Co-Exposure with the Herbicide 2,4-D Does Not Exacerbate Batrachochytrium salamandrivorans Infection in the Italian Crested Newt (Triturus carnifex)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Morphological Metrics
2.3. Bsal Load, Infection Intensity and Disease Severity Quantification
2.4. Telomere Length Assay
2.5. Statistical Analysis
3. Results
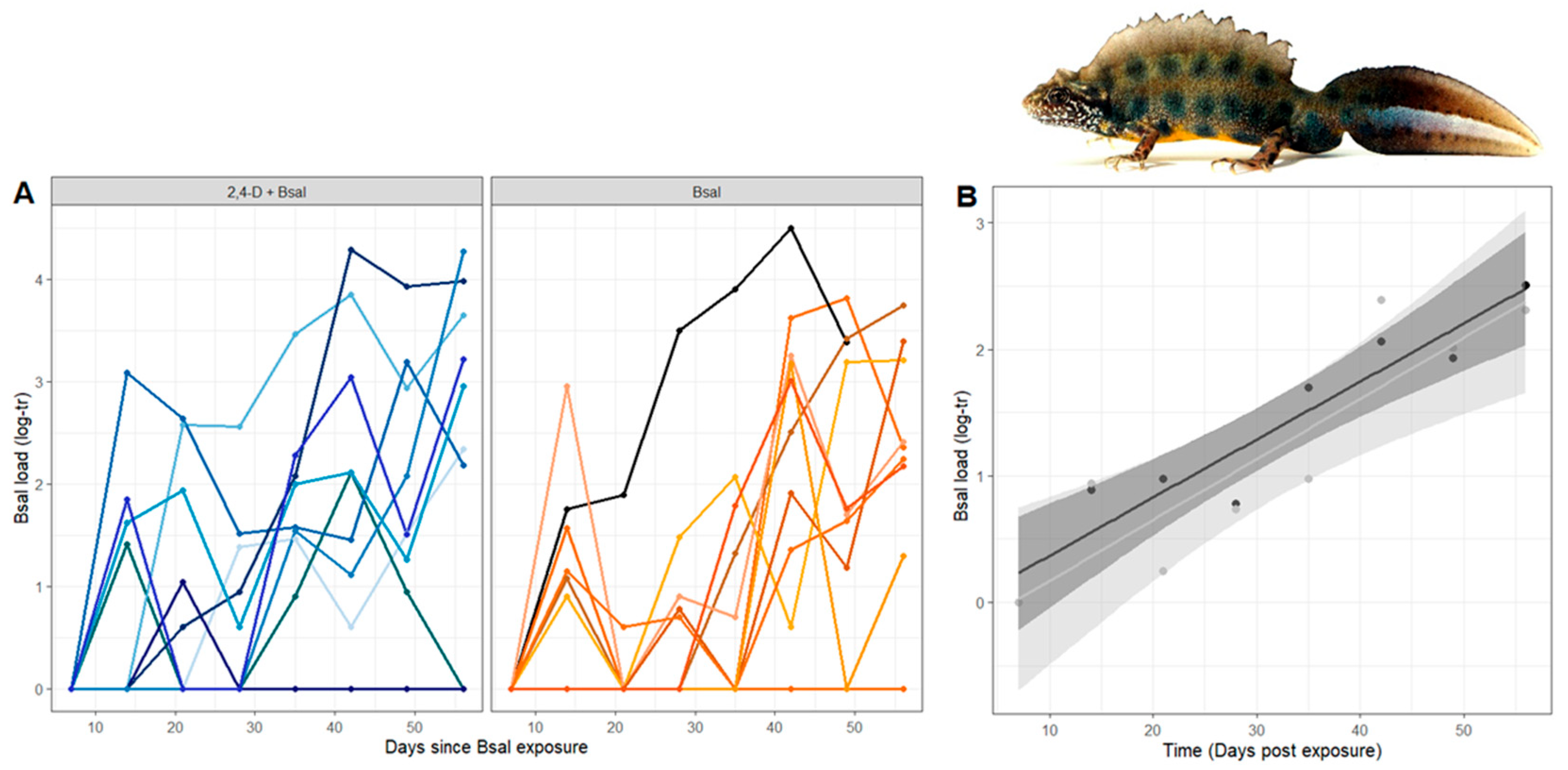
3.1. Susceptibility to Bsal Infection Varies Between Individual Italian Crested Newts
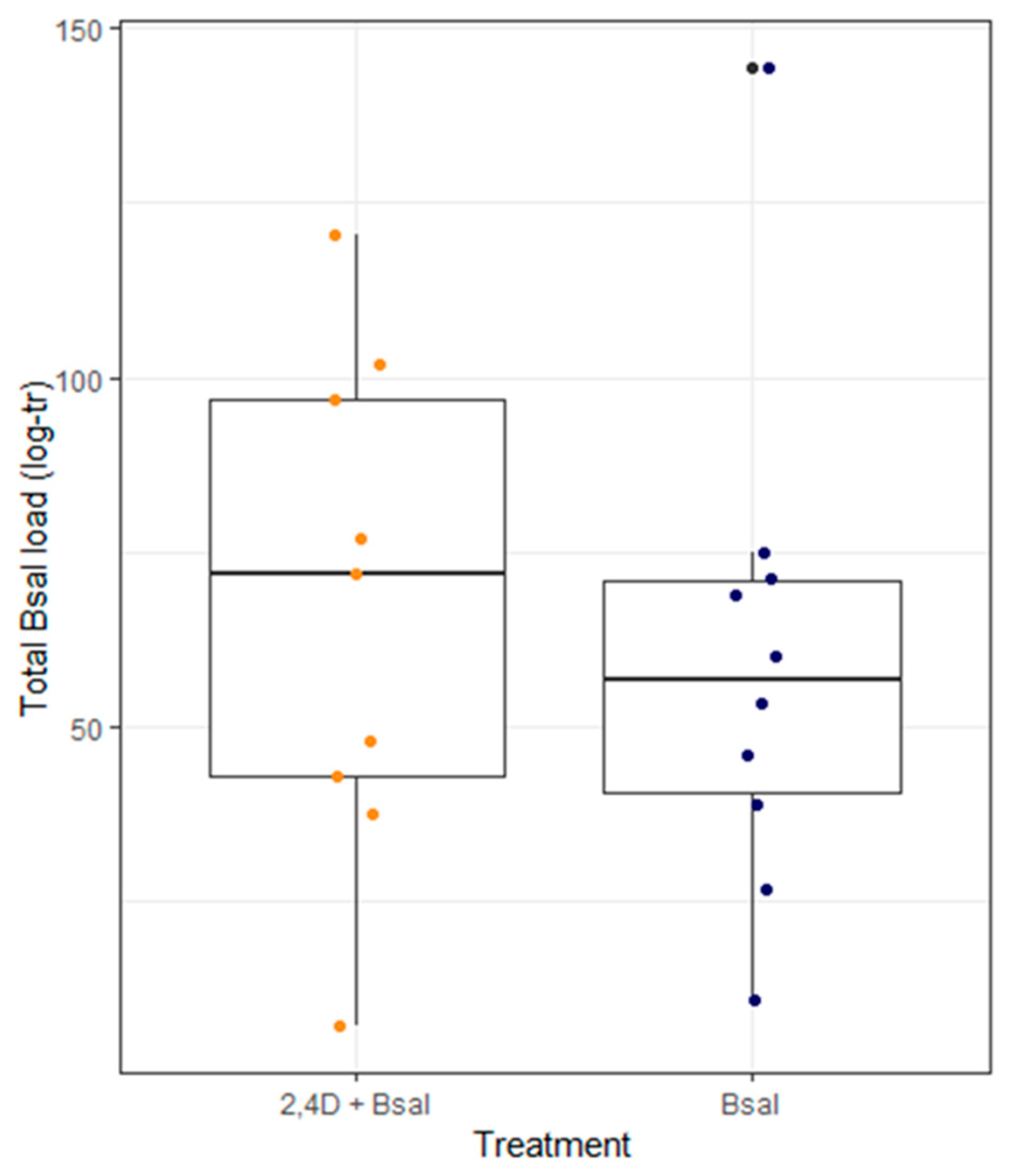
3.2. Bsal Load, Intensity of Infection and Severity of Chytridiomycosis Are Not Affected by Short-Term 2,4-D Exposure
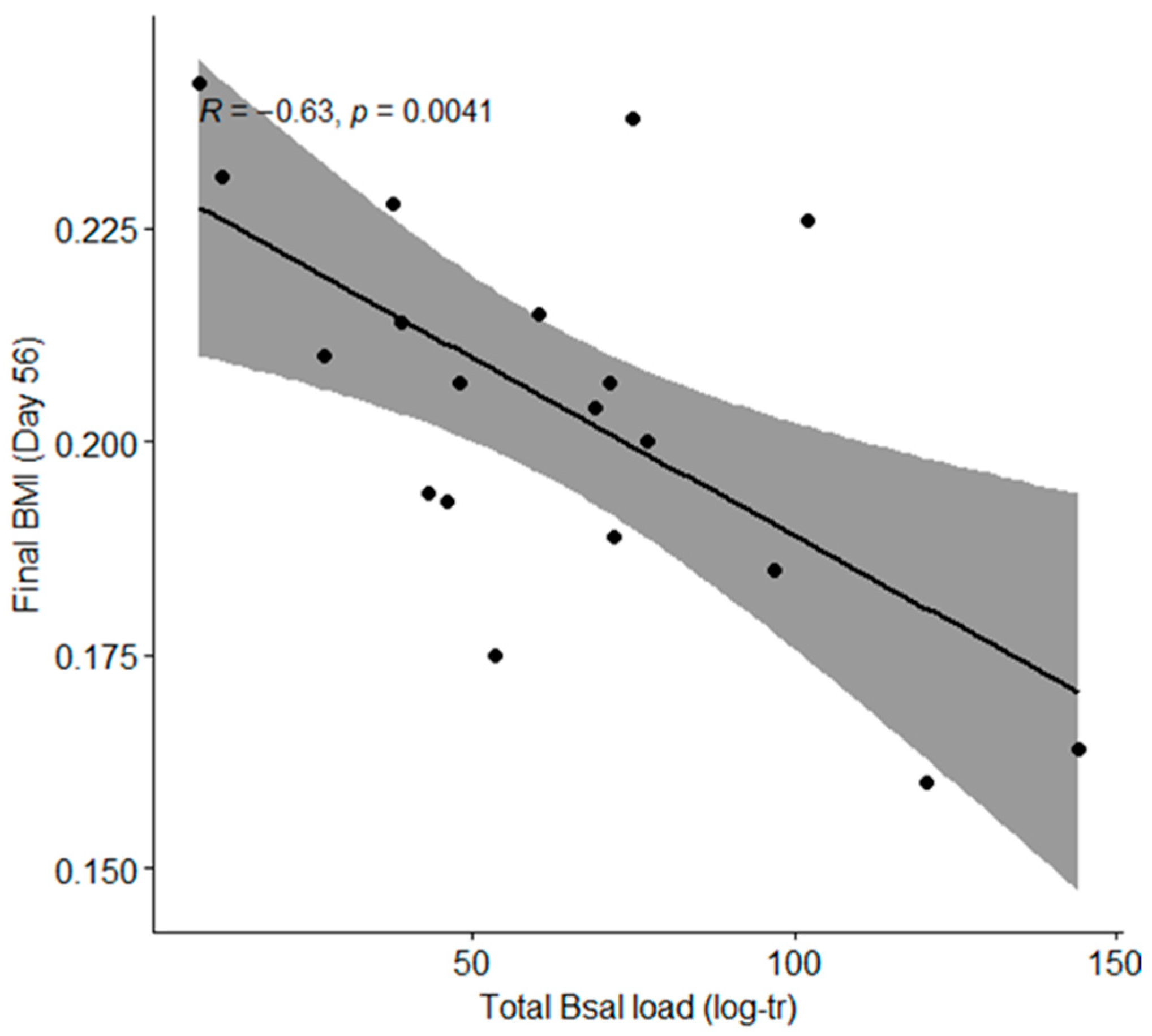
3.3. Newt Health Is Determined by the Total Bsal Load but Not by Exposure to 2,4-D
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bsal | Batrachochytrium salamandrivorans |
| Bd | Batrachochytrium dendrobatidis |
| 2,4-D | 2,4-dichlorophenoxyacetic Acid |
| BMI | Body Mass Index |
| qPCR | Quantitative Polymerase Chain Reaction |
| GE | Genomic Equivalent |
| SVL | Snout–Vent Length |
| UCE | Ultra-Conserved Element |
| ANOVA | Analysis of Variance |
| ANCOVA | Analysis of Covariance |
| DNA | Deoxyribonucleic Acid |
| T/S | Telomere/Single-copy Gene Ratio |
References
- Stuart, S.; Chanson, J.; Cox, N.; Young, B.; Rodrigues, A.; Fischman, D.; Waller, R. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- IUCN SSC Amphibian Specialist Group. Triturus cristatus. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 11 December 2024).
- Swandby, L.; Chanson, J.; Kelsey, J.; Hall, R.; Marris, A.; Wilson, K.; McCallum, H.; Gillespie, G.; Garnham, L.; Waller, R. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar]
- Brunner, J.L.; Olson, D.H.; Gray, M.J.; Miller, D.L.; Duffus, A.L. Global patterns of ranavirus detections. Facets 2021, 6, 912–924. [Google Scholar] [CrossRef]
- Lisachova, L.S.; Lisachov, A.P.; Ermakov, O.A.; Svinin, A.O.; Chernigova, P.I.; Lyapkov, S.M.; Zamaletdinov, R.I.; Pavlov, A.V.; Zaks, S.S.; Fayzulin, A.I. Continent-wide distribution of CMTV-like ranavirus, from the Urals to the Atlantic Ocean. Ecohealth 2025, 263, e5949. [Google Scholar] [CrossRef] [PubMed]
- Svinin, A.O.; Vedernikov, A.A.; Drobot, G.P.; Bashinskiy, I.V.; Neymark, L.A.; Litvinchuk, S.N.; Ermakov, O.A.; Ivanov, A.Y.; Osipov, V.V.; Dubois, A. Strigea robusta causes polydactyly and severe forms of Rostand’s anomaly P in water frogs. Parasites Vectors 2020, 13, 931–940. [Google Scholar] [CrossRef]
- Trimpert, J.; Eichhorn, I.; Vladimirova, D.; Haake, A.; Schink, A.K.; Klopfleisch, R.; Lubke-Becker, A. Elizabethkingia miricola infection in multiple anuran species. Transbound. Emerg. Dis. 2021, 68, 931–940. [Google Scholar] [CrossRef]
- Hof, C.; Araújo, M.B.; Jetz, W.; Rahbek, C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 2011, 480, 516–519. [Google Scholar] [CrossRef]
- Alton, L.; Franklin, C. Drivers of amphibian declines: Effects of ultraviolet radiation and interactions with other environmental factors. Clim. Chang. Resp. 2017, 4, 6. [Google Scholar] [CrossRef]
- Gaietto, K.; Rumschlag, S.; Boone, M. Effects of pesticide exposure and the amphibian chytrid fungus on gray treefrog (Hyla chrysoscelis) metamorphosis. Environ. Toxicol. Chem. 2014, 33, 2358–2362. [Google Scholar] [CrossRef]
- Hanlon, S.; Kerby, J.; Parris, M. Unlikely remedy: Fungicide clears infection from pathogenic fungus in larval southern leopard frogs (Lithobates sphenocephalus). PLoS ONE 2012, 8, e43573. [Google Scholar] [CrossRef]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphib. Fungal Panzootic Causes Catastrophic Ongoing Loss biodiversity. Science 2019, 363, 1459–1463. [Google Scholar]
- Berger, L.; Speare, R.; Daszak, P.; Green, D.E.; Cunningham, A.A.; Goggin, C.L.; Slocombe, R.; Ragan, M.A.; Hyatt, A.D.; McDonald, K.R.; et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rainforests of Australia and Central America. Proc. Natl. Acad. Sci. USA 1998, 95, 9031–9036. [Google Scholar] [CrossRef] [PubMed]
- Martel, A.; Blooi, M.; Adriaensen, C.; Van Rooij, P.; Beukema, W.; Fisher, M.C.; Schmidt, B.R.; Spitzen-van der Sluijs, A.; Salvidio, S.; van Praet, S. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 2014, 346, 630–631. [Google Scholar] [CrossRef]
- Islam, M.R.; Gray, M.J.; Peace, A. Identifying the dominant transmission pathway in a multi-stage infection model of the emerging fungal pathogen batrachochytrium salamandrivorans on the eastern newt. In Infectious Diseases and Our Planet; Teboh-Ewungkem, M.I., Ngwa, G.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; p. 7. [Google Scholar]
- Carter, E.D.; DeMarchi, J.A.; Wilber, M.Q.; Miller, D.L.; Gray, M.J. Batrachochytrium salamandrivorans is necronotic: Carcasses could play a role in Bsal transmission. Front. Amphib. Reptile Sci. 2024, 2, 1284608. [Google Scholar] [CrossRef]
- Fisher, M.; Pasmans, F.; Martel, A. Virulence and pathogenicity of chytrid fungi causing amphibian extinctions. Annu. Rev. Microbiol. 2021, 75, 10. [Google Scholar] [CrossRef]
- O’Hanlon, S.J.; Rieux, A.; Farrer, R.A.; Rosa, G.M.; Waldman, B.; Bataille, A.; Kosch, T.A.; Murray, K.A.; Brankovics, B.; Fumagalli, M.; et al. Recent Asian Origin of Chytrid Fungi Causing Global Amphibian Declines. Science 2018, 360, 621–627. [Google Scholar] [CrossRef]
- Byrne, A.Q.; Vredenburg, V.T.; Martel, A.; Pasmans, F.; Bell, R.C.; Blackburn, D.C.; Bletz, M.C.; Bosch, J.; Briggs, C.J.; Brown, R.M.; et al. Cryptic diversity of a widespread global pathogen reveals expanded threats to amphibian conservation. Proc. Natl. Acad. Sci. USA 2019, 116, 20382–20387. [Google Scholar] [CrossRef]
- Spitzen-van der Sluijs, A.; Martel, A.; Asselberghs, J.; Devisscher, S.; van den Berghe, J.; Stegen, G.; Schmeller, D.S.; Crottini, A. Expanding distribution of lethal amphibian fungus Batrachochytrium salamandrivorans in Europe. Emerg. Infect. Dis. 2016, 22, 1286–1288. [Google Scholar] [CrossRef] [PubMed]
- Lötters, S.; Wagner, N.; Albaladejo, P.; Boening, L.; Dalbeck, L.; Duessel, H.; Feldmeier, M.; Guschal, K.; Kirst, D.; Ohlhoff, K.; et al. The amphibian pathogen batrachochytrium salamandrivorans in the hotspot of its European invasive range: Past–present–future. Salamandra 2020, 56, 173–188. [Google Scholar]
- Bosch, J.; Martel, A.; Sopniewski, J.; Fernández-Loras, A.; Fernández-Giberteau, D.; Fisher, M.C.; Martínez-Solano, I.; Bielby, J.; Garner, T.W.J. Batrachochytrium Salamandrivorans Threat to the Iberian Urodele Hotspot. J. Fungi 2021, 7, 644. [Google Scholar] [CrossRef]
- Dondero, L.; Allaria, G.; Rosa, G.M.; Costa, A.; Ficetola, G.F.; Cogoni, R.E.; Grasselli, E.; Salvidio, S. Threats of the emerging pathogen batrachochytrium salamandrivorans (Bsal) to Italian wild salamander populations. Acta Herpetol. 2023, 18, 3–9. [Google Scholar] [CrossRef]
- Buck, J.C.; Hua, J.; Brogan, W.R.; Dang, T.D.; Rohr, J.R. Effects of pesticide mixtures on host-pathogen dynamics of the amphibian chytrid fungus. PLoS ONE 2015, 10, e0132832. [Google Scholar] [CrossRef]
- Jones, D.; Dang, T.; Urbina, J.; Rodriguez, S.; Cadiz, N.; Davidson, C.; Rollins-Smith, L. Effect of simultaneous amphibian exposure to pesticides and an emerging fungal pathogen, Batrachochytrium dendrobatidis. Environ. Sci. Technol. 2017, 51, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Barbi, A.; Goessens, T.; Strubbe, D.; Gilbert, M.; Pasmans, F.; Martel, A. Widespread triazole pesticide use affects infection dynamics of a global amphibian pathogen. Ecol. Lett. 2023, 26, 313–322. [Google Scholar] [CrossRef]
- Rohr, J.; Raffel, T.; Halstead, N.; McMahon, T.; Johnson, S.; Boughton, R.; Martin, L. Early life exposure to a herbicide has enduring effects on pathogen-induced mortality. Proc. R. Soc. B 2013, 280, 20131502. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. Consumption of Pesticides. Available online: https://ec.europa.eu/eurostat/databrowser/view/aei_fm_salpest09/default/table?lang=en (accessed on 7 July 2024).
- Maggi, F.; Tang, F.; La Cecilia, D.; McBratney, A.; Padarian, J.; Bailey, R. Pest-Chemgrids, global gridded maps of the top 20 crop-specific pesticide application rates from 2015 to 2025. Sci. Data 2019, 6, 170. [Google Scholar] [CrossRef]
- Islam, F.; Wang, J.; Farooq, M.; Khan, M.S.; Xu, L.; Zhu, J.; Li, Q.X.; Zhou, W. Potential impact of the herbicide 2,4-dichlorophenoxyacetic acid on human and ecosystems. Environ. Int. 2018, 111, 332–351. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.; Girotto, L.; Goulart, B.; Oliveira, F.; Martinez, C.; Almeida, E.A.; Costa, M. Effects of 2,4-D-based herbicide (DMA® 806) on sensitivity, respiration rates, energy reserves and behavior of tadpoles. Ecotoxicol. Environ. Saf. 2019, 182, 109446. [Google Scholar] [CrossRef]
- Bolis, A.; Gazzola, A.; Pellitteri-Rosa, D.; Canova, L.; Bonato, L. Exposure during embryonic development to Roundup® Power 2.0 affects lateralization, level of activity and growth, but not defensive behaviour of marsh frog tadpoles. Environ. Pollut. 2020, 263, 114395. [Google Scholar] [CrossRef]
- Pavan, F.; Samojeden, C.; Rutkoski, C.; da Silva, L.B.; Lemos, P.; Oliveira, R.; Hartmann, P. Morphological, behavioral and genotoxic effects of glyphosate and 2,4-D mixture in tadpoles of two native species of South American amphibians. Environ. Toxicol. Pharmacol. 2021, 85, 103637. [Google Scholar] [CrossRef]
- Ryan, T.; Scott, C.; Douthitt, B. Sub-lethal effects of 2,4-D exposure on golf course amphibians. USGA Turfgrass Environ. Res. Online 2006, 5, 1–14. [Google Scholar]
- Hou, L.; Andreotti, G.; Baccarelli, A.; Tarantini, L.; Bonzini, M.; Hoxha, M.; Cravo, V.M.; Zanobetti, A.; Schwartz, J.; Hoppin, J.A.; et al. Lifetime pesticide use and telomere shortening among male pesticide applicators in the Agricultural Health Study. Environ. Health Perspect. 2013, 121, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Molbert, N.; Angelier, F.; Alliot, F.; Guillon, S.; Veyssière, C.; Lourdais, O. Fish from urban rivers and with high pollutant levels have shorter telomeres. Biol. Lett. 2021, 17, 20200819. [Google Scholar] [CrossRef]
- Erickson, P.A.; Chang, V.C.; He, S.; Dagnall, C.; Teshome, K.; Machiela, M.J.; Barry, K.H.; Pereira, E.F.R.; Gadalla, S.M.; Parks, C.G.; et al. Occupational pesticide use and relative leukocyte telomere length in the biomarkers of exposure and effect in agriculture study. Environ. Res. 2024, 241, 117996. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Johnson, V.J.; Blakley, B.R. The effect of exposure to a commercial 2,4-D formulation during gestation on the immune response in CD-1 mice. Toxicology 2001, 165, 79–89. [Google Scholar] [CrossRef]
- Ville, P.; Roch, P.; Cooper, E.L.; Narbonne, J.-F. Immuno-modulator effects of carbaryl and 2,4-D in the earthworm Eisenia fetida andrei. Arch. Environ. Contam. Toxicol. 1997, 32, 291–297. [Google Scholar] [CrossRef]
- Schabetsberger, R.; Jehle, R.; Maletzky, A.; Pesta, J.; Sztatecsny, M. Delineation of terrestrial reserves for amphibians: Post-breeding migrations of Italian crested newts (Triturus c. carnifex) at high altitude. Biol. Conserv. 2004, 117, 95–104. [Google Scholar] [CrossRef]
- Dufresnes, C.; Pellet, J.; Bettinelli-Riccardi, S.; Speybroeck, J.; Wellenreuther, M.; Perrin, N.; Stöck, M. Massive genetic introgression in threatened northern crested newts (Triturus cristatus) by an invasive congener (T. carnifex) in Western Switzerland. Conserv. Genet. 2016, 17, 325–338. [Google Scholar]
- Meilink, W.; Arntzen, J.; van Delft, J.; Gubbels, J.; de Rooij, P.; Spitzen-van der Sluijs, A. Genetic pollution of a threatened native crested newt species through hybridization with an invasive congener in the Netherlands. Biol. Conserv. 2015, 184, 145–153. [Google Scholar] [CrossRef]
- Wielstra, B.; Burke, T.; Butlin, R.; Arntzen, J. Efficient screening for ‘genetic pollution’ in an anthropogenic crested newt hybrid zone. Conserv. Genet. Resour. 2016, 8, 553–560. [Google Scholar] [CrossRef]
- Gilbert, M.; Spitzen-van der Sluijs, A.; Canessa, S.; Martel, A.; Pasmans, F. Mitigating Batrachochytrium Salamandrivorans in Europe. Batrachochytrium Salamandrivorans Action Plan for European Urodeles; RAVON: Nijmegen, The Netherlands, 2020. [Google Scholar]
- EFSA European Food Safe Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance 2,4-D. EFSA J. 2014, 12, 3812. [Google Scholar]
- Vardia, H.K.; Rao, P.S.; Durve, V.S. Sensitivity of toad larvae to 2,4-D and endosulfan pesticides. Arch. Hydrobiol. 1984, 100, 395–400. [Google Scholar]
- Garrow, J.S.; Webster, J. Quetelet’s index (W/H2) as a measure of fatness. Int. J. Obes. 1985, 9, 147–153. [Google Scholar]
- Stevenson, R.D.; Woods, W.A., Jr. Condition indices for conservation: New uses for evolving tools. Integr. Comp. Biol. 2006, 46, 1169–1190. [Google Scholar] [CrossRef]
- Blooi, M.; Pasmans, F.; Longcore, J.E.; Spitzen-van der Sluijs, A.; Vercammen, F.; Martel, A. Duplex real-time PCR for rapid simultaneous detection of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans in amphibian samples. J. Clin. Microbiol. 2013, 51, 4173. [Google Scholar] [CrossRef]
- Smith, K.G. Use of quantitative PCR assay for amphibian chytrid detection: Comment on Kriger et al.(2006a, b). Dis. Aquat. Org. 2007, 73, 253. [Google Scholar] [CrossRef] [PubMed]
- Clare, F.; Daniel, O.; Garner, T.; Fisher, M. Assessing the ability of swab data to determine the true burden of infection for the amphibian pathogen Batrachochytrium dendrobatidis. EcoHealth 2016, 13, 360–367. [Google Scholar] [CrossRef]
- Fieschi-Méric, L.; Pasmans, F.; Fernández Meléndez, E.; Bruyckere, S.; Blomme, E.; Verbrugghe, E.; Martel, A. Bsal susceptibility depends on host source population but not on skin microbiota in Pleurodeles waltl. Anim. Microbiome 2025. in revision. [Google Scholar]
- Sánchez-Montes, G.; Martínez-Solano, I.; Díaz-Paniagua, C.; Rivera, D.; Fitze, P. Telomere Attrition Age A Wild Amphib. population. Biol. Lett. 2020, 16, 20200168. [Google Scholar] [CrossRef]
- Hudon, S.; Hurtado, E.; Beck, J.; Lewis, A.; Waters, P.; Bartels, S.; Reichert, S. Universal assay for measuring vertebrate telomeres by real-time quantitative PCR. bioRxiv 2019. [Google Scholar] [CrossRef]
- Giraudeau, M.; Heidinger, B.; Bonneaud, C.; Sepp, T. Telomere shortening as a mechanism of long-term cost of infectious diseases in natural animal populations. Biol. Lett. 2019, 15, 20190190. [Google Scholar] [CrossRef]
- Voyles, J.; Young, S.; Berger, L.; Campbell, C.; Voyles, W.F.; Dinudom, A.; Cook, D.; Webb, R.; Alford, R.A.; Skerratt, L.F. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 2009, 326, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Brannelly, L.A.; Chatfield, M.W.H.; Richards-Zawacki, C.L. Epidermal loss and heterothermy drive nutrient allocation trade-offs in a facultatively cutaneous amphibian. Funct. Ecol. 2018, 32, 2072–2083. [Google Scholar]
- Bletz, M.C.; Kelly, M.; Sabino-Pinto, J.; Bales, E.; Van Praet, S.; Bert, W.; Boyen, F.; Vences, M.; Steinfartz, S.; Pasmans, F. Amphibian skin microbiota exhibits temporal variation in community structure but stability of predicted Bd-inhibitory function. Front. Microbiol. 2018, 9, 1632. [Google Scholar] [CrossRef] [PubMed]
- Canessa, S.; Bozzuto, C.; Grant, E.H.C.; Cruickshank, S.S.; Fisher, M.C.; Garner, T.W.J.; Bielby, J. Decision-making for mitigating wildlife diseases: From theory to practice for an emerging fungal pathogen of amphibians. J. Appl. Ecol. 2019, 56, 2428–2440. [Google Scholar] [CrossRef]
- Santos, G.; Rutkoski, C.F.; Folador, A.; Skovronski, V.J.; Müller, C.; Pompermaier, A.; Hartmann, P.A.; Hartmann, M. 2,4-D-based herbicide underdoses cause mortality, malformations, and nuclear abnormalities in Physalaemus cuvieri tadpoles. Comp. Biochem. Physiol. C 2024, 109, 109840. [Google Scholar] [CrossRef]
- Ortiz-Santaliestra, M.E.; Maia, J.P.; Egea-Serrano, A.; Brühl, C.A.; Lopes, I. Biological relevance of the magnitude of effects (considering mortality, sub-lethal and reproductive effects) observed in studies with amphibians and reptiles in view of population level impacts on amphibians and reptiles. EFSA Support. Publ. 2017, 14, EN-1251. [Google Scholar] [CrossRef]
- Aronzon, C.M.; Sandoval, M.T.; Herkovits, J.; Pérez-Coll, C.S. Stage-dependent toxicity of 2,4-dichlorophenoxyacetic on the embryonic development of a South American toad, Rhinella arenarum. Environ. Toxicol. 2011, 26, 373–381. [Google Scholar] [CrossRef]
- Van Meter, R.J.; Glinski, D.A.; Purucker, S.T.; Henderson, W.M. Influence of exposure to pesticide mixtures on the metabolomic profile in post-metamorphic green frogs (Lithobates clamitans). Sci. Total Environ. 2018, 624, 1663–1671. [Google Scholar] [CrossRef]
- Zaffaroni, N.; Zavanella, T.; Cattaneo, A.; Traina, V.; Tagliavini, G.; Fassina, G.; Lucchini, G. The toxicity of 2,4-dichlorophenoxyacetic acid to the adult crested newt. Environ. Res. 1986, 41, 79–87. [Google Scholar] [CrossRef]
- Lajmanovich, R.C.; Attademo, A.M.; Simoniello, M.F.; Poletta, G.L.; Junges, C.M.; Peltzer, P.M.; Grenón, P.; Cabagna-Zenklusen, M.C. Harmful Effects of the Dermal Intake of Commercial Formulations Containing Chlorpyrifos, 2,4-D, and Glyphosate on the Common Toad Rhinella arenarum (Anura: Bufonidae). Water Air Soil. Pollut. 2015, 226, 427. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meléndez, E.F.; Fieschi-Méric, L.; Verbrugghe, E.; Blomme, E.; Fahrbach, M.; Ortiz-Santaliestra, M.E.; Pasmans, F.; Martel, A. Co-Exposure with the Herbicide 2,4-D Does Not Exacerbate Batrachochytrium salamandrivorans Infection in the Italian Crested Newt (Triturus carnifex). Animals 2025, 15, 1777. https://doi.org/10.3390/ani15121777
Meléndez EF, Fieschi-Méric L, Verbrugghe E, Blomme E, Fahrbach M, Ortiz-Santaliestra ME, Pasmans F, Martel A. Co-Exposure with the Herbicide 2,4-D Does Not Exacerbate Batrachochytrium salamandrivorans Infection in the Italian Crested Newt (Triturus carnifex). Animals. 2025; 15(12):1777. https://doi.org/10.3390/ani15121777
Chicago/Turabian StyleMeléndez, Eduardo Fernández, Léa Fieschi-Méric, Elin Verbrugghe, Ellen Blomme, Michael Fahrbach, Manuel E. Ortiz-Santaliestra, Frank Pasmans, and An Martel. 2025. "Co-Exposure with the Herbicide 2,4-D Does Not Exacerbate Batrachochytrium salamandrivorans Infection in the Italian Crested Newt (Triturus carnifex)" Animals 15, no. 12: 1777. https://doi.org/10.3390/ani15121777
APA StyleMeléndez, E. F., Fieschi-Méric, L., Verbrugghe, E., Blomme, E., Fahrbach, M., Ortiz-Santaliestra, M. E., Pasmans, F., & Martel, A. (2025). Co-Exposure with the Herbicide 2,4-D Does Not Exacerbate Batrachochytrium salamandrivorans Infection in the Italian Crested Newt (Triturus carnifex). Animals, 15(12), 1777. https://doi.org/10.3390/ani15121777








