Heat Stress from Calving to Mating: Mechanisms and Impact on Cattle Fertility
Simple Summary
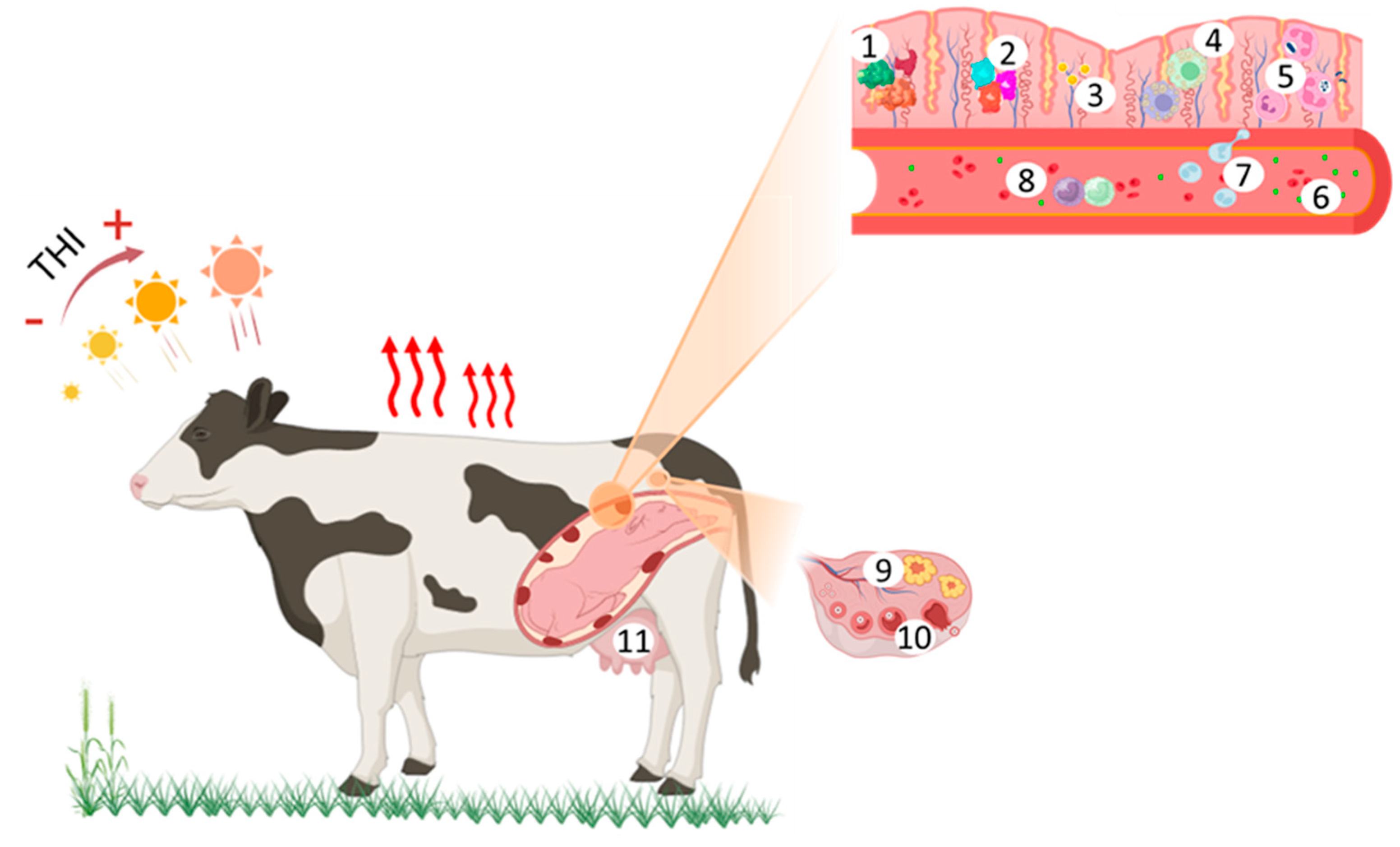
Abstract
1. Introduction
2. Prepartum and Calving
3. Uterine Immunity and Self-Defense
4. Uterine Involution and Placental Expulsion
5. Resumption of Ovarian Cyclicity
6. Oocyte Competence, Fertilization and Embryonic Development
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thornton, P.; Nelson, G.; Mayberry, D.; Herrero, M. Impacts of heat stress on global cattle production during the 21st century: A modelling study. Lancet Planet. Health 2022, 6, e192–e201. [Google Scholar] [CrossRef] [PubMed]
- Rashamol, V.; Sejian, S. Climate Resilient Livestock Production: Way Forward. J. Dairy. Vet. Sci. 2018, 5, 5556673. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.F. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Bernabucci, U. Climate change: Impact on livestock and how can we adapt. Anim. Front. 2019, 9, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Mcmanus, C.M.; Rezende Paiva, S.; Faria, D. Genomics and climate change: La génomique et le changement climatique -ES- Genómica y cambio climático. Rev. Sci. Tech. OIE 2020, 39, 481–490. [Google Scholar] [CrossRef]
- Ravagnolo, O.; Misztal, I. Genetic Component of Heat Stress in Dairy Cattle, Parameter Estimation. J. Dairy Sci. 2000, 83, 2126–2130. [Google Scholar] [CrossRef]
- Molinari, P.C.C.; Dahl, G.E.; Sheldon, I.M.; Bromfield, J.J. Effect of calving season on metritis incidence and bacterial content of the vagina in dairy cows. Theriogenology 2022, 191, 67–76. [Google Scholar] [CrossRef]
- Capela, L.; Leites, I.; Romão, R.; Lopes-da-Costa, L.; Pereira, R.M.L.N. Impact of Heat Stress on Bovine Sperm Quality and Competence. Animals 2022, 12, 975. [Google Scholar] [CrossRef]
- Pascottini, O.B.; LeBlanc, S.J. Modulation of immune function in the bovine uterus peripartum. Theriogenology 2020, 150, 193–200. [Google Scholar] [CrossRef]
- Fabris, T.F.; Laporta, J.; Corra, F.N.; Torres, Y.M.; Kirk, D.J.; McLean, D.J.; Chapman, J.; Dahl, G.E. Effect of nutritional immunomodulation and heat stress during the dry period on subsequent performance of cows. J. Dairy Sci. 2017, 100, 6733–6742. [Google Scholar] [CrossRef]
- Tao, S.; Monteiro, A.P.A.; Thompson, I.M.; Hayen, M.J.; Dahl, G.E. Effect of late-gestation maternal heat stress on growth and immune function of dairy calves. J. Dairy Sci. 2012, 95, 7128–7136. [Google Scholar] [CrossRef] [PubMed]
- Capuco, A.V.; Ellis, S.E.; Hale, S.A.; Long, E.; Erdman, R.A.; Zhao, X.; Paape, M.J. Lactation persistency: Insights from mammary cell proliferation studies. J. Anim. Sci. 2003, 81 (Suppl. S3), 18–31. [Google Scholar] [CrossRef]
- Kühl, N.M.; Rensing, L. Heat shock effects on cell cycle progression. Cell. Mol. Life Sci. 2000, 57, 450–463. [Google Scholar] [CrossRef]
- Petrova, N.V.; Velichko, A.K.; Razin, S.V.; Kantidze, O.L. Early S-phase cell hypersensitivity to heat stress. Cell Cycle 2016, 15, 337–344. [Google Scholar] [CrossRef]
- Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, I.V.; Denisenko, T.V.; Imreh, G.; Tyurin-Kuzmin, P.A.; Kaminskyy, V.O.; Gogvadze, V.; Zhivotovsky, B. Involvement of autophagy in the outcome of mitotic catastrophe. Sci. Rep. 2017, 7, 14571. [Google Scholar] [CrossRef] [PubMed]
- Noakes, D. Pregnancy and Parturition. In Em: Veterinary Reproduction and Obstetrics, 9th ed.; Saunders Elsevier: Amsterdam, The Netherlands, 2019; pp. 61–205. [Google Scholar]
- Whittle, W.L.; Holloway, A.C.; Lye, S.J.; Gibb, W.; Challis, J.R.G. Prostaglandin Production at the Onset of Ovine Parturition Is Regulated by Both Estrogen-Independent and Estrogen-Dependent Pathways. Endocrinology 2000, 141, 3783–3791. [Google Scholar] [CrossRef]
- Sakai, S.; Hagihara, N.; Kuse, M.; Kimura, K.; Okuda, K. Heat stress affects prostaglandin synthesis in bovine endometrial cells. J. Reprod. Dev. 2018, 64, 311–317. [Google Scholar] [CrossRef]
- Monteiro, A.P.A.; Tao, S.; Thompson, I.M.; Dahl, G.E. Effect of heat stress during late gestation on immune function and growth performance of calves: Isolation of altered colostral and calf factors. J. Dairy Sci. 2014, 97, 6426–6439. [Google Scholar] [CrossRef]
- Tao, S.; Bubolz, J.W.; Do Amaral, B.C.; Thompson, I.M.; Hayen, M.J.; Johnson, S.E. Effect of heat stress during the dry period on mammary gland development. J. Dairy Sci. 2011, 94, 5976–5986. [Google Scholar] [CrossRef]
- Ouellet, V.; Laporta, J.; Dahl, G.E. Late gestation heat stress in dairy cows: Effects on dam and daughter. Theriogenology 2020, 150, 471–479. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Collier, R.J.; Doelger, S.G.; Head, H.H.; Thatcher, W.W.; Wilcox, C.J. Effects of Heat Stress during Pregnancy on Maternal Hormone Concentrations, Calf Birth Weight and Postpartum Milk Yield of Holstein Cows. J. Anim. Sci. 1982, 54, 309–319. [Google Scholar] [CrossRef]
- Van Eetvelde, M.; Kamal, M.M.; Hostens, M.; Vandaele, L.; Fiems, L.O.; Opsomer, G. Evidence for placental compensation in cattle. Animal 2016, 10, 1342–1350. [Google Scholar] [CrossRef]
- Do Amaral, B.C.; Connor, E.E.; Tao, S.; Hayen, J.; Bubolz, J.; Dahl, G.E. Heat-stress abatement during the dry period: Does cooling improve transition into lactation? J. Dairy Sci. 2009, 92, 5988–5999. [Google Scholar] [CrossRef]
- Kumarasamy, V.; Mitchell, M.D.; Bloomfield, F.H.; Oliver, M.H.; Campbell, M.E.; Challis, J.R.G. Effects of periconceptional undernutrition on the initiation of parturition in sheep. Am. J. Physiol-Regul. Integr. Comp. Physiol. 2005, 288, R67–R72. [Google Scholar] [CrossRef] [PubMed]
- Gernand, E.; König, S.; Kipp, C. Influence of on-farm measurements for heat stress indicators on dairy cow productivity, female fertility, and health. J. Dairy Sci. 2019, 102, 6660–6671. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Williams, E.J.; Miller, A.N.A.; Nash, D.M.; Herath, S. Uterine diseases in cattle after parturition. Vet. J. 2008, 176, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, M.; Sawamukai, Y. Specific Localization of Macrophages in Pregnant Bovine Caruncles. Reprod. Domest. Anim. 2004, 39, 125–128. [Google Scholar] [CrossRef]
- Oliveira, L.J.; Hansen, P.J. Phenotypic Characterization of Macrophages in the Endometrium of the Pregnant Cow. Am. J. Reprod. Immunol. 2009, 62, 418–426. [Google Scholar] [CrossRef]
- Catozzi, C.; Ávila, G.; Zamarian, V.; Pravettoni, D.; Sala, G.; Ceciliani, F. In-vitro effect of heat stress on bovine monocytes lifespan and polarization. Immunobiology 2020, 225, 151888. [Google Scholar] [CrossRef]
- Dahl, G.E.; Tao, S.; Laporta, J. Heat Stress Impacts Immune Status in Cows Across the Life Cycle. Front. Vet. Sci. 2020, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.O.; Santos, N.R. Dynamics of postpartum endometrial cytology and bacteriology and their relationship to fertility in dairy cows. Theriogenology 2016, 85, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Furze, R.C.; Rankin, S.M. Neutrophil mobilization and clearance in the bone marrow. Immunology 2008, 125, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, S.; Ricci, E.; Migliorati, G.; Gentili, M.; Riccardi, C. How Glucocorticoids Affect the Neutrophil Life. Int. J. Mol. Sci. 2018, 19, 4090. [Google Scholar] [CrossRef]
- Burton, J.L.; Kehrli, M.E.; Kapil, S.; Horst, R.L. Regulation of L-selectin and CD18 on bovine neutrophils by glucocorticoids: Effects of cortisol and dexamethasone. J. Leukoc. Biol. 1995, 57, 317–325. [Google Scholar] [CrossRef]
- Capela, L.; Leites, I.; Mateus, L.; Romão, R.; Pereira, R.M.L.N.; Lopes-da-Costa, L. Effects of seasonal chronic heat stress on body thermoregulation, cortisol release and uterine health in postpartum native Alentejana and Mertolenga beef cattle. BMC Vet. Res. 2025, 21, 404, accepted, in press. [Google Scholar] [CrossRef]
- Koch, F.; Otten, W.; Sauerwein, H.; Reyer, H.; Kuhla, B. Mild heat stress–induced adaptive immune response in blood mononuclear cells and leukocytes from mesenteric lymph nodes of primiparous lactating Holstein cows. J. Dairy Sci. 2023, 106, 3008–3022. [Google Scholar] [CrossRef]
- Koch, F.; Reyer, H.; Görs, S.; Hansen, C.; Wimmers, K.; Kuhla, B. Heat stress and feeding effects on the mucosa-associated and digesta microbiome and their relationship to plasma and digesta fluid metabolites in the jejunum of dairy cows. J. Dairy Sci. 2024, 107, 5162–5177. [Google Scholar] [CrossRef] [PubMed]
- Molinari, P.C.C.; Bromfield, J.J. Inflammatory responses of bovine endometrial epithelial cells are increased under in vitro heat stress conditions. J. Therm. Biol. 2023, 114, 103564. [Google Scholar] [CrossRef]
- Bai, H.; Ukita, H.; Kawahara, M.; Mitani, T.; Furukawa, E.; Yanagawa, Y.; Yabuuchi, N.; Kim, H.; Takahashi, M. Effect of summer heat stress on gene expression in bovine uterine endometrial tissues. Anim. Sci. J. 2020, 91, e13474. [Google Scholar] [CrossRef]
- Molinari, P.C.C.; Davidson, B.D.; Laporta, J.; Dahl, G.E.; Sheldon, I.M.; Bromfield, J.J. Prepartum heat stress in dairy cows increases postpartum inflammatory responses in blood of lactating dairy cows. J. Dairy Sci. 2023, 106, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Capela, L.; Leites, I.; Mateus, L.; Silva, E.; Pissarra, H.; Romão, R.; Pereira, R.M.L.N.; Lopes-da-Costa, L. Seasonal chronic heat stress, body temperatures, metabolic profiles, hair cortisol concentrations and uterine immune cell populations in postpartum dairy cows. National Institute of Agrarian and Veterinarian Research: Santarém, Portugal, 2025; to be submitted. [Google Scholar]
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Qu, Y.; Li, J.; Cui, L.; Wang, Y.; Lin, J. Cortisol inhibits NF-κB and MAPK pathways in LPS activated bovine endometrial epithelial cells. Int. Immunopharmacol. 2018, 56, 71–77. [Google Scholar] [CrossRef]
- Gwazdauskas, F.C.; Wilcox, C.J.; Thatcher, W.W. Environmental and Managemental Factors Affecting Conception Rate in a Subtropical Climate. J. Dairy Sci. 1975, 58, 88–92. [Google Scholar] [CrossRef]
- Roman-Ponce, H.; Thatcher, W.W.; Caton, D.; Barron, D.H.; Wilcox, C.J. Thermal Stress Effects on Uterine Blood Flow in Dairy Cows. J. Anim. Sci. 1978, 46, 175–180. [Google Scholar] [CrossRef]
- McNaughton, A.P.; Murray, R.D. Structure and function of the bovine fetomaternal unit in relation to the causes of retained fetal membranes. Vet. Rec. 2009, 165, 615–622. [Google Scholar] [CrossRef]
- Streyl, D.; Kenngott, R.; Herbach, N.; Wanke, R.; Blum, H.; Sinowatz, F. Gene expression profiling of bovine peripartal placentomes: Detection of molecular pathways potentially involved in the release of foetal membranes. Reproduction 2012, 143, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Solary, E. A role of HSPs in apoptosis through “protein triage”? Cell Death Differ. 2003, 10, 619–620. [Google Scholar] [CrossRef]
- Takagi, M.; Yamamoto, D.; Ohtani, M.; Miyamoto, A. Quantitative analysis of messenger RNA expression of matrix metalloproteinases (MMP-2 and MMP-9), tissue inhibitor-2 of matrix metalloproteinases (TIMP-2), and steroidogenic enzymes in bovine placentomes during gestation and postpartum. Mol. Reprod. Dev. 2007, 74, 801–807. [Google Scholar] [CrossRef]
- Walter, I.; Boos, A. Matrix Metalloproteinases (MMP-2 and MMP-9) and Tissue Inhibitor-2 of Matrix Metalloproteinases (TIMP-2) in the Placenta and Interplacental Uterine Wall in Normal Cows and in Cattle with Retention of Fetal Membranes. Placenta 2001, 22, 473–483. [Google Scholar] [CrossRef]
- Rispoli, L.A.; Payton, R.R.; Gondro, C.; Saxton, A.M.; Nagle, K.A.; Jenkins, B.W. Heat stress effects on the cumulus cells surrounding the bovine oocyte during maturation: Altered matrix metallopeptidase 9 and progesterone production. Reproduction 2013, 146, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, Y.J.; Kim, J.Y.; Lee, W.T.; Yenari, M.A.; Giffard, R.G. The 70 kDa heat shock protein suppresses matrix metalloproteinases in astrocytes. NeuroReport 2004, 15, 499–502. [Google Scholar] [CrossRef]
- Inaba, R.; Kawahara-Miki, R.; Shinozawa, A.; Yasuhara, T.; Fujii, T.; Koyama, K. Impaired placentomal interferon signaling as the possible cause of retained fetal membrane in parturition-induced cows. J. Reprod. Dev. 2022, 68, 30–37. [Google Scholar] [CrossRef]
- Collier, R.J.; Gebremedhin, K.G. Thermal Biology of Domestic Animals. Annu. Rev. Anim. Biosci. 2015, 3, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Morera, P.; Basiricò, L.; Hosoda, K.; Bernabucci, U. Chronic heat stress up-regulates leptin and adiponectin secretion and expression and improves leptin, adiponectin and insulin sensitivity in mice. J. Mol. Endocrinol. 2012, 48, 129–138. [Google Scholar] [CrossRef]
- Magdub, A.; Johnson, H.D.; Belyea, R.L. Effect of Environmental Heat and Dietary Fiber on Thyroid Physiology of Lactating Cows. J. Dairy Sci. 1982, 65, 2323–2331. [Google Scholar] [CrossRef] [PubMed]
- Block, J.; Chase, C.C.; Hansen, P.J. Inheritance of resistance of bovine preimplantation embryos to heat shock: Relative importance of the maternal versus paternal contribution. Mol. Reprod. Dev. 2002, 63, 32–37. [Google Scholar] [CrossRef]
- Cavestany, D.; El-Wishy, A.B.; Foote, R.H. Effect of Season and High Environmental Temperature on Fertility of Holstein Cattle. J. Dairy Sci. 1985, 68, 1471–1478. [Google Scholar] [CrossRef]
- Pennington, J.A.; Albright, J.L.; Diekman, M.A.; Callahan, C.J. Sexual Activity of Holstein Cows: Seasonal Effects. J. Dairy Sci. 1985, 68, 3023–3030. [Google Scholar] [CrossRef]
- Alnimer, M.; De Rosa, G.; Grasso, F.; Napolitano, F.; Bordi, A. Effect of climate on the response to three oestrous synchronisation techniques in lactating dairy cows. Anim. Reprod. Sci. 2002, 71, 157–168. [Google Scholar] [CrossRef]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic Losses from Heat Stress by US Livestock Industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Gilad, E.; Meidan, R.; Berman, A.; Graber, Y.; Wolfenson, D. Effect of heat stress on tonic and GnRH-induced gonadotrophin secretion in relation to concentration of oestradiol in plasma of cyclic cows. Reproduction 1993, 99, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.P.; Boland, M.P. Frequency of twin births among Holstein-Friesian cows in a warm dry climate. Theriogenology 1991, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bridges, P.J.; Brusie, M.A.; Fortune, J.E. Elevated temperature (heat stress) in vitro reduces androstenedione and estradiol and increases progesterone secretion by follicular cells from bovine dominant follicles. Domest. Anim. Endocrinol. 2005, 29, 508–522. [Google Scholar] [CrossRef]
- De Rensis, F.; Garcia-Ispierto, I.; López-Gatius, F. Seasonal heat stress: Clinical implications and hormone treatments for the fertility of dairy cows. Theriogenology 2015, 84, 659–666. [Google Scholar] [CrossRef]
- Kawano, K.; Yanagawa, Y.; Nagano, M.; Katagiri, S. Effects of heat stress on the endometrial epidermal growth factor profile and fertility in dairy cows. J. Reprod. Dev. 2022, 68, 144–151. [Google Scholar] [CrossRef]
- Katagiri, S.; Moriyoshi, M.; Yanagawa, Y. Endometrial epidermal growth factor profile and its abnormalities in dairy cows. J. Reprod. Dev. 2016, 62, 465–470. [Google Scholar] [CrossRef]
- Rensis, F.D.; Scaramuzzi, R.J. Heat stress and seasonal effects on reproduction in the dairy cow—A review. Theriogenology 2003, 60, 1139–1151. [Google Scholar] [CrossRef]
- Sigdel, A.; Liu, L.; Abdollahi-Arpanahi, R.; Aguilar, I.; Peñagaricano, F. Genetic dissection of reproductive performance of dairy cows under heat stress. Anim. Genet. 2020, 51, 511–520. [Google Scholar] [CrossRef]
- Vasques, M.I.; Horta, A.E.M.; Marques, C.C.; Sasser, R.G.; Humblot, P. Levels of bPSPB throughout single and twin pregnancies after AI or transfer of IVM/IVF cattle embryos. Anim. Reprod. Sci. 1995, 38, 279–289. [Google Scholar] [CrossRef]
- Paula-Lopes, F.F.; Lima, R.S.; Satrapa, R.A.; Barros, C.M. Physiology and Endocrinology Symposium: Influence of cattle genotype (Bos indicus vs. Bos taurus) on oocyte and preimplantation embryo resistance to increased temperature 1,2. J. Anim. Sci. 2013, 91, 1143–1153. [Google Scholar]
- Rahman, M.B.; Schellander, K.; Luceño, N.L.; Van Soom, A. Heat stress responses in spermatozoa: Mechanisms and consequences for cattle fertility. Theriogenology 2018, 113, 102–112. [Google Scholar] [CrossRef]
- Roth, Z. Stress-induced alterations in oocyte transcripts are further expressed in the developing blastocyst. Mol. Reprod. Dev. 2018, 85, 821–835. [Google Scholar] [CrossRef]
- Gendelman, M.; Roth, Z. In vivo vs. in vitro models for studying the effects of elevated temperature on the GV-stage oocyte, subsequent developmental competence and gene expression. Anim. Reprod. Sci. 2012, 134, 125–134. [Google Scholar] [CrossRef]
- Pavani, K.C.; Baron, E.; Correia, P.; Lourenço, J.; Bettencourt, B.F.; Sousa, M. Gene expression, oocyte nuclear maturation and developmental competence of bovine oocytes and embryos produced after in vivo and in vitro heat shock. Zygote 2016, 24, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z.; Arav, A.; Bor, A.; Zeron, Y.; Braw-Tal, R.; Wolfenson, D. Improvement of quality of oocytes collected in the autumn by enhanced removal of impaired follicles from previously heat-stressed cows. Reprod. Camb. Engl. 2001, 122, 737–744. [Google Scholar] [CrossRef]
- Baruselli, P.S.; Ferreira, R.M.; Vieira, L.M.; Souza, A.H.; Bó, G.A.; Rodrigues, C.A. Use of embryo transfer to alleviate infertility caused by heat stress. Theriogenology 2020, 155, 1–11. [Google Scholar] [CrossRef]
- Sartori, R.; Sartor-Bergfelt, R.; Mertens, S.A.; Guenther, J.N.; Parrish, J.J.; Wiltbank, M.C. Fertilization and Early Embryonic Development in Heifers and Lactating Cows in Summer and Lactating and Dry Cows in Winter. J. Dairy Sci. 2002, 85, 2803–2812. [Google Scholar] [CrossRef]
- Gómez-Guzmán, J.A.; Parra-Bracamonte, G.M.; Velazquez, M.A. Impact of Heat Stress on Oocyte Developmental Competence and Pre-Implantation Embryo Viability in Cattle. Animals 2024, 14, 2280. [Google Scholar] [CrossRef]
- Stamperna, K.; Giannoulis, T.; Cañon-Beltrán, K.; Dovolou, E.; Kalemkeridou, M.; Nanas, I.; Rizos, D.; Moutou, K.A.; Mamuris, Z.; Amiridis, G.S. Oviductal epithelial cells transcriptome and extracellular vesicles characterization during thermoneutral and heat stress conditions in dairy cows. Theriogenology 2022, 187, 152–163. [Google Scholar] [CrossRef]
- Camargo, L.S.; Viana, J.H.; Ramos, A.A.; Serapião, R.V.; de Sa, W.F.; Ferreira, A.M.; Guimarães, M.F.; do Vale Filho, V.R. Developmental competence and expression of the Hsp 70.1 gene in oocytes obtained from Bos indicus and Bos taurus dairy cows in a tropical environment. Theriogenology 2007, 68, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Sakatani, M.; Bonilla, L.; Dobbs, K.B.; Block, J.; Ozawa, M.; Shanker, S.; Yao, J.; Hansen, P.J. Changes in the transcriptome of morula-stage bovine embryos caused by heat shock: Relationship to developmental acquisition of thermotolerance. Reprod. Biol. Endocrinol. 2013, 11, 3. [Google Scholar] [CrossRef]
- Zhang, B.; Peñagaricano, F.; Driver, A.; Chen, H.; Khatib, H. Differential expression of heat shock protein genes and their splice variants in bovine preimplantation embryos. J. Dairy Sci. 2011, 94, 4174–4182. [Google Scholar] [CrossRef] [PubMed]
- Demetrio, D.G.B.; Santos, R.M.; Demetrio, C.G.B.; Vasconcelos, J.L.M. Factors Affecting Conception Rates Following Artificial Insemination or Embryo Transfer in Lactating Holstein Cows. J. Dairy Sci. 2007, 90, 5073–5082. [Google Scholar] [CrossRef]
- Hansen, P.J. The incompletely fulfilled promise of embryo transfer in cattle—Why aren’t pregnancy rates greater and what can we do about it? J. Anim. Sci. 2020, 98, skaa288. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, J.L.; Demétrio, D.G.; Santos, R.M.; Chiari, J.R.; Rodrigues, C.A.; Sá Filho, O.G. Factors potentially affecting fertility of lactating dairy cow recipients. Theriogenology 2006, 65, 192–200. [Google Scholar] [CrossRef]
- Nanas, I.; Chouzouris, T.; Dadouli, K.; Dovolou, E.; Stamperna, K.; Barbagianni, M. A study on stress response and fertility parameters in phenotypically thermotolerant and thermosensitive dairy cows during summer heat stress. Reprod. Domest. Anim. 2020, 55, 1774–1783. [Google Scholar] [CrossRef]
- Nanas, I.; Chouzouris, T.M.; Dovolou, E.; Dadouli, K.; Stamperna, K.; Kateri, I.; Barbagianni, M.; Amiridis, G.S. Early embryo losses, progesterone and pregnancy associated glycoproteins levels during summer heat stress in dairy cows. J. Therm. Biol. 2021, 98, 102951. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capela, L.; Leites, I.; Pereira, R.M.L.N. Heat Stress from Calving to Mating: Mechanisms and Impact on Cattle Fertility. Animals 2025, 15, 1747. https://doi.org/10.3390/ani15121747
Capela L, Leites I, Pereira RMLN. Heat Stress from Calving to Mating: Mechanisms and Impact on Cattle Fertility. Animals. 2025; 15(12):1747. https://doi.org/10.3390/ani15121747
Chicago/Turabian StyleCapela, Luís, Inês Leites, and Rosa M. L. N. Pereira. 2025. "Heat Stress from Calving to Mating: Mechanisms and Impact on Cattle Fertility" Animals 15, no. 12: 1747. https://doi.org/10.3390/ani15121747
APA StyleCapela, L., Leites, I., & Pereira, R. M. L. N. (2025). Heat Stress from Calving to Mating: Mechanisms and Impact on Cattle Fertility. Animals, 15(12), 1747. https://doi.org/10.3390/ani15121747




