Effects of Different Rearing Methods on the Intestinal Morphology, Intestinal Metabolites, and Gut Microbiota of Lueyang Black-Bone Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval Statement
2.2. Animal Resources and Feeding Program
2.3. Sample Collection
2.4. Histological Analysis
2.5. Nontargeted LC-MS Metabolomics Analysis
2.6. Intestinal Microbiome Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of Different Rearing Modes on the Intestinal Morphology of Lueyang Black-Bone Chickens
3.2. Untargeted Metabolomics Analysis
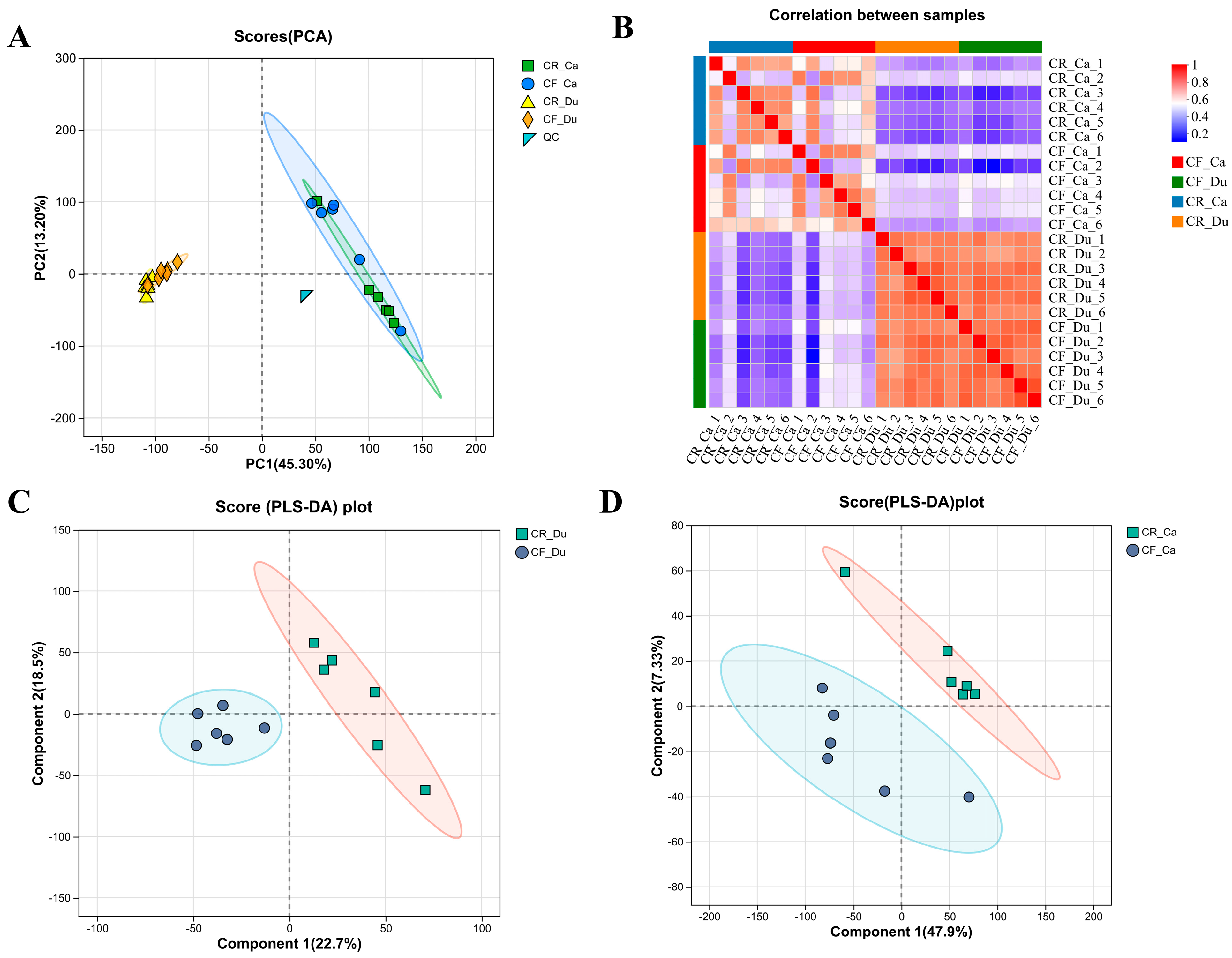
3.2.1. Quality Control of Metabolomes Under Different Rearing Methods
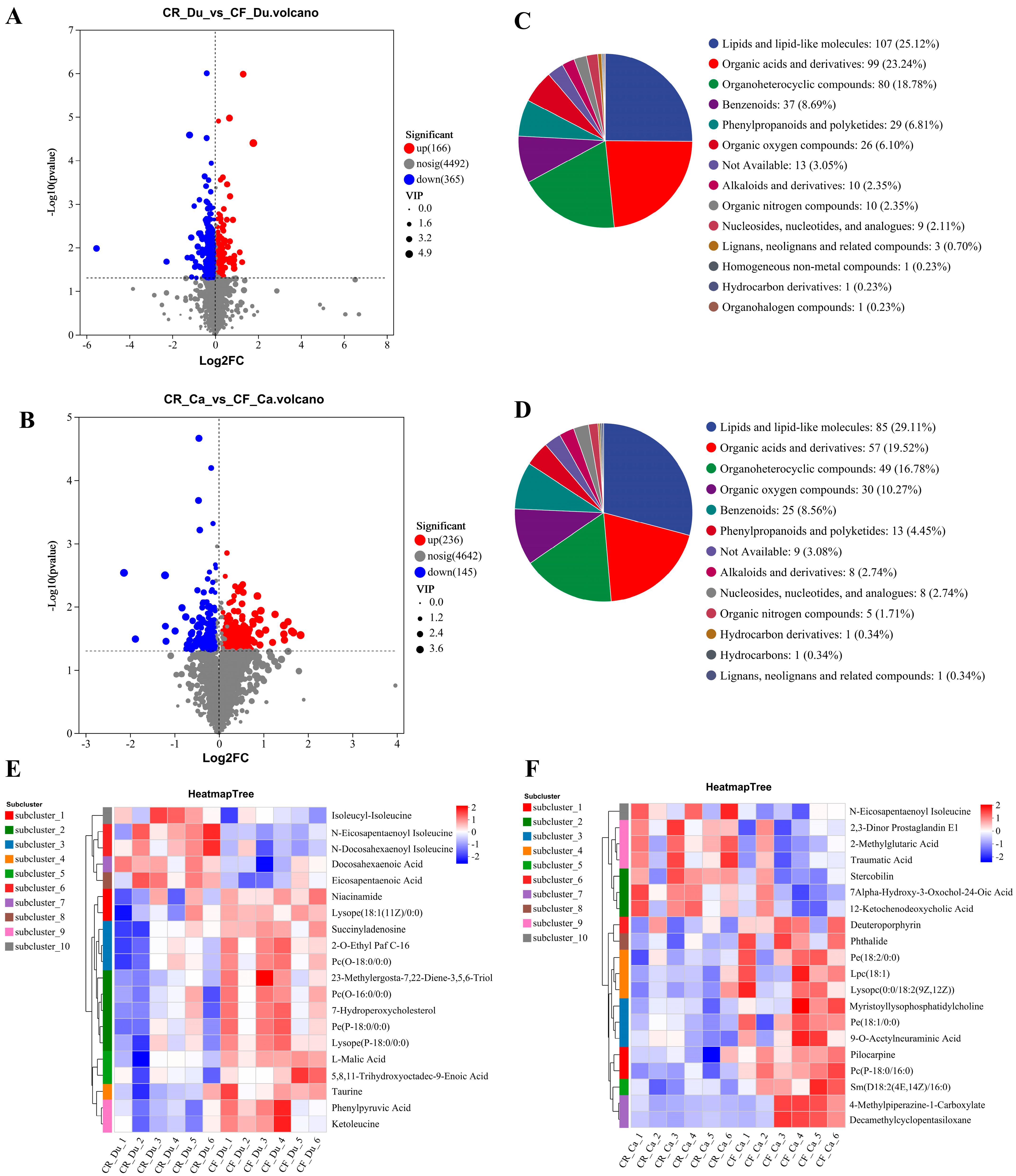
3.2.2. Analysis of Differential Metabolites
Screening and Identification of Differential Metabolites
Cluster Analysis of Differential Metabolites
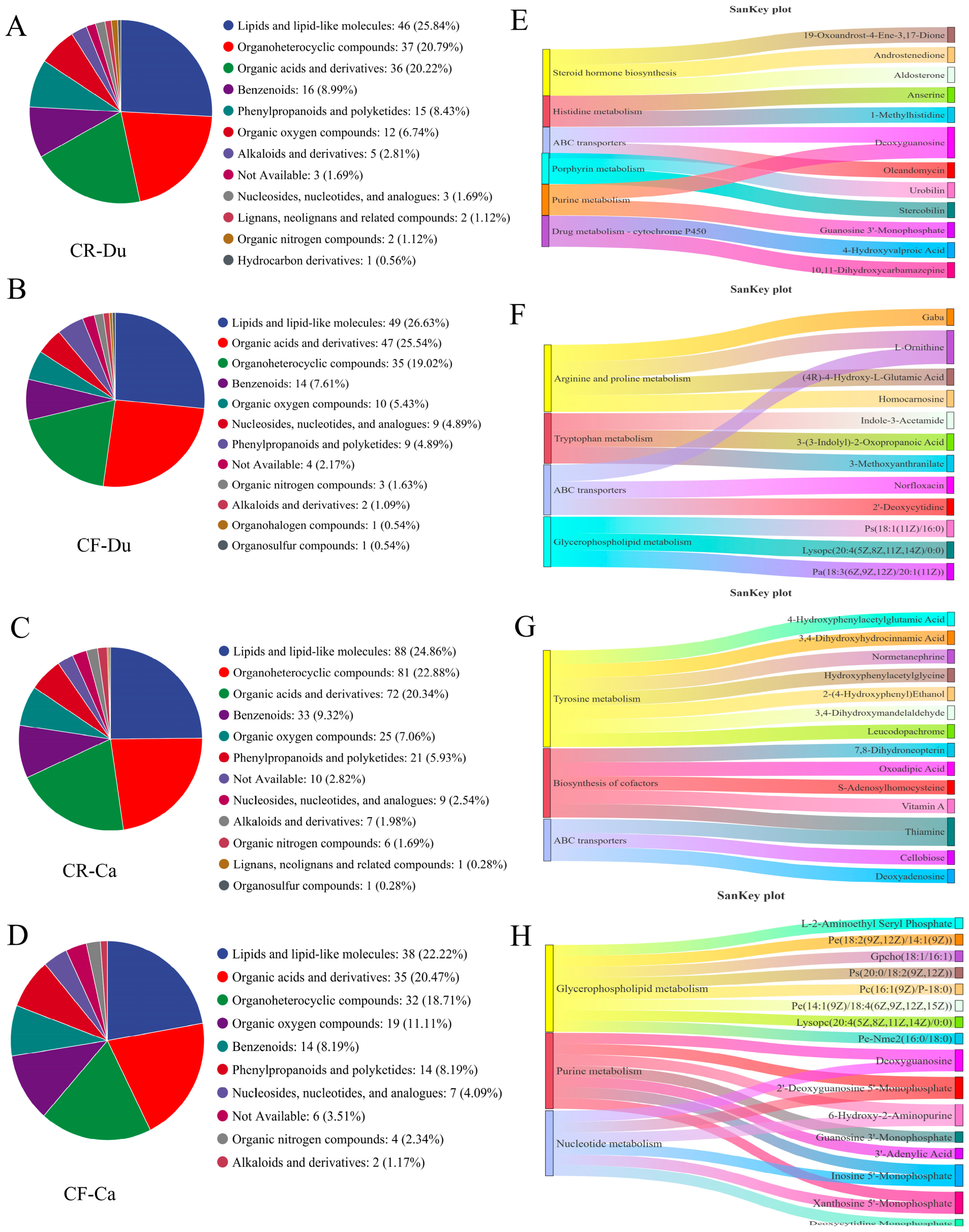
Enrichment Analysis of Differential Metabolites
3.2.3. Unique Metabolites Analysis
Screening and Identification of Unique Metabolites
Function Analysis of Unique Metabolites
3.3. Effects of Different Rearing Modes on the Gut Microbiota of Chickens
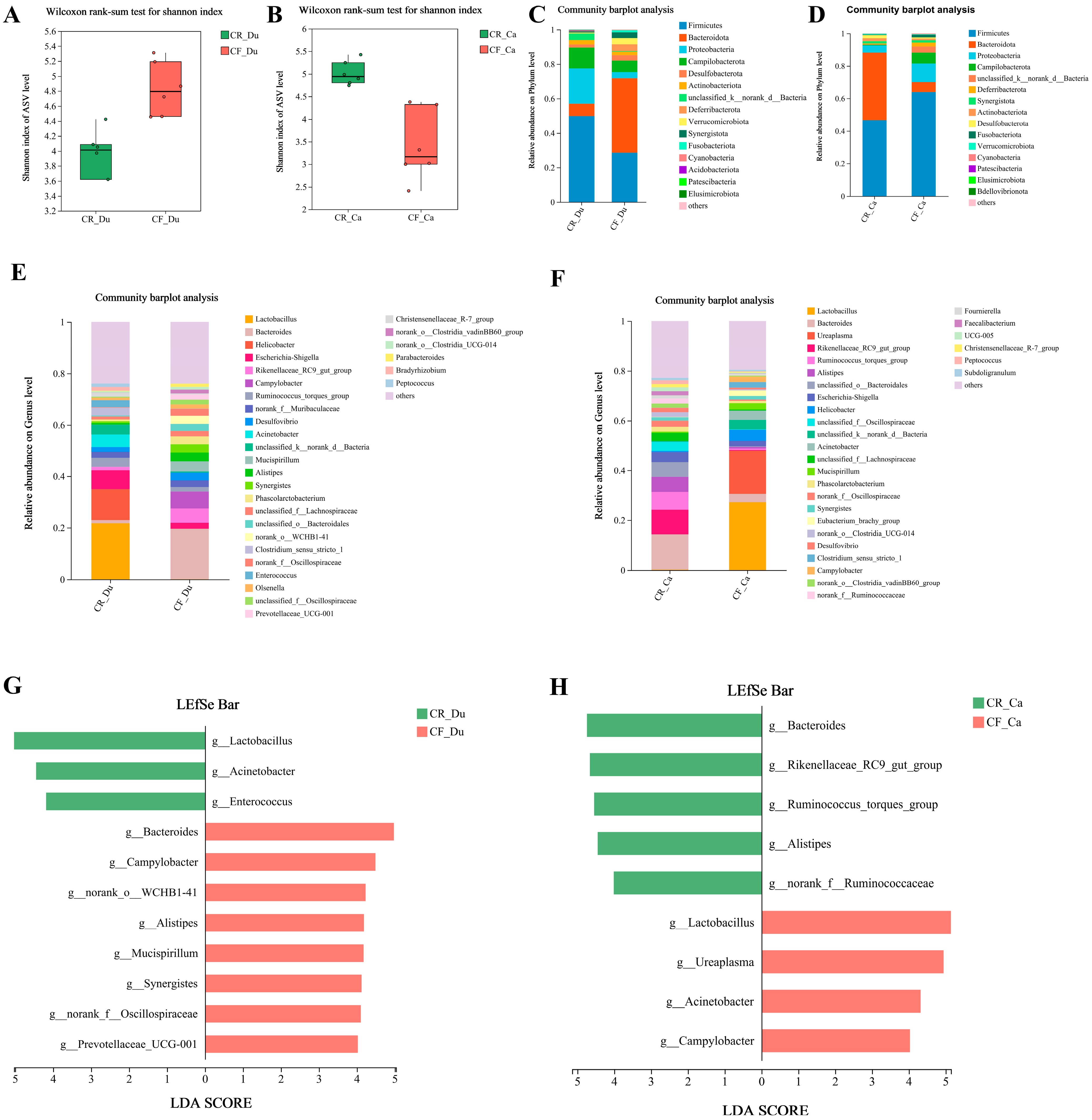
3.3.1. Analysis of Alpha Diversity of Gut Microbiota
3.3.2. Analysis of Gut Microbiota Structure
3.3.3. Differential Microbiota Screening
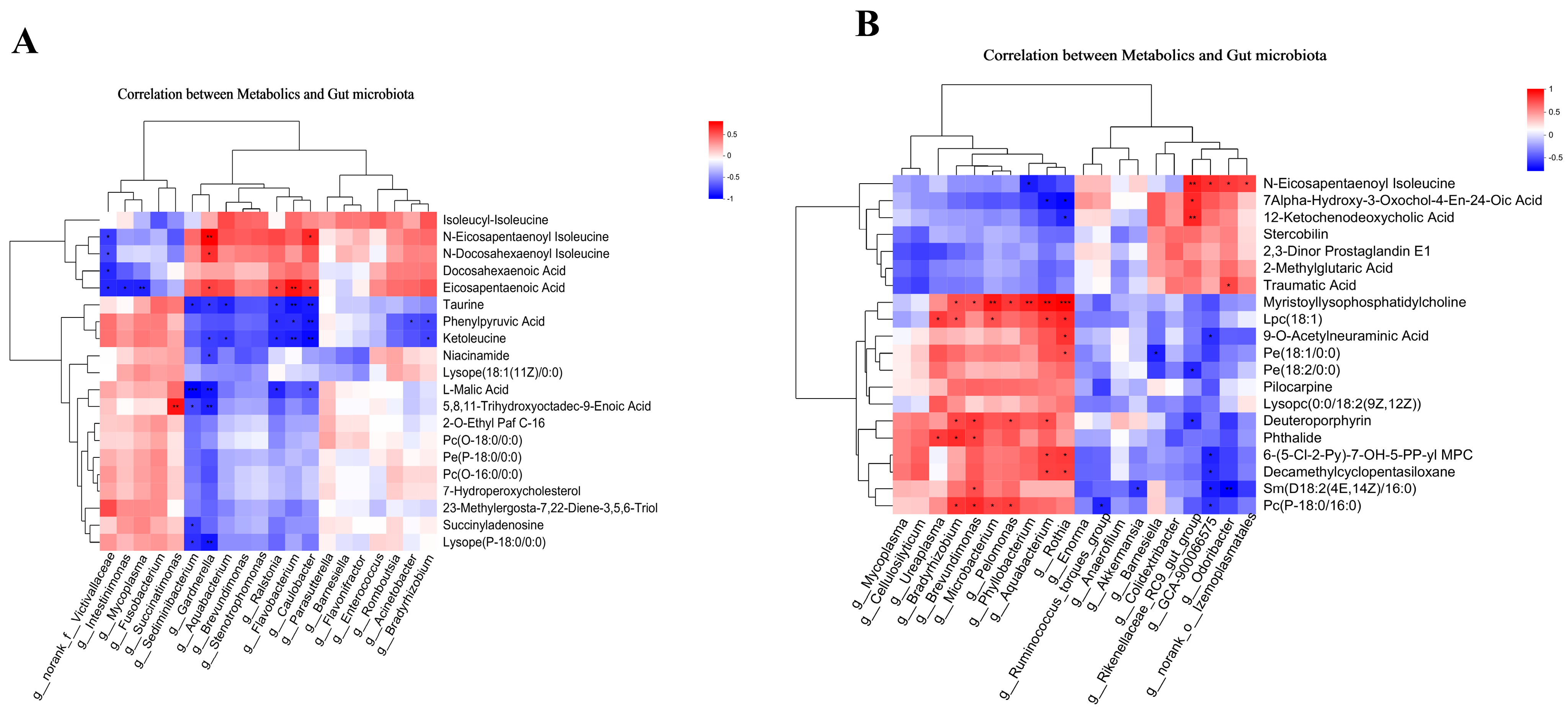
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, P.B. Evolution of the modern broiler and feed efficiency. Annu. Rev. Anim. Biosci. 2014, 2, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.H.; Shi, K.; Zhao, Q.; Duan, Y.; Shen, Y.Y.; Tian, J.J.; He, K.; Li, D.F.; Yu, M.L.; Lu, Y.Q.; et al. Transcriptome Analysis Reveals the Differentially Expressed Genes Associated with Growth in Guangxi Partridge Chickens. Genes 2022, 13, 798. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Zhao, Y.; Li, H.; Li, S.; Gu, J.; Zhang, H.; Zhang, Y.; Li, H.; Tian, Y.; Li, G.; et al. Weighted gene co-expression network analysis identified hub genes critical to fatty acid composition in Gushi chicken breast muscle. BMC Genom. 2023, 24, 594. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Cao, C.; Cai, Y.; Li, Y.; Song, Y.; Bao, X.; Zhang, J. Effects of Different Rearing Systems on Lueyang Black-Bone Chickens: Meat Quality, Amino Acid Composition, and Breast Muscle Transcriptome. Genes 2022, 13, 1898. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liao, J.H.; Liang, Z.L.; Balasubramanian, B.; Liu, W.C. Hepatic lipid metabolomics in response to heat stress in local broiler chickens breed (Huaixiang chickens). Vet. Med. Sci. 2021, 7, 1369–1378. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Li, Q.; Wang, Q.; Wen, J.; Zhao, G. Comparison of the Efficiency of BLUP and GBLUP in Genomic Prediction of Immune Traits in Chickens. Animals 2020, 10, 419. [Google Scholar] [CrossRef]
- Muyyarikkandy, M.S.; Parzygnat, J.; Thakur, S. Uncovering changes in microbiome profiles across commercial and backyard poultry farming systems. Microbiol. Spectr. 2023, 11, e0168223. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Q.; Liu, S.; Sun, R.; Zhou, Y.; Li, Y. Age-Related Variations in Intestinal Microflora of Free-Range and Caged Hens. Front. Microbiol. 2017, 8, 1310. [Google Scholar] [CrossRef]
- Zhang, W.; Jian, X.; Ding, S.; Chang, J.; Ji, S.; Chi, Y. Insights into the gut microbiota characteristics between the organic and traditional feeding chickens based on amplicon and metagenomic sequencing. Front. Microbiol. 2024, 15, 1509461. [Google Scholar] [CrossRef]
- Li, T.; Wang, P.; Zhi, Z.; Guo, T.; Zhou, J.; Zhang, H.; Cao, C.; Cai, Y.; Li, Y.; Zhang, J. Free-caged rearing modes regulate chicken intestinal metabolism by influencing gut microbial homeostasis. Poult. Sci. 2025, 104, 104381. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.F.D. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: A review. Anim. Feed. Sci. Technol. 2016, 212, 18–26. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, L.; Qin, S. Gut Microbiota Modulation on Intestinal Mucosal Adaptive Immunity. J. Immunol. Res. 2019, 2019, 4735040. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Van Hul, M. Do diet and microbes really ‘PREDICT’ cardiometabolic risks? Nat. Rev. Endocrinol. 2021, 17, 259–260. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, B.; Hu, C.; Liu, Y.; Wang, X.; Zhang, J.; Wang, F.; Zhu, M. Short-chain fatty acids as a target for prevention against food allergy by regulatory T cells. JGH Open 2019, 3, 190–195. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Xin, J.; Zhang, T.; Sun, N.; Ni, X.; Zeng, D.; Bai, Y. Lactobacillus johnsonii BS15 Prevents Psychological Stress-Induced Memory Dysfunction in Mice by Modulating the Gut-Brain Axis. Front. Microbiol. 2020, 11, 1941. [Google Scholar] [CrossRef]
- Rios-Arce, N.D.; Collins, F.L.; Schepper, J.D.; Steury, M.D.; Raehtz, S.; Mallin, H.; Schoenherr, D.T.; Parameswaran, N.; McCabe, L.R. Epithelial Barrier Function in Gut-Bone Signaling. Adv. Exp. Med. Biol. 2017, 1033, 151–183. [Google Scholar] [CrossRef]
- He, L.; Yang, H.; Lan, F.; Chen, R.; Jiang, P.; Jin, W. Use of GC-IMS and Stoichiometry to Characterize Flavor Volatiles in Different Parts of Lueyang Black Chicken during Slaughtering and Cutting. Foods 2024, 13, 1885. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, C.; Chen, Y.; Zheng, B. Purification and Characterization of Antioxidant Peptides of Pseudosciaena crocea Protein Hydrolysates. Molecules 2016, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Inkanuwat, A.; Sukaboon, R.; Reamtong, O.; Asawanonda, P.; Pattaratanakun, A.; Saisavoey, T.; Sangtanoo, P.; Karnchanatat, A. Nitric Oxide Synthesis Inhibition and Anti-Inflammatory Effect of Polypeptide Isolated from Chicken Feather Meal in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Food Technol. Biotechnol. 2019, 57, 200–212. [Google Scholar] [CrossRef]
- Kong, X.; Guo, Z.; Yao, Y.; Xia, L.; Liu, R.; Song, H.; Zhang, S. Acetic acid alters rhizosphere microbes and metabolic composition to improve willows drought resistance. Sci. Total Environ. 2022, 844, 157132. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Lee, H.J.; Ko, H.J.; Song, D.K.; Jung, Y.J. Lysophosphatidylcholine Promotes Phagosome Maturation and Regulates Inflammatory Mediator Production Through the Protein Kinase A-Phosphatidylinositol 3 Kinase-p38 Mitogen-Activated Protein Kinase Signaling Pathway During Mycobacterium tuberculosis Infection in Mouse Macrophages. Front. Immunol. 2018, 9, 920. [Google Scholar] [CrossRef]
- Mohammed-Saeid, W.; Soudy, R.; Tikoo, R.; Kaur, K.; Verrall, R.E.; Badea, I. Design and Evaluation of Gemini Surfactant-Based Lipoplexes Modified with Cell-Binding Peptide for Targeted Gene Therapy in Melanoma Model. J. Pharm. Pharm. Sci. 2018, 21, 363–375. [Google Scholar] [CrossRef]
- Li, F.; Chong, Z.Z.; Maiese, K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide. Front. Biosci. 2004, 9, 2500–2520. [Google Scholar] [CrossRef]
- Méndez-Lara, K.A.; Letelier, N.; Farré, N.; Diarte-Añazco, E.M.G.; Nieto-Nicolau, N.; Rodríguez-Millán, E.; Santos, D.; Pallarès, V.; Escolà-Gil, J.C.; Vázquez Del Olmo, T.; et al. Nicotinamide Prevents Apolipoprotein B-Containing Lipoprotein Oxidation, Inflammation and Atherosclerosis in Apolipoprotein E-Deficient Mice. Antioxidants 2020, 9, 1162. [Google Scholar] [CrossRef]
- Li, Y.; Shi, C.W.; Zhang, Y.T.; Huang, H.B.; Jiang, Y.L.; Wang, J.Z.; Cao, X.; Wang, N.; Zeng, Y.; Yang, G.L.; et al. Riboflavin Attenuates Influenza Virus Through Cytokine-Mediated Effects on the Diversity of the Gut Microbiota in MAIT Cell Deficiency Mice. Front. Microbiol. 2022, 13, 916580. [Google Scholar] [CrossRef]
- Zhao, D.D.; Gai, Y.D.; Li, C.; Fu, Z.Z.; Yin, D.Q.; Xie, M.; Dai, J.Y.; Wang, X.X.; Li, Y.X.; Wu, G.F.; et al. Dietary taurine effect on intestinal barrier function, colonic microbiota and metabolites in weanling piglets induced by LPS. Front. Microbiol. 2023, 14, 1259133. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.A.; Ito, M.K.; Maki, K.C.; Orringer, C.E.; Bays, H.E.; Jones, P.H.; McKenney, J.M.; Grundy, S.M.; Gill, E.A.; Wild, R.A.; et al. National lipid association recommendations for patient-centered management of dyslipidemia: Part 1—full report. J. Clin. Lipidol. 2015, 9, 129–169. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.R.; Warner, J.B.; Hardesty, J.E.; Song, Y.L.; King, T.N.; Kang, J.X.; Chen, C.Y.; Xie, S.; Yuan, F.; Prodhan, M.A.I.; et al. Decreased ω-6:ω-3 PUFA ratio attenuates ethanol-induced alterations in intestinal homeostasis, microbiota, and liver injury. J. Lipid. Res. 2019, 60, 2034–2049. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T. Sphingolipids as Functional Food Components: Benefits in Skin Improvement and Disease Prevention. J. Agric. Food Chem. 2022, 70, 9597–9609. [Google Scholar] [CrossRef]
- Grösch, S.; Alessenko, A.V.; Albi, E. The Many Facets of Sphingolipids in the Specific Phases of Acute Inflammatory Response. Mediat. Inflamm. 2018, 2018, 5378284. [Google Scholar] [CrossRef]
- Degagné, E.; Saba, J.D. S1pping fire: Sphingosine-1-phosphate signaling as an emerging target in inflammatory bowel disease and colitis-associated cancer. Clin. Exp. Gastroenterol. 2014, 7, 205–214. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Shi, Q.; Yuan, X.; Zeng, Y.; Wang, J.; Zhang, Y.; Xue, C.; Li, L. Crosstalk between Gut Microbiota and Bile Acids in Cholestatic Liver Disease. Nutrients 2023, 15, 2411. [Google Scholar] [CrossRef]
- Vera-Álava, J.O.; Arteaga-Solórzano, J.G.; Reyna-Gallegos, S.L. Organic acids, microbiota, gut health and productive response in broilers chickens. Rev. Colomb. Cienc. Anim.-RECIA 2023, 2, e1019. [Google Scholar] [CrossRef]
- Butts, C.L.; Jones, Y.L.; Lim, J.K.; Salter, C.E.; Belyavskaya, E.; Sternberg, E.M. Tissue expression of steroid hormone receptors is associated with differential immune responsiveness. Brain Behav. Immun. 2011, 25, 1000–1007. [Google Scholar] [CrossRef]
- Munir, N.; Cheng, C.; Xia, C.; Xu, X.; Nawaz, M.A.; Iftikhar, J.; Chen, Y.; Lin, Y.; Lai, Z. RNA-Seq analysis reveals an essential role of tyrosine metabolism pathway in response to root-rot infection in Gerbera hybrida. PLoS ONE 2019, 14, e0223519. [Google Scholar] [CrossRef] [PubMed]
- Neustaeter, A.; Leibovitzh, H.; Turpin, W.; Croitoru, K. Understanding Predictors of Crohn’s Disease: Determinants of Altered Barrier Function in Pre-Disease Phase of Crohn’s Disease. J. Can. Assoc. Gastroenterol. 2024, 7, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Mutafova-Yambolieva, V.N.; Durnin, L. The purinergic neurotransmitter revisited: A single substance or multiple players? Pharmacol. Ther. 2014, 144, 162–191. [Google Scholar] [CrossRef]
- Hope, H.C.; Salmond, R.J. The Role of Non-essential Amino Acids in T Cell Function and Anti-tumour Immunity. Arch. Immunol. Ther. Exp. (Warsz) 2021, 69, 29. [Google Scholar] [CrossRef]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Haskó, G. The Purinergic System as a Pharmacological Target for the Treatment of Immune-Mediated Inflammatory Diseases. Pharmacol. Rev. 2019, 71, 345–382. [Google Scholar] [CrossRef]
- Straube, H.; Straube, J.; Rinne, J.; Fischer, L.; Niehaus, M.; Witte, C.P.; Herde, M. An inosine triphosphate pyrophosphatase safeguards plant nucleic acids from aberrant purine nucleotides. New Phytol. 2023, 237, 1759–1775. [Google Scholar] [CrossRef]
- Duley, J.A.; Florin, T.H. Thiopurine therapies: Problems, complexities, and progress with monitoring thioguanine nucleotides. Ther. Drug Monit. 2005, 27, 647–654. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine Oxidoreductase in Drug Metabolism: Beyond a Role as a Detoxifying Enzyme. Curr. Med. Chem. 2016, 23, 4027–4036. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- Stumpff, F.; Manneck, D.; Martens, H. Unravelling the secrets of the caecum. Pflug. Arch. 2019, 471, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Agace, W.W.; McCoy, K.D. Regionalized Development and Maintenance of the Intestinal Adaptive Immune Landscape. Immunity 2017, 46, 532–548. [Google Scholar] [CrossRef]
- Chen, G.Y.; O’Leary, B.R.; Du, J.; Carroll, R.S.; Steers, G.J.; Buettner, G.R.; Cullen, J.J. Pharmacologic Ascorbate Radiosensitizes Pancreatic Cancer but Radioprotects Normal Tissue: The Role of Oxidative Stress-Induced Lipid Peroxidation. Antioxidants 2024, 13, 361. [Google Scholar] [CrossRef]
- Santos, J.M. Influência de Dieta Desbalanceada Hipoproteica na Imunidade Tecido-Específica da Mucosa Intestinal. Master’s Thesis, Instituto de Ciências Biomédicas, Universidade de São Paulo, São Paulo, Brazil, 2020. [Google Scholar]
- Graudenzi, A.; Caravagna, G.; De Matteis, G.; Antoniotti, M. Investigating the relation between stochastic differentiation, homeostasis and clonal expansion in intestinal crypts via multiscale modeling. PLoS ONE 2014, 9, e97272. [Google Scholar] [CrossRef]
- Rabbi, M.F.; Labis, B.; Metz-Boutigue, M.H.; Bernstein, C.N.; Ghia, J.E. Catestatin decreases macrophage function in two mouse models of experimental colitis. Biochem. Pharmacol. 2014, 89, 386–398. [Google Scholar] [CrossRef]
- Cader, M.Z.; Kaser, A. Recent advances in inflammatory bowel disease: Mucosal immune cells in intestinal inflammation. Gut 2013, 62, 1653–1664. [Google Scholar] [CrossRef]
- Gancarcikova, S.; Lauko, S.; Hrckova, G.; Andrejcakova, Z.; Hajduckova, V.; Madar, M.; Kolesar Fecskeova, L.; Mudronova, D.; Mravcova, K.; Strkolcova, G.; et al. Innovative Animal Model of DSS-Induced Ulcerative Colitis in Pseudo Germ-Free Mice. Cells 2020, 9, 2571. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A.; Cosme, F.; Inês, A. Wine and Non-Dairy Fermented Beverages: A Novel Source of Pro- and Prebiotics. Fermentation 2020, 6, 113. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Tomaszewska, E.; Donaldson, J.; Jachimowicz, K. The Role of Nutritional Factors in the Modulation of the Composition of the Gut Microbiota in People with Autoimmune Diabetes. Nutrients 2022, 14, 2498. [Google Scholar] [CrossRef]
- Fidanza, M.; Panigrahi, P.; Kollmann, T.R. Lactiplantibacillus plantarum-Nomad and Ideal Probiotic. Front. Microbiol. 2021, 12, 712236. [Google Scholar] [CrossRef]
- Gou, H.Z.; Zhang, Y.L.; Ren, L.F.; Li, Z.J.; Zhang, L. How do intestinal probiotics restore the intestinal barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef]
- Palkovicsné Pézsa, N.; Kovács, D.; Gálfi, P.; Rácz, B.; Farkas, O. Effect of Enterococcus faecium NCIMB 10415 on Gut Barrier Function, Internal Redox State, Proinflammatory Response and Pathogen Inhibition Properties in Porcine Intestinal Epithelial Cells. Nutrients 2022, 14, 1486. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yan, Y.; Zhong, J.; Zhao, Y.; Xu, Y.; Zhang, T.; Xiong, H.; Chen, Y.; Wang, X.; Zhang, K. Enterococcus durans 98D alters gut microbial composition and function to improve DSS-induced colitis in mice. Heliyon 2024, 10, e28486. [Google Scholar] [CrossRef]
- Elnar, A.G.; Kim, M.G.; Lee, J.E.; Han, R.H.; Yoon, S.H.; Lee, G.Y.; Yang, S.J.; Kim, G.B. Acinetobacter pullorum sp. nov., Isolated from Chicken Meat. J. Microbiol. Biotechnol. 2020, 30, 526–532. [Google Scholar] [CrossRef]
- Glover, J.S.; Browning, B.D.; Ticer, T.D.; Engevik, A.C.; Engevik, M.A. Acinetobacter calcoaceticus is Well Adapted to Withstand Intestinal Stressors and Modulate the Gut Epithelium. Front. Physiol. 2022, 13, 880024. [Google Scholar] [CrossRef]
- Thomas, F.; Hehemann, J.H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, J.; He, Z.; Li, H. Bacteroides and NAFLD: Pathophysiology and therapy. Front. Microbiol. 2024, 15, 1288856. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Fu, Y.; Wang, H.; Alenezi, T.; Almansour, A.; Jia, Z. The Role of Immune Response and Microbiota on Campylobacteriosis. In Campylobacter; Téllez-Isaías, G., El-Ashram, S., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Kiarie, A.; Bebora, L.; Gitao, G.; Ochien’g, L.; Okumu, N.; Mutisya, C.; Wasonga, J.; Masudi, S.P.; Moodley, A.; Amon-Tanoh, M.A.; et al. Prevalence and risk factors associated with the occurrence of Campylobacter sp. in children aged 6-24 months in peri-urban Nairobi, Kenya. Front. Public Health 2023, 11, 1147180. [Google Scholar] [CrossRef]
- Xu, G.; Huang, J.; Chen, W.; Zhao, A.; Pan, J.; Yu, F. The Influence of Increasing Roughage Content in the Diet on the Growth Performance and Intestinal Flora of Jinwu and Duroc × Landrace × Yorkshire Pigs. Animals 2024, 14, 1913. [Google Scholar] [CrossRef]
- You, H.; Chang, F.; Chen, H.; Wang, Y.; Han, W. Exploring the role of CBLB in acute myocardial infarction: Transcriptomic, microbiomic, and metabolomic analyses. J. Transl. Med. 2024, 22, 654. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, M.; Löber, U.; Adamek, K.; Węgrzyn, D.; Skonieczna-Żydecka, K.; Malinowski, D.; Łoniewski, I.; Markó, L.; Ulas, T.; Forslund, S.K.; et al. The gut microbiota is associated with the small intestinal paracellular permeability and the development of the immune system in healthy children during the first two years of life. J. Transl. Med. 2021, 19, 177. [Google Scholar] [CrossRef]
- Ji, H.; Chen, L.; Ma, Y.; Degen, A.A.; Yuan, Z.; Chen, H.; Zhou, J. A Comparison of Growth Performance, Blood Parameters, Rumen Fermentation, and Bacterial Community of Tibetan Sheep When Fattened by Pasture Grazing versus Stall Feeding. Microorganisms 2024, 12, 1967. [Google Scholar] [CrossRef]
- Pang, K.; Wang, J.; Chai, S.; Yang, Y.; Wang, X.; Liu, S.; Ding, C.; Wang, S. Ruminal microbiota and muscle metabolome characteristics of Tibetan plateau yaks fed different dietary protein levels. Front. Microbiol. 2024, 15, 1275865. [Google Scholar] [CrossRef]
- Viscardi, R.M.; Kallapur, S.G. Role of Ureaplasma Respiratory Tract Colonization in Bronchopulmonary Dysplasia Pathogenesis: Current Concepts and Update. Clin. Perinatol. 2015, 42, 719–738. [Google Scholar] [CrossRef] [PubMed]
- Crossman, H.; Tavakol, M.; Freise, C.; Chin-Hong, P. 1757. Hepatitis C-Infected Donors and Hepatitis C-Infected Recipients: Analysis of Renal Transplant Outcomes. Open Forum Infect. Dis. 2019, 6, S645–S646. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136. [Google Scholar] [CrossRef]
- Takeuchi, T.; Nakanishi, Y.; Ohno, H. Microbial Metabolites and Gut Immunology. Annu. Rev. Immunol. 2024, 42, 153–178. [Google Scholar] [CrossRef]
- Roussel, C.; Anunciação Braga Guebara, S.; Plante, P.L.; Desjardins, Y.; Di Marzo, V.; Silvestri, C. Short-term supplementation with ω-3 polyunsaturated fatty acids modulates primarily mucolytic species from the gut luminal mucin niche in a human fermentation system. Gut Microbes 2022, 14, 2120344. [Google Scholar] [CrossRef]
- Vijay, A.; Astbury, S.; Le Roy, C.; Spector, T.D.; Valdes, A.M. The prebiotic effects of omega-3 fatty acid supplementation: A six-week randomised intervention trial. Gut Microbes 2021, 13, 1–11. [Google Scholar] [CrossRef]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci. Rep. 2017, 7, 11079. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Ahmed, M. Functional, Diagnostic and Therapeutic Aspects of Bile. Clin. Exp. Gastroenterol. 2022, 15, 105–120. [Google Scholar] [CrossRef]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe 2020, 27, 659–670. [Google Scholar] [CrossRef]
- Li, N.; Ma, P.; Li, Y.; Shang, X.; Nan, X.; Shi, L.; Han, X.; Liu, J.; Hong, Y.; Li, Q.; et al. Gut microbiota-derived 12-ketolithocholic acid suppresses the IL-17A secretion from colonic group 3 innate lymphoid cells to prevent the acute exacerbation of ulcerative colitis. Gut Microbes 2023, 15, 2290315. [Google Scholar] [CrossRef]
- Mocan, T.; Kang, D.W.; Molloy, B.J.; Jeon, H.; Spârchez, Z.A.; Beyoğlu, D.; Idle, J.R. Plasma fetal bile acids 7α-hydroxy-3-oxochol-4-en-24-oic acid and 3-oxachola-4,6-dien-24-oic acid indicate severity of liver cirrhosis. Sci. Rep. 2021, 11, 8298. [Google Scholar] [CrossRef]
- Wang, L.; Gao, J.; Li, G.; Cheng, J.; Yuan, G.; Zhang, T.; Zeng, W.; Lu, H. Identification of Metabolites in Muscles of Lueyang Black-Bone Chickens: A Comparative Analysis of Caged and Cage-Free Rearing Modes Using Untargeted Metabolomic Techniques. Animals 2024, 14, 2041. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yuan, F.; Rong, L.; Cai, J.; Yang, S.; Jia, Z.; Li, S. Transcriptomic and Metabolomic Profile Analysis of Muscles Reveals Pathways and Biomarkers Involved in Flavor Differences between Caged and Cage-Free Chickens. Foods 2022, 11, 2890. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Amevor, F.K.; Lan, X.; Tang, B.; Qin, S.; Fu, P.; Liu, A.; Liu, L. Integrative metabolomics and transcriptomics analysis revealed specific genes and metabolites affecting meat quality of chickens under different rearing systems. Poult. Sci. 2024, 103, 103994. [Google Scholar] [CrossRef]
- He, S.; Lin, J.; Jin, Q.; Ma, X.; Liu, Z.; Chen, H.; Ma, J.; Zhang, H.; Descovich, K.; Phillips, C.J.C.; et al. The Relationship between Animal Welfare and Farm Profitability in Cage and Free-Range Housing Systems for Laying Hens in China. Animals 2022, 12, 2090. [Google Scholar] [CrossRef]
- Holt, P.S. Centennial Review: A revisiting of hen welfare and egg safety consequences of mandatory outdoor access for organic egg production. Poult. Sci. 2021, 100, 101436. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, S.; Shao, L.; Zhao, M.; Wang, L.; Cheng, J.; Zhang, T.; Lu, H. Effects of Different Rearing Methods on the Intestinal Morphology, Intestinal Metabolites, and Gut Microbiota of Lueyang Black-Bone Chickens. Animals 2025, 15, 1758. https://doi.org/10.3390/ani15121758
Zeng S, Shao L, Zhao M, Wang L, Cheng J, Zhang T, Lu H. Effects of Different Rearing Methods on the Intestinal Morphology, Intestinal Metabolites, and Gut Microbiota of Lueyang Black-Bone Chickens. Animals. 2025; 15(12):1758. https://doi.org/10.3390/ani15121758
Chicago/Turabian StyleZeng, Shuang, Linqing Shao, Mingming Zhao, Ling Wang, Jia Cheng, Tao Zhang, and Hongzhao Lu. 2025. "Effects of Different Rearing Methods on the Intestinal Morphology, Intestinal Metabolites, and Gut Microbiota of Lueyang Black-Bone Chickens" Animals 15, no. 12: 1758. https://doi.org/10.3390/ani15121758
APA StyleZeng, S., Shao, L., Zhao, M., Wang, L., Cheng, J., Zhang, T., & Lu, H. (2025). Effects of Different Rearing Methods on the Intestinal Morphology, Intestinal Metabolites, and Gut Microbiota of Lueyang Black-Bone Chickens. Animals, 15(12), 1758. https://doi.org/10.3390/ani15121758





