Genotypic and Allelic Frequencies of Hereditary Cataract in the Italian Population of Australian Shepherd and Miniature American Shepherd Dogs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. DNA Genotyping
2.3. Statistical Analyses
3. Results
3.1. Temporal Trend
3.2. Sex
3.3. Coat Color
3.4. Age
3.5. Miniature American Shepherds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ASCA: The Australian Shepherd. Available online: https://asca.org/aussies/about-aussies/the-australian-shepherd/ (accessed on 25 February 2025).
- ENCI: Ente Nazionale Cinofilia Italiana. Available online: https://www.enci.it/libro-genealogico/razze/australian-shepherd (accessed on 25 February 2025).
- Donner, J.; Anderson, H.; Davison, S.; Hughes, A.M.; Bouirmane, J.; Lindqvist, J.; Lytle, K.M.; Ganesan, B.; Ottka, C.; Ruotanen, P.; et al. Frequency and distribution of 152 genetic disease variants in over 100,000 mixed breed and purebred dogs. PLoS Genet. 2018, 14, e1007361. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.G.; Nelms, S.R. Diseases of the lens and cataract formation. In Veterinary Ophthalmology, 3rd ed.; Gelatt, K.N., Ed.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 1999; pp. 797–825. [Google Scholar]
- Rubin, L.F. Inherited Eye Diseases in Purebred Dogs; Williams & Wilkins: Baltimore, MD, USA, 1989. [Google Scholar]
- Barnett, K.C. Hereditary cataract in the dog. J. Small. Anim. Pract. 1978, 19, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Gelatt, K.N.; MacKay, E.O. Prevalence of primary breed-related cataracts in the dog in North America. Vet. Ophthalmol. 2005, 8, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Online Mendelian Inheritance in Animals (OMIA). Cataract, Early-Onset, HSF4-Related in Canis lupus familiaris (OMIA:001758-9615). Available online: https://omia.org/OMIA001758/9615/ (accessed on 27 May 2025).
- Mellersh, C.S.; Pettitt, L.; Forman, O.P.; Vaudin, M.; Barnett, K.C. Identification of mutations in HSF4 in dogs of three different breeds with hereditary cataracts. Vet. Ophthalmol. 2006, 9, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Mellersh, C.S.; Graves, K.T.; McLaughlin, B.; Ennis, R.B.; Pettitt, L.; Vaudin, M.; Barnett, K.C. Mutation in HSF4 associated with early but not late-onset hereditary cataract in the Boston Terrier. J. Hered. 2007, 98, 531–533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mellersh, C.S.; McLaughlin, B.; Ahonen, S.; Pettitt, L.; Lohi, H.; Barnett, K.C. Mutation in HSF4 is associated with hereditary cataract in the Australian Shepherd. Vet. Ophthalmol. 2009, 12, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Diehl, K.A.; Asif, S.K.; Mowat, F. Ophthalmic Disease and Screening in Breeding Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2023, 53, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Genetics Committee of the American College of Veterinary Ophthalmologists. Ocular Disorders Presumed to Be Inherited in Purebred Dogs, 13th ed.; American College of Veterinary Ophthalmologists: Meridian, ID, USA, 2021. [Google Scholar]
- Bu, L.; Jin, Y.; Shi, Y.; Chu, R.; Ban, A.; Eiberg, H.; Andres, L.; Jiang, H.; Zheng, G.; Qian, M.; et al. Mutant DNA-binding domain of HSF4 is associated with autosomal dominant lamellar and Marner cataract. Nat. Gen. 2002, 31, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Forshew, T.; Johnson, C.A.; Khaliq, S.; Pasha, S.; Willis, C.; Abbasi, R.; Tee, L.; Smith, U.; Trembath, R.C.; Mehdi, S.Q.; et al. Locus heterogeneity in autosomal recessive congenital cataracts: Linkage to 9q and germline HSF4 mutations. Hum. Gen. 2005, 117, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Ke, T.; Wang, Q.K.; Ji, B.; Wang, X.; Liu, P.; Zhang, X.; Tang, Z.; Ren, X.; Liu, M. Novel HSF4 mutation causes congenital total white cataract in a Chinese family. Am. J. Ophthalmol. 2006, 142, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, A.; Wohlke, A.; Distl, O. Evaluation of canine heat-shock transcription factor 4 as a candidate for primary cataracts in English Cocker Spaniels and wire-haired Kromfohrlanders. J. Anim. Breed. Genet. 2007, 124, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, K.K.L.; Lingaas, F. Cataracts in Havanese: Genome wide association study reveals two loci associated with posterior polar cataract. Canine Med. Genet. 2023, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, S.L.; Pettitt, L.; McLaughlin, B.; Jenkins, C.A.; Mellersh, C.S. A novel locus on canine chromosome 13 is associated with cataract in the Australian Shepherd breed of domestic dog. Mamm. Genome 2015, 26, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Agresti, A. An Introduction to Categorical Data Analysis, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2007; p. 39. [Google Scholar]
- Majchrakova, Z.; Hrckova Turnova, E.; Bielikova, M.; Turna, J.; Dudas, A. The incidence of genetic disease alleles in Australian Shepherd dog breed in European countries. PLoS ONE 2023, 18, e0281215. [Google Scholar] [CrossRef] [PubMed]
- Beckers, E.; Van Poucke, M.; Ronsyn, L.; Peelman, L. Frequency estimation of disease-causing mutations in the Belgian population of some dog breeds—Part 1: Shepherds. Vlaams Diergeneeskd. Tijdschr. 2016, 85, 175–184. [Google Scholar]
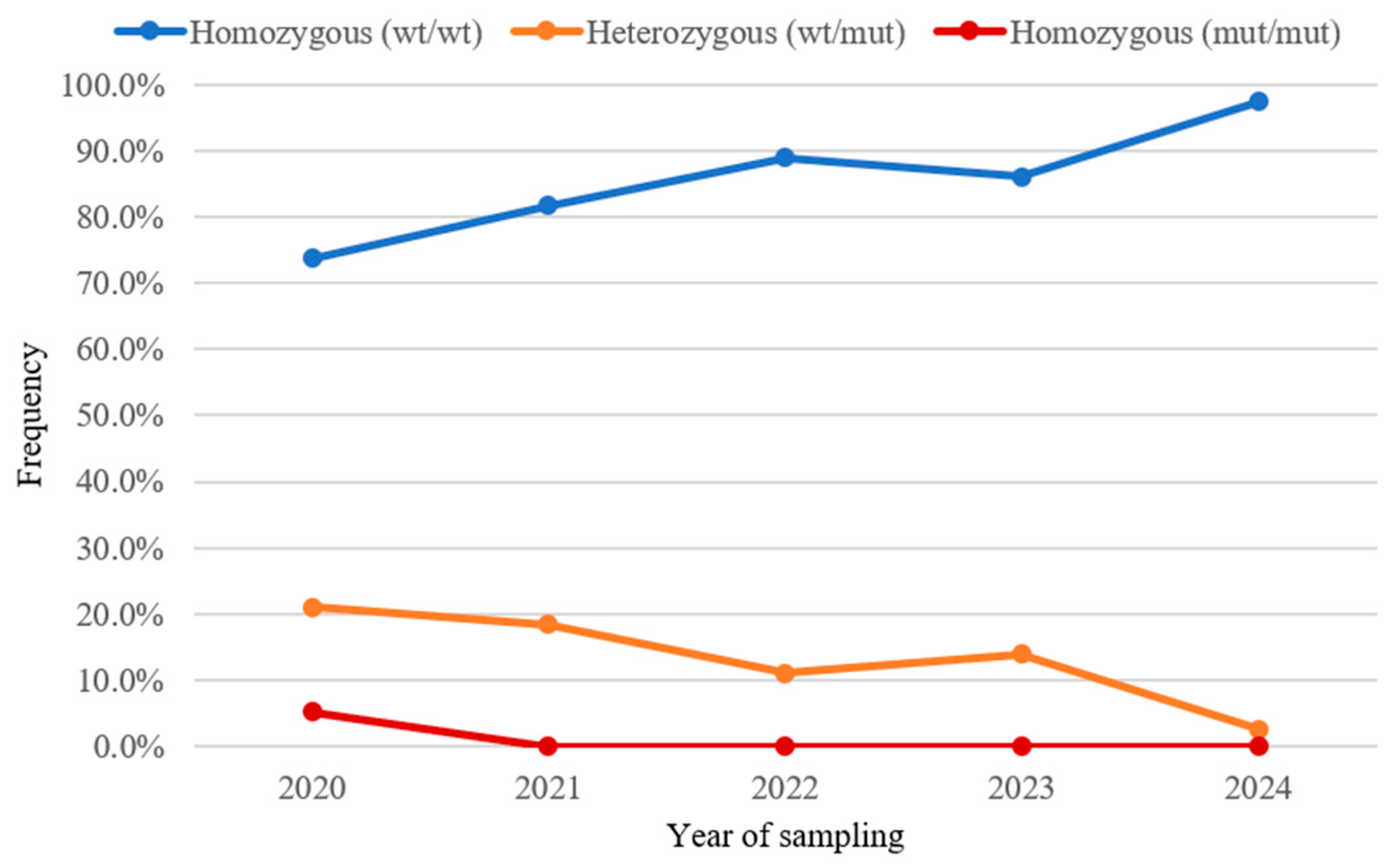
| Year of Sampling | N of Samples | Homozygous (WT/WT) | Heterozygous (WT/mut) | Homozygous (mut/mut) | |||
|---|---|---|---|---|---|---|---|
| n | freq | n | freq | n | freq | ||
| 2020 | 19 | 14 | 73.6% | 4 | 21.1% | 1 | 5.3% |
| 2021 | 49 | 40 | 81.6% | 9 | 18.4% | 0 | 0.0% |
| 2022 | 45 | 40 | 88.9% | 5 | 11.1% | 0 | 0.0% |
| 2023 | 43 | 37 | 86.0% | 6 | 14.0% | 0 | 0.0% |
| 2024 | 77 | 75 | 97.4% | 2 | 2.6% | 0 | 0.0% |
| total | 233 | 206 | 88.4% | 26 | 11.2% | 1 | 0.4% |
| Comparison | p-Value | Adjusted p-Value |
|---|---|---|
| 2020 vs. 2021 | 0.33 | 1 |
| 2020 vs. 2022 | 0.16 | 1 |
| 2020 vs. 2023 | 0.18 | 1 |
| 2020 vs. 2024 | 0.003 | 0.03 * |
| 2021 vs. 2022 | 0.39 | 1 |
| 2021 vs. 2023 | 0.78 | 1 |
| 2021 vs. 2024 | 0.003 | 0.03 * |
| 2022 vs. 2023 | 0.75 | 1 |
| 2022 vs. 2024 | 0.09 | 0.99 |
| 2023 vs. 2024 | 0.02 | 0.24 |
| Sex | N of Samples | Homozygous (WT/WT) | Heterozygous (WT/mut) | Homozygous (mut/mut) | |||
|---|---|---|---|---|---|---|---|
| n | freq | n | freq | n | freq | ||
| Female | 146 | 127 | 87.0% | 18 | 12.3% | 1 | 0.7% |
| Male | 87 | 79 | 90.8% | 8 | 9.2% | 0 | 0.0% |
| Coat Color | N of Samples | Homozygous (WT/WT) | Heterozygous (WT/mut) | Homozygous (mut/mut) | |||
|---|---|---|---|---|---|---|---|
| n | freq | n | freq | n | freq | ||
| Black and white | 2 | 2 | 100.0% | 0 | 0.0% | 0 | 0.0% |
| Black tricolor | 67 | 56 | 83.6% | 10 | 14.9% | 1 | 1.5% |
| Blue merle | 78 | 70 | 89.7% | 8 | 10.3% | 0 | 0.0% |
| Black and tan | 10 | 10 | 100.0% | 0 | 0.0% | 0 | 0.0% |
| Red | 2 | 2 | 100.0% | 0 | 0.0% | 0 | 0.0% |
| Red merle | 30 | 30 | 100.0% | 0 | 0.0% | 0 | 0.0% |
| Red tricolor | 35 | 29 | 82.9% | 6 | 17.1% | 0 | 0.0% |
| Red and tan | 3 | 3 | 100.0% | 0 | 0.0% | 0 | 0.0% |
| Tricolor (undefined) | 5 | 4 | 80.0% | 1 | 20.0% | 0 | 0.0% |
| Undetermined | 1 | 0 | 0.0% | 1 | 100.0% | 0 | 0.0% |
| Age | N of Samples | Homozygous (WT/WT) | Heterozygous (WT/mut) | Homozygous (mut/mut) | |||
|---|---|---|---|---|---|---|---|
| n | freq | n | freq | n | freq | ||
| <1 year | 22 | 20 | 90.9% | 2 | 9.1% | 0 | 0.0% |
| 1 year | 67 | 57 | 85.1% | 10 | 14.9% | 0 | 0.0% |
| 2 years | 72 | 64 | 88.9% | 8 | 11.1% | 0 | 0.0% |
| 3 years | 40 | 37 | 92.5% | 3 | 7.5% | 0 | 0.0% |
| 4 years | 18 | 15 | 83.3% | 2 | 11.1% | 1 | 5.6% |
| 5 years | 8 | 7 | 87.5% | 1 | 12.5% | 0 | 0.0% |
| 6 years | 3 | 3 | 100.0% | 0 | 0.0% | 0 | 0.0% |
| 7 years | 1 | 1 | 100.0% | 0 | 0.0% | 0 | 0.0% |
| 8 years | 1 | 1 | 100.0% | 0 | 0.0% | 0 | 0.0% |
| 12 years | 1 | 1 | 100.0% | 0 | 0.0% | 0 | 0.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Iorio, M.G.; Minozzi, G.; Ghilardi, S.; Frattini, S.; Bagardi, M.; Brambilla, P.G.; Paganelli, A.; Cozzi, M.C.; Vecchi, F.; Polli, M. Genotypic and Allelic Frequencies of Hereditary Cataract in the Italian Population of Australian Shepherd and Miniature American Shepherd Dogs. Animals 2025, 15, 1778. https://doi.org/10.3390/ani15121778
De Iorio MG, Minozzi G, Ghilardi S, Frattini S, Bagardi M, Brambilla PG, Paganelli A, Cozzi MC, Vecchi F, Polli M. Genotypic and Allelic Frequencies of Hereditary Cataract in the Italian Population of Australian Shepherd and Miniature American Shepherd Dogs. Animals. 2025; 15(12):1778. https://doi.org/10.3390/ani15121778
Chicago/Turabian StyleDe Iorio, Maria Grazia, Giulietta Minozzi, Sara Ghilardi, Stefano Frattini, Mara Bagardi, Paola Giuseppina Brambilla, Alessandra Paganelli, Maria Cristina Cozzi, Francesca Vecchi, and Michele Polli. 2025. "Genotypic and Allelic Frequencies of Hereditary Cataract in the Italian Population of Australian Shepherd and Miniature American Shepherd Dogs" Animals 15, no. 12: 1778. https://doi.org/10.3390/ani15121778
APA StyleDe Iorio, M. G., Minozzi, G., Ghilardi, S., Frattini, S., Bagardi, M., Brambilla, P. G., Paganelli, A., Cozzi, M. C., Vecchi, F., & Polli, M. (2025). Genotypic and Allelic Frequencies of Hereditary Cataract in the Italian Population of Australian Shepherd and Miniature American Shepherd Dogs. Animals, 15(12), 1778. https://doi.org/10.3390/ani15121778





