Instruments to Assess Disease-Specific Quality of Life in Dogs: A Scoping Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Definition of Terms
2.2. Search Methods
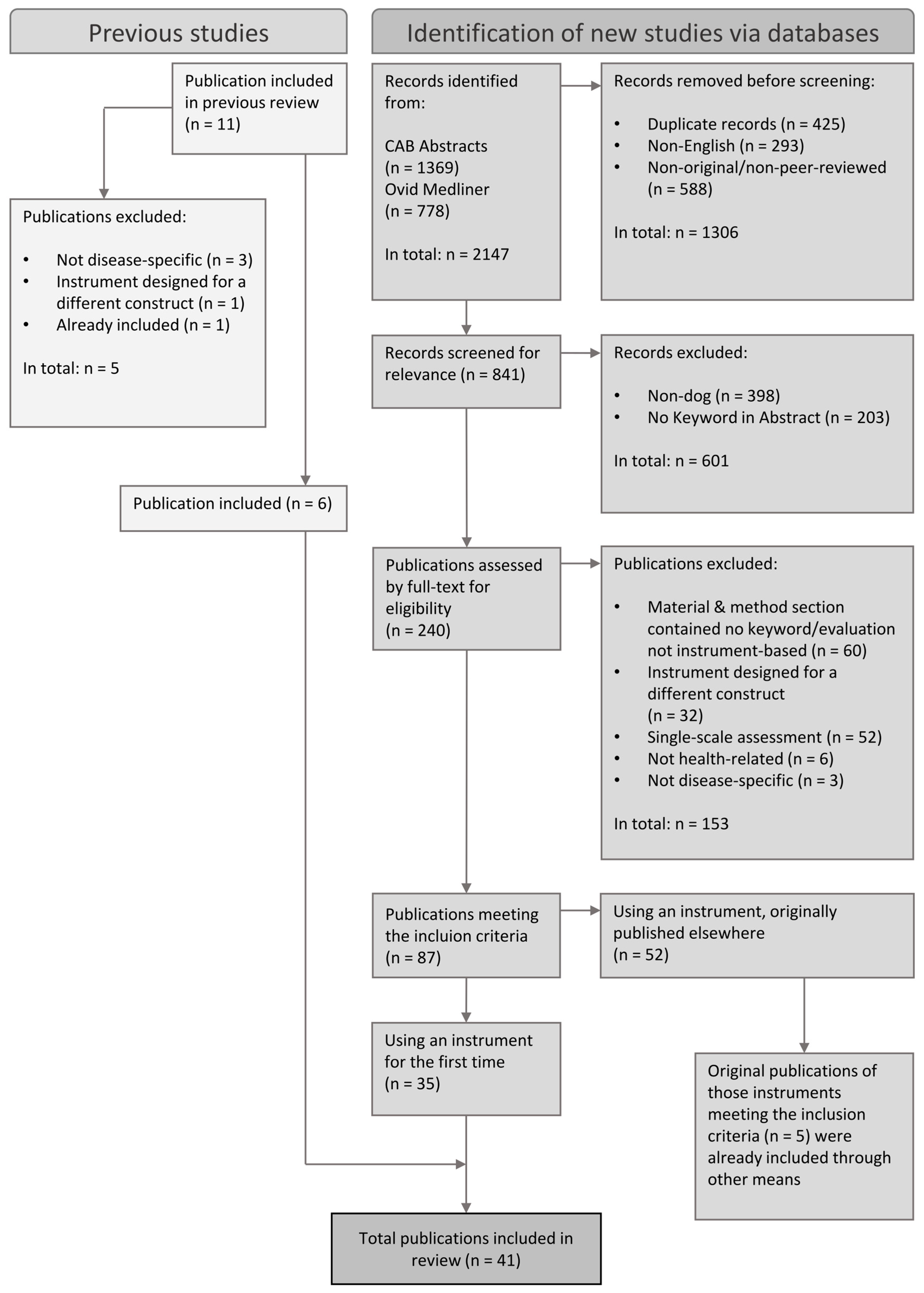
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Further Selection Process
2.6. Data Collection
2.7. Extraction of Disease Specification and Construct Measured
2.8. Extraction of Instrument Availability
2.9. Evaluation of the Item Generation and/or Selection Process and Any Form of Evaluation and/or Testing
3. Results
3.1. Disease Specification and Construct Measured
3.2. Instrument Availability
3.3. Information About Item Generation and/or Selection
3.4. Information About Instrument Testing
3.5. Characteristics of the Available Instruments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| QoL | Quality of Life |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews |
| FDA | US Food and Drug Administration |
| COSMIN | Consensus-based Standards for the Selection of Health Status Measurement Instruments |
| ICC | Intraclass Correlation Coefficient |
| VAS | Visual Analogue Scale |
| NRS | Numerical Rating Scale |
| PCA | Principal Component Analysis |
| FA | Exploratory Factor Analysis |
| HRQoL | Health-related Quality of Life |
References
- Yeates, J.; Main, D. Assessment of companion animal quality of life in veterinary practice and research. J. Small Anim. Pract. 2009, 50, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Belshaw, Z.; Asher, L.; Harvey, N.D.; Dean, R.S. Quality of life assessment in domestic dogs: An evidence-based rapid review. Vet. J. 2015, 206, 203–212. [Google Scholar] [CrossRef] [PubMed]
- The WHOQOL Group. The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- McMillan, F.D. Quality of life in animals. J. Am. Vet. Med. Assoc. 2000, 216, 1904–1910. [Google Scholar] [CrossRef]
- Belshaw, Z. Quality of life assessment in companion animals: What, why, who, when and how. Companion Anim. 2018, 23, 264–268. [Google Scholar] [CrossRef]
- McDowell, I. Measuring Health: A Guide to Rating Scales and Questionnaires, 3rd ed.; Oxford University Press: Oxford, UK, 2006; ISBN 978-0-19-516567-8. [Google Scholar]
- Guyatt, G.H. Measuring Health-Related Quality of Life: General Issues. Can. Respir. J. 1997, 4, 123–130. [Google Scholar] [CrossRef]
- Scott, E.M.; Nolan, A.M.; Reid, J.; Wiseman-Orr, M.L. Can we really measure animal quality of life? Methodologies for measuring quality of life in people and other animals. Anim. Welf. 2007, 16, 17–24. [Google Scholar] [CrossRef]
- Streiner, D.L.; Norman, G.R. Health Measurement Scales: A Practical Guide to Their Development and Use, 4th ed.; Oxford University Press: Oxford, UK, 2008; ISBN 9780199231881. [Google Scholar]
- Kirkley, A.; Griffin, S. Development of disease-specific quality of life measurement tools. Arthroscopy 2003, 19, 1121–1128. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009. Available online: https://www.fda.gov/media/77832/download (accessed on 29 January 2025).
- Fulmer, A.E.; Laven, L.J.; Hill, K.E. Quality of Life Measurement in Dogs and Cats: A Scoping Review of Generic Tools. Animals 2022, 12, 400. [Google Scholar] [CrossRef]
- Greene, L.M.; Royal, K.D.; Bradley, J.M.; Lascelles, B.D.X.; Johnson, L.R.; Hawkins, E.C. Severity of Nasal Inflammatory Disease Questionnaire for Canine Idiopathic Rhinitis Control: Instrument Development and Initial Validity Evidence. J. Vet. Intern. Med. 2017, 31, 134–141. [Google Scholar] [CrossRef]
- Beths, T.; Munn, R.; Bauquier, S.H.; Mitchell, P.; Whittem, T. A pilot study of 4CYTE™ Epiitalis® Forte, a novel nutraceutical, in the management of naturally occurring osteoarthritis in dogs. Aust. Vet. J. 2020, 98, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- DeVon, H.A.; Block, M.E.; Moyle-Wright, P.; Ernst, D.M.; Hayden, S.J.; Lazzara, D.J.; Savoy, S.M.; Kostas-Polston, E. A psychometric toolbox for testing validity and reliability. J. Nurs. Scholarsh. 2007, 39, 155–164. [Google Scholar] [CrossRef]
- Prinsen, C.A.C.; Mokkink, L.B.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; de Vet, H.C.W.; Terwee, C.B. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual. Life Res. 2018, 27, 1147–1157. [Google Scholar] [CrossRef]
- Terwee, C.B.; Prinsen, C.A.C.; Chiarotto, A.; Westerman, M.J.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; de Vet, H.C.W.; Mokkink, L.B. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: A Delphi study. Qual. Life Res. 2018, 27, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Mokkink, L.B.; de Vet, H.C.W.; Prinsen, C.A.C.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; Terwee, C.B. COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual. Life Res. 2018, 27, 1171–1179. [Google Scholar] [CrossRef]
- Mokkink, L.B.; Boers, M.; van der Vleuten, C.P.M.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; de Vet, H.C.W.; Terwee, C.B. COSMIN Risk of Bias tool to assess the quality of studies on reliability or measurement error of outcome measurement instruments: A Delphi study. BMC Med. Res. Methodol. 2020, 20, 293. [Google Scholar] [CrossRef]
- Budke, C.M.; Levine, J.M.; Kerwin, S.C.; Levine, G.J.; Hettlich, B.F.; Slater, M.R. Evaluation of a questionnaire for obtaining owner-perceived, weighted quality-of-life assessments for dogs with spinal cord injuries. J. Am. Vet. Med. Assoc. 2008, 233, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Favrot, C.; Linek, M.; Mueller, R.; Zini, E. Development of a questionnaire to assess the impact of atopic dermatitis on health-related quality of life of affected dogs and their owners. Vet. Dermatol. 2010, 21, 64–70. [Google Scholar] [CrossRef]
- Freeman, L.M.; Rush, J.E.; Farabaugh, A.E.; Must, A. Development and evaluation of a questionnaire for assessing health-related quality of life in dogs with cardiac disease. J. Am. Vet. Med. Assoc. 2005, 226, 1864–1868. [Google Scholar] [CrossRef]
- Giuffrida, M.A.; Brown, D.C.; Ellenberg, S.S.; Farrar, J.T. Development and psychometric testing of the Canine Owner-Reported Quality of Life questionnaire, an instrument designed to measure quality of life in dogs with cancer. J. Am. Vet. Med. Assoc. 2018, 252, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulou, M.A.; Kitchell, B.E.; Yuzbasiyan-Gurkan, V. Development of a survey instrument to assess health-related quality of life in small animal cancer patients treated with chemotherapy. J. Am. Vet. Med. Assoc. 2013, 242, 1679–1687. [Google Scholar] [CrossRef]
- Lynch, S.; Savary-Bataille, K.; Leeuw, B.; Argyle, D.J. Development of a questionnaire assessing health-related quality-of-life in dogs and cats with cancer. Vet. Comp. Oncol. 2011, 9, 172–182. [Google Scholar] [CrossRef]
- Marchetti, V.; Gori, E.; Mariotti, V.; Gazzano, A.; Mariti, C. The impact of chronic inflammatory enteropathy on dogs’ quality of life and dog-owner relationship. Vet. Sci. 2021, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Noli, C.; Minafò, G.; Galzerano, M. Quality of life of dogs with skin diseases and their owners. Part 1: Development and validation of a questionnaire. Vet. Dermatol. 2011, 22, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Schofield, I.; O’Neill, D.G.; Brodbelt, D.C.; Church, D.B.; Geddes, R.F.; Niessen, S.J.M. Development and evaluation of a health-related quality-of-life tool for dogs with Cushing’s syndrome. J. Vet. Intern. Med. 2019, 33, 2595–2604. [Google Scholar] [CrossRef]
- Weiske, R.; Sroufe, M.; Quigley, M.; Pancotto, T.; Werre, S.; Rossmeisl, J.H. Development and evaluation of a caregiver reported quality of life assessment instrument in dogs with intracranial disease. Front. Vet. Sci. 2020, 7, 537. [Google Scholar] [CrossRef]
- Wessmann, A.; Volk, H.A.; Parkin, T.; Ortega, M.; Anderson, T.J. Evaluation of quality of life in dogs with idiopathic epilepsy. J. Vet. Intern. Med. 2014, 28, 510–514. [Google Scholar] [CrossRef]
- Yazbek, K.V.B.; Fantoni, D.T. Validity of a health-related quality-of-life scale for dogs with signs of pain secondary to cancer. J. Am. Vet. Med. Assoc. 2005, 226, 1354–1358. [Google Scholar] [CrossRef]
- Coughlin, S.S. Recall bias in epidemiologic studies. J. Clin. Epidemiol. 1990, 43, 87–91. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Dossier Requirements for Anticancer Medicinal Products for Dogs and Cats. 2021. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-dossier-requirements-anticancer-medicinal-products-dogs-and-cats-revision-1_en.pdf (accessed on 28 January 2025).
- Wiseman, M.L.; Nolan, A.M.; Reid, J.; Scott, E.M. Preliminary study on owner-reported behaviour changes associated with chronic pain in dogs. Vet. Rec. 2001, 149, 423–424. [Google Scholar] [CrossRef] [PubMed]
- Doit, H.; Dean, R.S.; Duz, M.; Brennan, M.L. A systematic review of the quality of life assessment tools for cats in the published literature. Vet. J. 2021, 272, 105658. [Google Scholar] [CrossRef] [PubMed]
- de Vet, H.C.W.; Terwee, C.B.; Mokkink, L.B.; Knol, D.L. Measurement in Medicine: A Practical Guide; Cambridge University Press: New York, NY, USA, 2011; p. 181. ISBN 978-0-521-11820-0. [Google Scholar]
- Mokkink, L.B.; Terwee, C.B.; Knol, D.L.; Stratford, P.W.; Alonso, J.; Patrick, D.L.; Bouter, L.M.; de Vet, H.C.W. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: A clarification of its content. BMC Med. Res. Methodol. 2010, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Mokkink, L.B.; Terwee, C.B.; Patrick, D.L.; Alonso, J.; Stratford, P.W.; Knol, D.L.; Bouter, L.M.; de Vet, H.C.W. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J. Clin. Epidemiol. 2010, 63, 737–745. [Google Scholar] [CrossRef]
- Karimi, M.; Brazier, J. Health, Health-Related Quality of Life, and Quality of Life: What is the Difference? Pharmacoeconomics 2016, 34, 645–649. [Google Scholar] [CrossRef]
- Terwee, C.B.; Prinsen, C.A.C.; Chiarotto, A.; de Vet, H.C.W.; Bouter, L.M.; Alonso, J.; Westerman, M.J.; Patrick, D.L.; Mokkink, L.B. COSMIN Methodology for Assessing the Content Validity of PROMs—User Manual. 2018. Available online: https://www.cosmin.nl/wp-content/uploads/COSMIN-methodology-for-content-validity-user-manual-v1.pdf (accessed on 29 January 2025).
- Mokkink, L.B.; Prinsen, C.A.C.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; de Vet, H.C.W.; Terwee, C.B. COSMIN Methodology for Systematic Reviews of Patient-Reported Outcome Measures (PROMs)—User Manual. 2018. Available online: https://cosmin.nl/wp-content/uploads/COSMIN-syst-review-for-PROMs-manual_version-1_feb-2018.pdf (accessed on 29 January 2025).
| Criteria |
|---|
| Was the instrument based on/adapted from previously published veterinary questionnaires? |
| If so, were there any clues about the motivation for modification or of the criteria for item selection? |
| Was there any orientation towards humane medicine? |
| Was a literature review used to generate items? |
| Were experts involved in the development of the items? |
| Property | Aspects | Description |
|---|---|---|
| Comprehensibility | An attempt has been made to ensure that the instrument is understandable by the target group, e.g., qualitative focus group discussions, cognitive interviews with the target group, or quantitative assessments for clarity. | |
| Reliability | The reliability of a test indicates that the measurement of a test is subject to minimal errors, and results are independent of influences other than the object being measured itself [9] (p. 159). | |
| Test–retest reliability resp. intra-rater reliability | The ability of the instrument to produce repeatable results if the same person administers the tool to the same individual at two or more time points; the correlation should be calculated using the ICC * [10] or Cohen’s kappa when no underlying continuum exists [9] (pp. 174–178). | |
| Inter-rater reliability | The reproducibility of a score when different people administer the instrument to the same individual. Calculation of the correlation should be performed using the ICC * [10] or Cohen’s kappa if no underlying continuum exists [9] (pp. 174–178). | |
| Internal consistency | The correlation degree between the individual items within a questionnaire. High internal consistency contributes to good reliability. It should be calculated using Cronbach’s alpha [9] (p. 88). | |
| Validity a | A test’s validity describes the test’s ability to measure what the test was supposed to measure [6] (p. 30). | |
| Content validity | The degree to which an instrument adequately incorporates every relevant aspect and leaves out unimportant aspects. Content validity should be established over every other aspect of validity. It can be established based on the judgement of experts [9] (p. 233), or when it is about an instrument measuring an outcome related to the subjective experience of patients. It can also be established through the input of patients given during the development process or via interviews wherein patients are asked whether the instrument encompasses all important aspects [11]. | |
| Face validity | The degree to which the instrument appears to measure what it intends to measure. It may increase the acceptance of people who are supposed to use it or lead respondents to consciously or subconsciously adjust their answers to satisfy expectations [9] (p. 79) [10]. | |
| Construct validity —hypothesis testing. | The degree to which an instrument measures previously formulated hypotheses: Known-groups comparison Comparison of two populations known to be different regarding the construct being measured, e.g., sick and healthy individuals [11,16] Comparison with a separately asked overall assessment Comparing a multi-item measure with a single-item question about the construct in general, where the results are expected to correlate. Convergent validity Testing how the evaluated construct behaves in relation to measurements of other constructs known to be related. Convergent validity describes that the measurements of two supposedly related constructs should behave similarly [6] (p. 34), e.g., the results of a QoL measurement are expected to correlate with the scores of an instrument to measure disease severity, hypothesising that disease severity influences QoL. | |
| Construct validity—Structural validity/unidimensionality. | Statistical assessment of the underlying structure; principal component or factor analysis to identify the underlying dimensions, used to reduce the number of variables, aids in omitting redundant items, of which the underlying driving component is already sufficiently represented elsewhere; latent variables to discover sub-concepts of a construct and their relations. | |
| Responsiveness | Information has been provided on how responsive the tool is to changes in condition. | |
| Interpretability | Information has been provided on the meaning of a change in the score and a report of the minimal important difference. |
| Name | Score Calculation | Domains | Items |
|---|---|---|---|
| Was the instrument named? | Was there an overall score? | Were the items grouped into domains? | How many items were contained? |
| If yes, how was it created? | If yes, how many were there? | How were the items scaled? | |
| If yes, was there a weighted score? |
| Disease Type | Number of Instruments |
|---|---|
| Cancer | 11 |
| Cardiac disease | 7 |
| Orthopaedic disease/condition | 7 |
| Neurological disease | 3 |
| Portosystemic shunt | 3 |
| Skin disease | 3 |
| Respiratory disease | 2 |
| Enteric disease | 2 |
| Haematological disease | 1 |
| Obesity | 1 |
| Metabolic disease | 1 |
| Specific Focus | Number of Instruments |
|---|---|
| Cancer in general | 2 |
| Receiving chemotherapy | 1 |
| Appendicular osteosarcoma | 1 |
| Nasal carcinoma | 1 |
| Urinary bladder carcinoma | 1 |
| Spontaneous melanoma | 1 |
| Lung carcinoma | 1 |
| Oral malignant melanoma | 1 |
| Mast cell tumour | 1 |
| Pain secondary to cancer | 1 |
| Budke et al., 2008 [21] | Favrot et al., 2010 [22] | Freeman et al., 2005 [23] | Giuffrida et al., 2018 [24] | Iliopoulou et al., 2013 [25] | Lynch et al., 2011 [26] | Marchetti et al., 2021 [27] | Noli et al., 2011 [28] | Schofield et al., 2019 [29] | Weiske et al., 2020 [30] | Wessmann et al., 2014 [31] | Yazbek and Fantoni, 2005 [32] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Instrument available? | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | |
| Development described? | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | N | |
| Comprehensibility? | N | N | Y | Y | N | Y | N | Y | Y | Y | N | Y | |
| Reliability | Test–retest reliability/ Intra-rater reliability? | N | N | (Y) c | Y | N | N | N | N g | Y | Y j | N | N |
| Inter-rater reliability? | N | N | N | N | N | N | N | N | Y | N | N | N | |
| Internal consistency? | na a | N | Y | Y | N | N | Y f | (Y) h | Y | Y | Y | N | |
| Underlying structure | Statistical assessment of the underlying structure? | na a | N | N | Y | N | N | N | N | Y | Y | Y | N |
| Unidimensionality/ principal component analysis | N | Y | Y | Y | |||||||||
| Structural validity/ factor analysis | Y | N | N | N | |||||||||
| Validity | Face validity | Y | N | Y | N | N | Y | N | N | N | Y | N | N |
| Content validity | na a | Y | N | Y | N | Y | N | Y | Y | Y | Y | N | |
| Construct validity—hypothesis testing known-groups comparison | Y | N | N | N | Y e | N | Y | Y | Y | Y | N | Y | |
| Construct validity—hypothesis testing comparison instrument overall score with a single-question overall assessment | Y | na * | Y | N | N | na * | na * | N | Y | Y | na * | N | |
| Construct validity—hypothesis testing convergent validity with related constructs | N | N b | Y d | N | N | N | N | Y i | N | Y | N k | N | |
| Responsiveness? | N | N | N | Y | N | N | N | N | N | N | N | ||
| Interpretability? | N | N | N | N | N | N | N | N | N | N | N |
| Instrument Named? If Yes, How? | Field of Application | Number of Items | Item Scaling | Divided into Domains? | If Yes, How Many? | Overall Score Calculated? | Weighted Score? | Calculation Method | |
|---|---|---|---|---|---|---|---|---|---|
| Budke et al. (2008) [21] | No | Spinal cord injuries | 5 | VAS | No | Yes | Yes | Multiplying the weight percentage by the VAS score | |
| Favrot et al. (2010) [22] | No | Atopic dermatitis | 14 | 5-Point Likert | No | No | No | ||
| Freeman et al. (2005) [23] | Functional evaluation of cardiac health (FETCH) | Heart disease | 0–5 NRS | No | Yes | No | Item scores summarised | ||
| Giuffrida et al. (2018) [24] | Canine Owner-Reported Quality of Life (CORQ) | Cancer | 17 a | 0–7 NRS | Yes | 3 | Yes | No | The sum of item scores divided by the total number of items |
| Lynch et al. (2011) [26] | Cancer Treatment Form | Cancer | 22 a | 1–5 NRS + one VAS | Yes | 8 | ND | ||
| Marchetti et al. (2021) [27] | No | Chronic inflammatory enteropathy | 5 | 1–10 NRS | No | No | |||
| Noli et al. (2011) [28] | No | Skin diseases | 7 | 4-point adjectival | No | Yes | No | Item scores summarised | |
| Schofield et al. (2019) [29] | CushQol-pet | Cushing’s syndrome | 19 | 4-point adjectival | No | Yes | No | The sum of item scores divided by the maximum possible score | |
| Weiske et al. (2020) [30] | CanBrainQOL-24 | Intracranial diseases | 24 | 5-point adjectival b | Yes | 3 | Yes | No | Item scores summarised |
| Wessmann et al. (2014) [31] | Epilepsy disease-specific quality of life list of key questions (EpiQol) | Idiopathic epilepsy | 16 c | 5-point Likert | Yes c | 2 c | No | ||
| Yazbek and Fantoni (2005) [32] | No | Pain secondary to cancer | 12 | 4-point adjectival scale | No | Yes | No | Item scores summarised |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhein, F.F.; Klee, R.; Albrecht, B.; Krämer, S. Instruments to Assess Disease-Specific Quality of Life in Dogs: A Scoping Review. Animals 2025, 15, 1780. https://doi.org/10.3390/ani15121780
Rhein FF, Klee R, Albrecht B, Krämer S. Instruments to Assess Disease-Specific Quality of Life in Dogs: A Scoping Review. Animals. 2025; 15(12):1780. https://doi.org/10.3390/ani15121780
Chicago/Turabian StyleRhein, Friederike Felicitas, Rebecca Klee, Balazs Albrecht, and Stephanie Krämer. 2025. "Instruments to Assess Disease-Specific Quality of Life in Dogs: A Scoping Review" Animals 15, no. 12: 1780. https://doi.org/10.3390/ani15121780
APA StyleRhein, F. F., Klee, R., Albrecht, B., & Krämer, S. (2025). Instruments to Assess Disease-Specific Quality of Life in Dogs: A Scoping Review. Animals, 15(12), 1780. https://doi.org/10.3390/ani15121780







