar] [CrossRef]
- Harrington, J.M.; Young, D.J.; Essader, A.S.; Sumner, S.J.; Levine, K.E. Analysis of Human Serum and Whole Blood for Mineral Content by ICP-MS and ICP-OES: Development of a Mineralomics Method. Biol. Trace Elem. Res. 2014, 160, 132–142. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [CrossRef]
- Mert, H.; Yildirim, S.; Yoruk, I.H.; Irak, K.; Comba, B.; Mert, N.; Aysin, N.; Coomba, A. Retinol, α-tocopherol and vitamin D3 in White Muscle Disease. Med. Weter. 2018, 74, 441–444. Available online: https://dx.doi.org/10.21521/mw.6027 (accessed on 11 April 2025).
- Pappenheimer, A.M. Muscular dystrophy in mice on vitamin E-deficient diet. Am. J. Pathol. 1942, 18, 169–181. [Google Scholar] [PubMed]
- Zakeri, N.; Kelishadi, M.R.; Asbaghi, O.; Naeini, F.; Afsharfar, M.; Mirzadeh, E.; Naserizadeh, S.k. Selenium supplementation and oxidative stress: A review. PharmaNutrition 2021, 17, 100263. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, S.; Gavrilović, A.; Li, D.; Tang, R. Selenium alleviates cadmium-induced oxidative stress, endoplasmic reticulum stress, and apoptosis in L8824 cells. Ecotoxicol. Environ. Saf. 2023, 262, 115337. [Google Scholar] [CrossRef]
- Wang, L.; Yin, J.; Liao, C.; Cheng, R.; Chen, F.; Yu, H.; Zhang, X. Selenium deficiency-induced high concentration of reactive oxygen species restricts hypertrophic growth of skeletal muscle in juvenile zebrafish by suppressing TORC1-mediated protein synthesis. Br. J. Nutr. 2023, 130, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, P.A. Medical Nutrition Therapy for Infectious Diseases. In Krause and Mahan’s Food and the Nutrition Care Process; Raymond, J.L., Morrow, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000; ISBN 978-0-309-06949-6. Available online: http://www.ncbi.nlm.nih.gov/books/NBK225483/ (accessed on 4 June 2025).
- Yildirim, S.; Ozkan, C.; Huyut, Z.; Çınar, A. Detection of Se, Vit. E, Vit. A, MDA, 8-OHdG, and CoQ10 Levels and Histopathological Changes in Heart Tissue in Sheep with White Muscle Disease. Biol. Trace Elem. Res. 2019, 188, 419–423. [Google Scholar] [CrossRef]
- Lei, L.; Jing, M.; Yingce, Z.; Pei, Z.; Yun, L. Selenium deficiency causes oxidative stress and activates inflammation, apoptosis, and necroptosis in the intestine of weaned calves. Metallomics 2023, 15, mfad028. [Google Scholar] [CrossRef]
- Osaki, Y.; Nishino, I.; Murakami, N.; Matsubayashi, K.; Tsuda, K.; Yokoyama, Y.I.; Morita, M.; Onishi, S.; Goto, Y.I.; Nonaka, I. Mitochondrial abnormalities in selenium-deficient myopathy. Muscle Nerve 1998, 21, 637–639. [Google Scholar] [CrossRef]
- Li, S.; Sun, W.; Zhang, K.; Zhu, J.; Jia, X.; Guo, X.; Zhao, Q.; Tang, C.; Yin, J.; Zhang, J. Selenium deficiency induces spleen pathological changes in pigs by decreasing selenoprotein expression, evoking oxidative stress, and activating inflammation and apoptosis. J. Anim. Sci. Biotechnol. 2021, 12, 65. [Google Scholar] [CrossRef]
- Löfstedt, J. White Muscle Disease of Foals. Vet. Clin. N. Am. Equine Pract. 1997, 13, 169–185. [Google Scholar] [CrossRef]
- National Agricultural Statistics Service. Sheep and Goats (January 2025); US Department of Agriculture: Washington, DC, USA, 2025. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/000000018/zk51xc07n/9593wq66x/shep0125.pdf (accessed on 15 May 2025).
- Filley, S.J.; Peters, A.; Bouska, C.; Pirelli, G.; Oldfield, J. Selenium Fertilization of Pastures for Improved Forage Selenium Content. Prof. Anim. Sci. 2007, 23, 144–147. [Google Scholar] [CrossRef]
- Hall, J.A.; Van Saun, R.J.; Nichols, T.; Mosher, W.; Pirelli, G. Comparison of selenium status in sheep after short-term exposure to high-selenium-fertilized forage or mineral supplement. Small Rumin. Res. 2009, 82, 40–45. [Google Scholar] [CrossRef]
- Langlands, J.P.; Donald, G.E.; Bowles, J.E.; Smith, A.J. Subclinical selenium insufficiency. 1. Selenium status and the response in liveweight and wool production of grazing ewes supplemented with selenium. Aust. J. Exp. Agric. 1991, 31, 25–31. [Google Scholar] [CrossRef]
- Ammerman, C.B.; Miller, S.M. Selenium in ruminant nutrition: A review. J. Dairy Sci. 1975, 58, 1561–1577. [Google Scholar] [CrossRef] [PubMed]
- Nasim, M.J.; Zuraik, M.M.; Abdin, A.Y.; Ney, Y.; Jacob, C. Selenomethionine: A Pink Trojan Redox Horse with Implications in Aging and Various Age-Related Diseases. Antioxidants 2021, 10, 882. [Google Scholar] [CrossRef]
- Van Ryssen, J.B.J.; Deagen, J.T.; Beilstein, M.A.; Whanger, P.D. Comparative metabolism of organic and inorganic selenium by sheep. J. Agric. Food Chem. 1989, 37, 1358–1363. [Google Scholar] [CrossRef]
- Hasan, D.; Ford, H.R.; Bionaz, M. Impact of maternal diet on the antioxidant status, immune function, and whole-blood selenium levels of lamb offspring. Ital. J. Anim. Sci. 2025, 24, 738–752. [Google Scholar] [CrossRef]
- Ford, H.R.; Hasan, D.; Ates, S.; Maria-Puerto-Hernandez, G.; Klopfenstein, J.; Trevisi, E.; Smallman, M.; Matra, M.; Bionaz, M. PREPRINT: Feeding Chicory Silage, But Not Se-Yeast or a Single Injection of Inorganic Se, Affects Metabolism, Fat in Milk, and Type I Immunity in Transition Ewes. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4616249 (accessed on 3 August 2024).
- Borobia, M.; Villanueva-Saz, S.; Ruiz de Arcaute, M.; Fernández, A.; Verde, M.T.; González, J.M.; Navarro, T.; Benito, A.A.; Arnal, J.L.; De las Heras, M.; et al. Copper Poisoning, a Deadly Hazard for Sheep. Animals 2022, 12, 2388. [Google Scholar] [CrossRef]
- Wiener, G.; Herbert, J.G.; Field, A.C. Variation in liver and plasma copper concentrations of sheep in relation to breed and haemoglobin type. J. Comp. Pathol. 1976, 86, 101–109. [Google Scholar] [CrossRef]
- Kolb, E. The Metabolism of Iron in Farm Animals Under Normal and Pathologic Conditions. Adv. Vet. Sci. 1963, 8, 49–114. [Google Scholar]
- Kozat, S.; Yüksek, N.; Göz, Y.; Keleş, İ. Serum Iron, Total Iron-Binding Capacity, Unbound Iron-Binding Capacity, Transferrin Saturation, Serum Copper, and Hematological Parameters in Pregnant Akkaraman Ewes Infected with Gastro-Intestinal Parasites. Turk. J. Vet. Anim. Sci. 2006, 30, 15. [Google Scholar]
- Abbott, E.M.; Parkins, J.J.; Holmes, P.H. Influence of dietary protein on the pathophysiology of haemonchosis in lambs given continuous infections. Res. Vet. Sci. 1988, 45, 41–49. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. Zinc: An essential but elusive nutrient. Am. J. Clin. Nutr. 2011, 94, 679S–684S. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.F. Overview of Zinc Absorption and Excretion in the Human Gastrointestinal Tract. J. Nutr. 2000, 130, 1374S–1377S. [Google Scholar] [CrossRef]
- Wessells, K.R.; Jorgensen, J.M.; Hess, S.Y.; Woodhouse, L.R.; Peerson, J.M.; Brown, K.H. Plasma Zinc Concentration Responds Rapidly to the Initiation and Discontinuation of Short-Term Zinc Supplementation in Healthy Men. J. Nutr. 2010, 140, 2128–2133. [Google Scholar] [CrossRef]
- Knez, M.; Stangoulis, J.C.R.; Glibetic, M.; Tako, E. The Linoleic Acid: Dihomo-γ-Linolenic Acid Ratio (LA:DGLA)—An Emerging Biomarker of Zn Status. Nutrients 2017, 9, 825. [Google Scholar] [CrossRef]
- Lowe, N.M.; Fekete, K.; Decsi, T. Methods of assessment of zinc status in humans: A systematic review. Am. J. Clin. Nutr. 2009, 89, 2040S–2051S. [Google Scholar] [CrossRef]
- Brown, T.F.; McCormick, M.E.; Morris, D.R.; Zeringue, L.K. Effects of dietary boron on mineral balance in sheep. Nutr. Res. 1989, 9, 503–512. [Google Scholar] [CrossRef]
- Nielsen, F.H. Update on human health effects of boron. J. Trace Elem. Med. Biol. 2014, 28, 383–387. [Google Scholar] [CrossRef]
- Office of Dietary Supplements—Boron. Available online: https://ods.od.nih.gov/factsheets/Boron-HealthProfessional/ (accessed on 16 March 2025).
- Zhang, Q.; Wang, Y.; Li, X.; Wang, Z.; Wang, H.; Yan, J. Metabolism of barium in the human body after suicidal ingestion: A CARE-compliant case report. Medicine 2022, 101, e30571. [Google Scholar] [CrossRef]
- Smith, D.B.; Cannon, W.F.; Woodruff, L.G.; Solano, F.; Kilburn, J.E.; Fey, D.L. Geochemical and Mineralogical Data for Soils of the Conterminous United States; Data Series; U.S. Geological Survey: Denver, CO, USA, 2013. Available online: https://pubs.usgs.gov/ds/801/pdf/ds801.pdf (accessed on 19 May 2025).
- Allen, V.G. Influence of dietary aluminum on nutrient utilization in ruminants. J. Anim. Sci. 1984, 59, 836–844. [Google Scholar] [CrossRef]
| Mineral | Unit | OSU | TAMU | SEM | p-Value | r | p-Value 1 |
|---|---|---|---|---|---|---|---|
| Co | ppb | 1.74 | 5.58 | 0.30 | <0.001 | 0.45 | 0.14 |
| Cu | ppb | 753 | 823 | 35.2 | 0.17 | 0.19 | 0.54 |
| Fe | ppm | 332 | 339 | 15.6 | 0.75 | 0.20 | 0.53 |
| Mn | ppb | 31.1 | 182.2 | 5.57 | <0.001 | 0.53 | 0.08 |
| Mo | ppb | 11.3 | 9.41 | 1.77 | 0.45 | 0.85 | <0.001 |
| Se | ppb | 153.8 | 170.3 | 30.2 | 0.70 | 0.98 | <0.001 |
| Zn | ppm | 2.13 | 2.33 | 1.35 | 0.30 | 0.46 | 0.13 |
| Percentile | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mineral | Units | n 1 | Min | Max | 25th | 50th | 75th | 90th | Mean | SD |
| Age | years | 284 | 2 | 7 | 3 | 3 | 4.2 | 5 | 3.5 | 1.17 |
| BCS 3 | 370 | 2.0 | 5.0 | 3.0 | 3.5 | 4.0 | 5.0 | 3.3 | 0.54 | |
| Co | ng/mL | 370 | 0.23 | 11.41 | 1.05 | 1.43 | 2.37 | 4.70 | 2.0 | 1.72 |
| Cu | ng/mL | 370 | 244 | 1355 | 641 | 739 | 868 | 1004 | 762 | 176 |
| Fe | µg/mL | 370 | 148 | 642 | 299 | 340 | 377 | 412 | 339 | 65 |
| Mn | ng/mL | 370 | 5.9 | 228 | 22.0 | 31.2 | 41.7 | 54.3 | 34.6 | 20.3 |
| Mo | ng/mL | 363 | 0.132 | 640 | 8.4 | 16.2 | 34.1 | 87.2 | 38.3 | 74.2 |
| Se | ng/mL | 370 | 2.1 | 519 | 105 | 213 | 289 | 354 | 205 | 113 |
| Zn | µg/mL | 370 | 0.94 | 4.72 | 1.97 | 2.29 | 2.67 | 3.07 | 2.34 | 0.57 |
| Minerals with Uncertain Dietary Requirement 2 | ||||||||||
| Al | ng/mL | 370 | 138 | 4897 | 645 | 960 | 1317 | 1841 | 1063 | 585 |
| As | ng/mL | 198 | 0.084 | 4.74 | 0.59 | 0.902 | 1.50 | 2.29 | 1.1 | 0.81 |
| B | ng/mL | 370 | 30 | 701 | 125 | 171 | 255 | 333 | 201 | 119 |
| Ba | ng/mL | 370 | 13.5 | 888 | 74 | 118 | 204 | 324 | 164 | 137 |
| Be | ng/mL | 280 | 0.001 | 0.206 | 0.023 | 0.046 | 0.078 | 0.106 | 0.055 | 0.04 |
| Cd | ng/mL | 367 | 0.001 | 1.076 | 0.025 | 0.041 | 0.069 | 0.131 | 0.070 | 0.11 |
| Cr | ng/mL | 370 | 3.23 | 100 | 13.2 | 25.3 | 26.6 | 28.7 | 21.4 | 10.6 |
| Hg | ng/mL | 273 | 0.01 | 8.05 | 0.10 | 0.17 | 0.28 | 0.64 | 0.36 | 0.71 |
| Pb | ng/mL | 370 | 0.29 | 258 | 1.00 | 1.51 | 2.88 | 5.38 | 4.80 | 19.8 |
| Tl | ng/mL | 261 | 0.002 | 0.200 | 0.010 | 0.017 | 0.029 | 0.040 | 0.022 | 0.02 |
| V | ng/mL | 370 | 0.052 | 6.41 | 0.28 | 0.45 | 0.89 | 2.26 | 0.87 | 1.10 |
| Adequacy | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mineral | Reference | Units | Reference | # Above | # Below | % Above | % Below | % Within |
| Sheep (n = 370) | ||||||||
| Co | 30–50 | ng/mL | [28] | 0 | 370 | 0 | 100 | 0 |
| Cu | 800–1200 | ng/mL | [27] | 7 | 238 | 2.0 | 64.0 | 34.0 |
| Fe | 360–420 | µg/mL | [27] | 31 | 223 | 8.4 | 60.2 | 31.4 |
| Mn | 20–40 | ng/mL | [25] | 106 | 70 | 28.6 | 18.9 | 52.5 |
| Se | 120–350 | ng/mL | [23] | 39 | 102 | 10.5 | 27.6 | 61.9 |
| Zn | 2.5–6.0 | µg/mL | [27] | 0 | 243 | 0 | 66.0 | 34.0 |
| As | <80 | ng/mL | [25] | 0 | NA | 0 | NA | 100 |
| Cd | <200 | ng/mL | [25] | 0 | NA | 0 | NA | 100 |
| Hg | <100 | ng/mL | [25] | 0 | NA | 0 | NA | 100 |
| Pb | <250 | ng/mL | [25] | 2 | NA | 0.54 | NA | 99.5 |
| Farms (n = 56) | ||||||||
| Co | 30–50 | ng/mL | [28] | 0 | 56 | 0 | 100 | 0 |
| Cu | 800–1200 | ng/mL | [27] | 0 | 35 | 0 | 62.5 | 37.5 |
| Fe | 360–420 | µg/mL | [27] | 5 | 34 | 9.0 | 61.0 | 30.0 |
| Mn | 20–40 | ng/mL | [25] | 15 | 5 | 27 | 9.0 | 64.0 |
| Se | 120–350 | ng/mL | [23] | 3 | 15 | 5.4 | 26.8 | 67.9 |
| Zn | 2.5–6.0 | µg/mL | [27] | 0 | 0 | 0 | 66.0 | 34.0 |
| As | <80 | ng/mL | [25] | 0 | NA | 0 | NA | 100 |
| Cd | <200 | ng/mL | [25] | 0 | NA | 0 | NA | 100 |
| Hg | <100 | ng/mL | [25] | 0 | NA | 0 | NA | 100 |
| Pb | <250 | ng/mL | [25] | 0 | NA | 0 | NA | 100 |
| Sample vs. Human TLV 4 | ||||||
|---|---|---|---|---|---|---|
| Mineral | Sheep Values 1 | Human Values 2 | Source | # Above | # Below | % Within |
| As | <80 | <23 1 | [29] 3 | 0 | 370 | 100 |
| B | - | <200 | [30] | 151 | 219 | 59.2 |
| Ba | - | <5.0 1 | [31] 3 | 370 | 0 | 0 |
| Be | - | <1.0 1 | [32] 3 | 0 | 370 | 100 |
| Cd | <200 | <5.0 1 | [33] 3 | 0 | 370 | 100 |
| Co | 30–50 | <1.8 | [34] 3 | 240 | 130 | 35.0 |
| Cr | - | <30.0 | [35] | 25 | 345 | 93.2 |
| Hg | <100 | <14.9 1 | [36] 3 | 0 | 370 | 100 |
| Pb | <250 | <34.0 1 | [37] 3 | 9 | 370 | 97.6 |
| Tl | - | <2.0 | [38] | 0 | 370 | 100 |
| Mineral | Range 1 | Plasma | WB | SEM | p-Value | R 2 | p-Value 2 | Formula 3 |
|---|---|---|---|---|---|---|---|---|
| Co | 0.18–2.0 | 2.25 | 5.58 | 0.46 | <0.001 | 0.90 | <0.001 | y = 0.5964x + 4.192 |
| Cu | 750–1700 | 0.93 | 0.82 | 0.05 | 0.11 | 0.45 | 0.017 | NA |
| Fe | 900–2700 | 253 | 34,474 | 838 | <0.001 | 0.52 | 0.01 | NA |
| Mn | 1.0–6.0 | 3.30 | 182.4 | 5.27 | <0.001 | 0.11 | 0.30 | NA |
| Mo | 1.0–50 | 7.97 | 9.15 | 1.75 | 0.64 | 0.94 | <0.001 | y = 0.7492x + 3.438 |
| Se | 60–200 | 49.6 | 170.5 | 24.3 | 0.002 | 0.99 | <0.001 | y = 3.821x − 28.81 |
| Zn | 550–1200 | 0.66 | 2.33 | 0.1 | <0.001 | 0.31 | 0.06 | NA |
| Mineral | Units | Blue | Purple | SEM | p-Value |
|---|---|---|---|---|---|
| Al | ng/mL | 1161 | 1081 | 519 | 0.58 |
| As | ng/mL | 0.81 | 0.74 | 0.18 | 0.71 |
| B | ng/mL | 104.7 | 96.6 | 5.78 | 0.18 |
| Ba | ng/mL | 104.1 | 106.0 | 6.12 | 0.76 |
| Be | ng/mL | 0.07 | 0.05 | 0.01 | 0.32 |
| Cd | ng/mL | 0.04 | 0.04 | 0.01 | 0.85 |
| Co | ng/mL | 1.67 | 1.57 | 0.15 | 0.52 |
| Cr | ng/mL | 7.63 | 7.48 | 1.50 | 0.92 |
| Cu | ng/mL | 606 | 595 | 23.3 | 0.66 |
| Fe | µg/mL | 279 | 273 | 14.7 | 0.67 |
| Hg | ng/mL | 0.19 | 0.25 | 0.04 | 0.16 |
| Mn | ng/mL | 29.0 | 29.0 | 1.58 | 0.99 |
| Mo | ng/mL | 12.0 | 12.0 | 0.84 | 0.92 |
| Pb | ng/mL | 0.80 | 0.81 | 0.05 | 0.85 |
| Se | ng/mL | 172.3 | 164.4 | 12.6 | 0.54 |
| Tl | ng/mL | 0.02 | 0.02 | 0.01 | 0.46 |
| V | ng/mL | 0.85 | 0.98 | 0.11 | 0.24 |
| Zn | ng/mL | 2087 | 2080 | 112 | 0.95 |
| Mineral | n | Range 1 | Units |
|---|---|---|---|
| Al | 362 | 382–2050 | ng/mL |
| As | 179 | 0.27–1.8 | ng/mL |
| B | 357 | 51–357 | ng/mL |
| Ba | 338 | 32–298 | ng/mL |
| Be | 277 | <0.120 | ng/mL |
| Cd | 320 | 0.011–0.088 | ng/mL |
| Co | 323 | 0.311–3.17 | ng/mL |
| Cr | 367 | 5.7–30.5 | ng/mL |
| Cu | 368 | 511–1052 | ng/mL |
| Fe | 368 | 244–435 | µg/mL |
| Hg | 235 | <0.352 | ng/mL |
| Mn | 360 | 14.2–60 | ng/mL |
| Mo | 302 | 3.0–41.7 | ng/mL |
| Pb | 322 | <3.32 | ng/mL |
| Se | 370 | 34–380 | ng/mL |
| Tl | 243 | <0.039 | ng/mL |
| V | 301 | <0.980 | ng/mL |
| Zn | 369 | 1494–3358 | ng/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, D.; Russo, C.J.; McLaughlin, K.R.; Pirelli, G.; Bionaz, M. Status of Selenium and Other Essential and Toxic Elements in Oregon Grazing Sheep. Animals 2025, 15, 1799. https://doi.org/10.3390/ani15121799
Hasan D, Russo CJ, McLaughlin KR, Pirelli G, Bionaz M. Status of Selenium and Other Essential and Toxic Elements in Oregon Grazing Sheep. Animals. 2025; 15(12):1799. https://doi.org/10.3390/ani15121799
Chicago/Turabian StyleHasan, Daniella, Christopher J. Russo, Katherine R. McLaughlin, Gene Pirelli, and Massimo Bionaz. 2025. "Status of Selenium and Other Essential and Toxic Elements in Oregon Grazing Sheep" Animals 15, no. 12: 1799. https://doi.org/10.3390/ani15121799
APA StyleHasan, D., Russo, C. J., McLaughlin, K. R., Pirelli, G., & Bionaz, M. (2025). Status of Selenium and Other Essential and Toxic Elements in Oregon Grazing Sheep. Animals, 15(12), 1799. https://doi.org/10.3390/ani15121799






