Simple Summary
The post-weaning period is critical for piglet growth and directly affects production efficiency in swine farming. Alpha-ketoglutaric acid (AKG) has shown potential in animal nutrition due to its role in energy metabolism, antioxidant defense, anti-inflammatory activity, and immune regulation. In this study, AKG supplementation significantly reduced the incidence of diarrhea, improved nutrient digestibility and amino acid utilization, and contributed to better overall health in weaned piglets.
Abstract
Alpha-ketoglutaric acid (AKG) is a key intermediate in the tricarboxylic acid cycle and plays a crucial role in energy production and amino acid metabolism. This study aimed to evaluate the effects of AKG on growth performance, nutrient digestibility, plasma biochemical parameters, and plasma amino acid profiles in weaned piglets. A total of 72 weaned piglets with an average weight of 7.33 kg (±0.50 kg) and an average age of 28 (±2 days) were randomly assigned to 3 dietary treatments with 6 replicates per group in a 42-day trial. The treatments included a basal diet (CT), a basal diet with 500 g/t AKG (AKG1), and a basal diet with 1000 g/t AKG (AKG2). Blood samples were collected on days 14 and 42, and fecal samples were collected on day 42. The results showed that diets including 500 g/t and 1000 g/t AKG significantly reduced diarrhea incidence in piglets compared to the CT group (p < 0.01). Moreover, diets including 1000 g/t AKG enhanced fecal dry matter digestibility, plasma albumin (ALB), and glucose (GLU) concentrations on day 42 compared to the AKG1 group (p < 0.05). In conclusion, diets including AKG reduced diarrhea incidence in weaned piglets, potentially through improved nutrient digestibility and enhanced systemic health. The 1000 g/t level exhibited greater beneficial effects than 500 g/t.
1. Introduction
Alpha-ketoglutarate (AKG) is a short-chain, non-toxic carboxylic acid with high stability in aqueous solutions. In biological systems, AKG serves as a crucial intermediate in the tricarboxylic acid (TCA) cycle. It is generated through the oxidative decarboxylation of isocitrate by isocitrate dehydrogenase and the oxidative deamination of glutamate by glutamate dehydrogenase, thereby playing a pivotal role in bridging carbon and nitrogen metabolism [1]. Furthermore, AKG serves as a precursor for the synthesis of L-glutamate, L-glutamine, L-proline, and L-arginine in various tissues, bridging carbohydrate and nitrogen metabolism and contributing to amino acid homeostasis and ammonia detoxification [2,3,4]. AKG facilitates the oxidation of nutrients such as amino acids, glucose, and fatty acids to generate cellular energy [5]. In particular, extracellular AKG serves as an important energy source for gastrointestinal cells. It is also recognized as a key molecule in regulating nitrogen transport, protein metabolism, gene expression, and the cellular redox state [6,7].
The biological functions of AKG extend to the regulation of protein synthesis, muscle and bone development, immune system homeostasis, and glucose and lipid metabolism. For example, AKG can inhibit glutamine degradation and activate the mammalian target of the rapamycin (mTOR) pathway to promote protein synthesis in porcine intestinal epithelial cells [8]. It also induces the phosphorylation of JNK, mTOR, S6K1, and S6, thereby promoting osteoblast differentiation [9]. Andersen et al. showed that dietary AKG supplementation in piglets enhances bone length, ultimate strength, and maximum elastic strength [10]. Furthermore, it can inhibit glucose-6-phosphate synthesis, reduce plasma leucine concentrations, and enhance nitric oxide production in endothelial cells, thus improving insulin sensitivity [11]. AKG is also involved in carnitine synthesis, facilitating mitochondrial β-oxidation of fatty acids, and promoting lipid degradation [12,13]. Previous studies demonstrated that dietary AKG supplementation alleviated lipopolysaccharide (LPS)-induced liver histopathological damage and improved liver amino acid profiles in weanling piglets [14].
Glutamine exhibits limitations such as poor stability and low solubility and can generate toxic by-products such as pyroglutamic acid and ammonia, which limit its application in animal production [15]. Dietary AKG supplementation has been reported to enhance glutamine metabolism and amino acid utilization in intestinal cells [16]. Moreover, AKG has been shown to promote bone growth and improve both apparent total tract digestibility (ATTD) and apparent ileal digestibility (AID) of phosphorus and calcium in the intestines of piglets [17]. It also alleviates intestinal inflammation, enhances epithelial repair under stress, and supports gut health during early weaning [18]. Additionally, AKG supplementation modulates the intestinal microbiota by promoting probiotic growth, increasing butyrate and valeric acid concentrations, and reducing intestinal ammonia levels in growing pigs [19]. Studies have shown that AKG can attenuate inflammation by suppressing the activation of M1 pro-inflammatory macrophages and promoting M2 anti-inflammatory polarization [20,21].
Given the pivotal role of AKG in energy metabolism, nitrogen balance, intestinal health, and immune regulation, dietary AKG supplementation may represent an effective strategy to support the growth and health of weaned piglets. However, comprehensive evaluations of its effects on growth performance, diarrhea incidence, plasma amino acid profiles, and nutrient digestibility remain limited. Therefore, the objective of this study was to investigate the effects of dietary AKG supplementation on growth performance, diarrhea incidence, plasma amino acid concentrations, and fecal apparent nutrient digestibility in weaned piglets.
2. Materials and Methods
2.1. Animal Ethics Approval
The trial was conducted at the Langfang Experimental Farm of Hebei Province, China. The animal procedures in this study were approved by the Animal Care and Use Committee of the Institute of Feed Research of the Chinese Academy of Agricultural Sciences (IFR-CAAS20240302).
2.2. Experimental Design
A total of 72 weaned barrows (Duroc × Landrace × Yorkshire) with similar initial body weight (BW, 7.33 ± 0.50 kg) and age (28 ± 2 days) were randomly assigned to 3 treatments with 6 replicates per treatment and 4 pigs per pen, and balanced for BW and litter of origin. All the piglets were purchased from a Langfang commercial farm and housed in a nursery room. The experiment lasted for 42 days. The control group was fed a corn–soybean meal basal diet, while the AKG1 and AKG2 groups received the same basal diet with the addition of 500 g/t and 1000 g/t of alpha-ketoglutaric acid, respectively (supplied by Sinovac Biotech Ltd., Shenzhen, China). The amino acid content in the diet was adjusted by supplementing crystalline amino acids (lysine, methionine, threonine, tryptophan, valine, and isoleucine) to meet the nutritional requirements of piglets. The initial temperature of the facility was set at 28 °C and decreased by 1 °C per week until reaching 26 °C. Relative humidity was maintained between 55% and 65%. Throughout the experiment, piglets had ad libitum access to feed and water and were vaccinated following routine protocols, and the pig house was cleaned regularly. The experimental feed was prepared according to the nutritional requirements of NRC (2012) [22], and the feed formula and nutritional contents are shown in Table 1.

Table 1.
Ingredient composition of the diets (%, as-fed basis).
2.3. Growth Performance and Diarrhea Incidence
The body weights of piglets were recorded on days 0, 14, 28, and 42 of the experiment. Daily feed intake was recorded by weighing the residual feed in the troughs. Average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) were calculated accordingly. When pigs died, the weight of the pigs and the residual feed weight in the trough were recorded for the correction of growth performance data.
Diarrhea incidence was monitored daily from days 1 to 14. Each piglet was individually assessed at 09:00 a.m. by observing perianal swelling and the presence of loose feces in the trough. A 5-point fecal scoring system was used for visual assessment and monitoring of each piglet every day: 1 point = hard, granular feces; 2 points = hard, formed feces; 3 points = soft, formed feces; 4 points = soft, unformed feces; 5 points = watery feces. Feces in liquid form (4–5 points) were considered diarrhea.
2.4. Apparent Digestibility of Nutrients
On days 40, 41, and 42, feces were collected for three consecutive days. Diets and feces samples were analyzed for moisture (method 930.15) (AOAC, 2005) [23], ether extract (method 920.39A) (AOAC, 2005), and crude protein (N × 6.25; method 990.03) (AOAC, 2005). Gross energy (GE) was determined using a Parr 6400 calorimeter (Parr Instrument Company, Moline, IL, USA). The apparent digestibility of nutrients was calculated using acid-insoluble ash (AIA) as an internal marker. The AIA content in the experimental diets and feces was determined according to the method described by (Newkirk et al., 2003) [24]. The apparent digestibility of crude protein, ether extract, and gross energy in feces was determined by the endogenous indicator method.
2.5. Plasma Biochemical Indices
On days 14 and 42, one piglet with a body weight close to the average was selected from each pen for anterior vena cava blood collection. The disposable blood collection needle and anticoagulant blood collection vessel containing heparin sodium were used. After blood collection, it was reversed and mixed evenly. It was left at room temperature for 30 min, then centrifuged for 15 min at 3000 rpm, and plasma biochemical indicators (ALB, ALP, ALT, AST, GLU, HDL, LDL, TC, TG, TP) were measured using an HTSH-8000 biochemical analyzer (Hitachi Ltd., Tokyo, Japan).
2.6. Plasma Amino Acid Level
Plasma was pretreated with 1.5 M perchloric acid and 2 M potassium carbonate, centrifuged at 10,000 rpm for 10 min to obtain supernatant and then filtered by a 0.22 μm filter membrane. The contents of 16 amino acids (Asp, Glu, Ser, His, Gly, Thr, Arg, Ala, Tyr, Val, Met, Trp, Ile, Phe, Lys, and Leu) were analyzed by Shimadzu SIL-20A high-performance liquid chromatography (Shimadzu Corporation, Kyoto, Japan). A C18 reversed-phase HPLC column (5 μm, 100 A, 4.6 × 150 mm) was used for the separation. We determined the amino acid analysis following the recommended methods [25].
2.7. Statistical Analysis
The data related to growth performance, digestibility, plasma biochemical indices, and amino acid levels were analyzed by one-way ANOVA using the SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Data are presented as means ± SEM. The dose-related effect of AKG was computed by GLM, using contrast command for the linear and quadratic effects. The chi-square test was utilized to analyze the incidence of diarrhea. Statistical significance was considered at p < 0.05, and a tendency was noted when 0.05 ≤ p < 0.10.
3. Results
3.1. Growth Performance and Diarrhea Incidence of Weaned Piglets
Table 2 illustrates the influence of varying doses of AKG on growth performance in weaned piglets. Diets containing AKG had no significant effects on BW, ADG, ADFI, and FCR of weaned piglets. Figure 1 demonstrates the influence of varying doses of AKG on the diarrhea rate in weaned piglets. Compared with the control, feeding piglets with both 500 g/t and 1000 g/t AKG significantly reduced the diarrhea incidence of piglets (p < 0.05).

Table 2.
Effects of AKG on growth performance of weaned piglets.
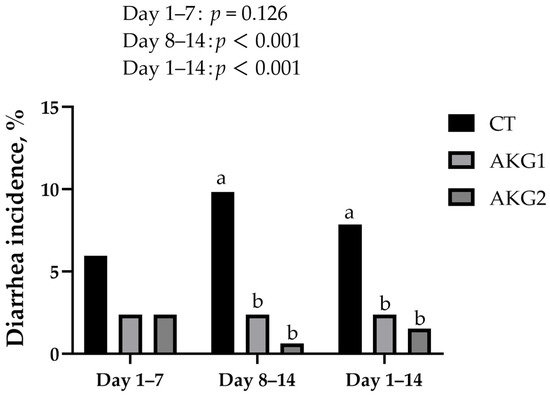
Figure 1.
Effects of AKG on the diarrhea incidence of weaned piglets. a,b Values with different superscripts are significantly different (p ≤ 0.05).
3.2. Nutrient Apparent Digestibility of Weaned Piglets
Table 3 shows the influence of alpha-ketoglutarate on fecal nutrient apparent digestibility of weaned piglets. The dry matter digestibility of weaned piglets consuming 1000 g/t AKG was significantly higher than that of 500 g/t (p < 0.05) group, and there was no significant change compared with the control group. As the AKG dose increases, the dry matter digestibility showed a significantly quadratic effect (p < 0.05), and the digestibility of crude protein and gross energy tended to increase (p = 0.056 and 0.054, respectively). In addition, the crude protein digestibility of weaned piglets in the AKG2 group tended to be higher than that in the CT group and AKG1 group (p = 0.092), while the energy utilization rate of piglets in the AKG2 group tended to be higher than that in the AKG1 group (p = 0.092).

Table 3.
Effect of AKG on the nutrient apparent digestibility (%) of weaned piglets.
3.3. Plasma Biochemistry Parameters of Weaned Piglets
Table 4 shows the influence of alpha-ketoglutarate on the fecal nutrient apparent digestibility of weaned piglets. The dry matter digestibility of weaned piglets consuming 1000 g/t AKG was significantly higher than that of the 500 g/t (p < 0.05) group, and there was no significant change compared with the control group. As the AKG dose increases, the dry matter digestibility showed a significantly quadratic effect (p < 0.05), and the digestibility of crude protein and gross energy tended to increase (p = 0.056 and 0.054, respectively). In addition, the crude protein digestibility of weaned piglets in the AKG2 group tended to be higher than that in the CT group and AKG1 group (p = 0.092), while the energy utilization rate of piglets in the AKG2 group tendend to be higher than that in the AKG1 group (p = 0.092).

Table 4.
Effects of AKG on the plasma biochemistry parameters of weaned piglets.
3.4. Plasma Amino Acid Level of Weaned Piglets
Table 5 demonstrates the influence of AKG on the plasma amino acid level of weaned piglets. Compared with the control group, AKG had no significant effect on the plasma amino acid content of weaned piglets. The Phe content in the plasma of the AKG1 group tended to be lower than that of the CT group on day 14 (p = 0.089). On day 42, the Tyr content in the plasma of the AKG1 group tended to be lower than that of the CT group (p = 0.091).

Table 5.
Effects of AKG on the plasma amino acid levels (mg/L) of weaned piglets.
4. Discussion
Weaning-induced gut dysfunction in piglets may result from alterations in intestinal structure and the specific loss of digestive enzymes [26]. At weaning, the gastrointestinal system is still immature, with insufficient secretion of gastric acid and digestive enzymes, limiting the digestion of solid feed. Consequently, undigested proteins can enter the large intestine, deteriorate, and promote the proliferation of pathogenic bacteria, leading to diarrhea [27]. Consistent with previous findings, dietary supplementation with 1% AKG alleviated LPS-induced diarrhea in weaned piglets [28]. The present study demonstrated that supplementation with 500 g/t and 1000 g/t AKG significantly reduced diarrhea incidence in weanling piglets.
In this study, AKG supplementation improved nutrient digestibility. Previous research reported that 10 g/kg AKG supplementation increased the apparent N-digestibility and net protein utilization of growing pigs [29]. Similarly, higher trypsin activity was observed in juvenile mirror carp fed a diet containing 0.6% AKG [30]. As a precursor of glutamine, AKG provides both energy and nitrogen sources for intestinal epithelial cells, reduces intestinal glutamine catabolism, supports gastrointestinal cellular metabolism, and ensures normal nutrient absorption [31,32]. These findings suggest that AKG may reduce diarrhea incidence by enhancing nutrient digestibility in weanling piglets.
Most plasma proteins are synthesized by the liver, with ALB being a major component responsible for maintaining plasma osmolality and reflecting both liver function and immune status. It has been reported that supplementing a low-protein diet with 1% and 1.5% AKG significantly increased serum ALB concentrations in piglets [33]. In this study, plasma ALB content was significantly higher in the AKG2 group compared to the AKG1 group on day 42, displaying a quadratic dose–response pattern. This suggests that AKG supplementation may promote protein synthesis and metabolism in piglets. Serum ALP, primarily derived from the liver, serves as an important indicator of hepatobiliary function or bone metabolism. Previous studies reported that supplementation with 0.2–1.0% AKG increased serum ALP activity in juvenile mirror carp [34]. In our study, although the difference was not statistically significant, plasma ALP levels in the AKG2 group were numerically higher than those in the AKG1 group on day 14, showing a similar direction of change. However, He et al. (2007) observed that 1% AKG supplementation significantly reduced serum ALP content in piglets, suggesting that the specific effects of AKG on liver function require further investigation [35]. An inverse relationship between serum HDL-C concentrations and coronary heart disease risk is well established [36]. Radzki et al. (2009) reported that AKG supplementation (0.01 M and 0.1 M) increased HDL concentrations in rats with experimentally induced hypercholesterolemia. In our study, plasma HDL concentrations showed a linear increase with AKG dosage on day 42, indicating that AKG supplementation may have the potential to reduce cardiovascular disease risk and promote overall health in piglets [37]. AKG has also been shown to stimulate amino acid absorption while decreasing plasma glucose absorption, thereby alleviating hyperglycemia by inhibiting hepatic gluconeogenesis [38]. GLU serves as a major direct energy source in animals, generating ATP through glycolysis and the TCA cycle. In the present study, supplementation with 1000 g/t AKG significantly increased plasma GLU concentrations on day 42, exhibiting a quadratic dose–response effect, suggesting that this dose may be optimal for promoting energy metabolism in weanling piglets.
In healthy animals, plasma amino acid (AA) concentrations are maintained within a relatively constant range. AA regulates key pathways essential for growth, development, immunity, and intestinal health, and serves as substrates for protein synthesis in intestinal mucosal cells [39]. He et al. (2016) observed that piglets fed diets supplemented with 1% AKG exhibited lower serum concentrations of Asp, Glu, Ala, Ile, Tyr, Phe, Lys, and Arg compared to controls, suggesting that AKG supplementation improves AA utilization [16]. Similarly, in our study, plasma concentrations of Phe on day 14 and Tyr on day 42 in the AKG1 group tendee to decrease and show a quadratic response, respectively, compared to the control group, although these differences were not statistically significant. Additionally, Phe activates the synthesis of BH4 and relates to neurological development and function, while Tyr participates in protein phosphorylation, nitrosation, and sulfation, and the degradation of both Phe and Tyr occurs primarily in the liver [40]. The observed decrease in Phe and Tyr values may reflect the effects of AKG supplementation on amino acid metabolism in piglets. Thus, these findings suggest that 500 g/t AKG may enhance the utilization of Phe and Tyr for tissue protein synthesis or promote their hepatic oxidation. However, most of these results showed positive trends, indicating certain limitations in our study, likely due to the small sample size. Therefore, future research will involve larger sample sizes and longer experimental durations to further validate these hypotheses.
5. Conclusions
In conclusion, dietary AKG showed no significant effect on the growth performance of piglets. However, both 500 g/t and 1000 g/t AKG significantly reduced the incidence of diarrhea in weaned piglets. Moreover, supplementation with 1000 g/t AKG improved fecal dry matter digestibility as well as plasma ALB and GLU concentrations on day 42. These findings suggest that AKG helps reduce diarrhea in piglets, potentially by promoting protein synthesis, enhancing energy metabolism, and improving overall health.
Author Contributions
Conceptualization, W.S. and X.J.; methodology, H.X. and X.J.; software, R.H.; validation, H.X. and X.J.; formal analysis, W.S. and W.C.; investigation, W.S.; resources, K.H. and X.J.; data curation, Y.L.; writing—original draft preparation, W.S.; writing—review and editing, Q.Z., X.L., V.B., X.G. and X.J.; supervision, X.J.; project administration, X.J.; funding acquisition, X.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Institute Animal Care and Use Committee of the Institute of Feed Research of the Chinese Academy of Agricultural Sciences (IFR-CAAS20240302, 2 March 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request from the authors.
Acknowledgments
We appreciate all crew members for their assistance during experiments at the swine base in Hebei province, China.
Conflicts of Interest
Authors Hongbo Xi and Kaikun Huang were employed by the company Shenzhen XinTianhe Biotechnology Co., Ltd, and author Qingchao Zhou was employed by the company Hebei Kangda Livestock and Poultry Breeding Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADFI | Average Daily Feed Intake |
| ADG | Average Daily Gain |
| AKG | Alpha-ketoglutaric acid |
| Ala | Alanine acid |
| ALB | Albumin |
| ALP | Alkaline Phosphatase |
| Arg | Arginine acid |
| Asp | Aspartic acid |
| AST | Aspartate Aminotransferase |
| FCR | Feed Conversion Ratio |
| Glu | Glutamic acid |
| GLU | Glucose |
| Gly | Glycine acid |
| HDL | High-Density Lipoprotein |
| His | Histidine acid |
| Ile | Isoleucine acid |
| LDL | Low-Density Lipoprotein |
| Leu | Leucine acid |
| Lys | Lysine acid |
| Met | Methionine acid |
| Phe | Phenylalanine acid |
| Ser | Serine acid |
| TC | Total Cholesterol |
| TG | Triglycerides |
| Thr | Threonine acid |
| TP | Total Protein |
| Trp | Tryptophan acid |
| Tyr | Tyrosine acid |
| Val | Valine acid |
References
- Baldwin, J.E.; Krebs, H. The evolution of metabolic cycles. Nature 1981, 291, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Lambert, B.D.; Filip, R.; Stoll, B.; Junghans, P.; Derno, M.; Hennig, U.; Souffrant, W.B.; Pierzynowski, S.; Burrin, D.G. First-pass metabolism limits the intestinal absorption of enteral α-ketoglutarate in young pigs. J. Nutr. 2006, 136, 2779–2784. [Google Scholar] [CrossRef] [PubMed]
- Pierzynowski, S.; Sjodin, A. Perspectives of glutamine and its derivatives as feed additives for farm animals. J. Anim. Feed. Sci. 1998, 7, 79–91. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Jungvid, H.; Fernández, J.A.; Pierzynowski, S. Absorption and metabolism of α-ketoglutarate in growing pigs. J. Anim. Physiol. Anim. Nutr. 2002, 86, 239–245. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Ding, B.; Liu, Y.; Zhu, H.; Liu, J.; Li, Y.; Kang, P.; Yin, Y.; Wu, G. Alpha-ketoglutarate and intestinal function. Front. Biosci. 2011, 16, 1186–1196. [Google Scholar] [CrossRef]
- Wu, G. Amino Acids: Biochemistry and Nutrition; CRC Press: Boca, Argentina, 2021. [Google Scholar]
- Filip, R.; Wdowiak, L.; Harrison, A.P.; Pierzynowski, S.G. Dietary supplementation with phytohemagglutinin in combination with alpha-ketoglutarate limits the excretion of nitrogen via urinary tract. Ann. Agric. Environ. Med. 2008, 15, 309–315. [Google Scholar] [PubMed]
- Yao, K.; Yin, Y.; Li, X.; Xi, P.; Wang, J.; Lei, J.; Hou, Y.; Wu, G. Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino Acids 2012, 42, 2491–2500. [Google Scholar] [CrossRef]
- Żurek, A.; Mizerska-Kowalska, M.; Sławińska-Brych, A.; Kaławaj, K.; Bojarska-Junak, A.; Kandefer-Szerszeń, M.; Zdzisińska, B. Alpha ketoglutarate exerts a pro-osteogenic effect in osteoblast cell lines through activation of JNK and mTOR/S6K1/S6 signaling pathways. Toxicol. Appl. Pharmacol. 2019, 374, 53–64. [Google Scholar] [CrossRef]
- Andersen, N.; Tatara, M.; Krupski, W.; Majcher, P.; Harrison, A. The long-term effect of α-ketoglutarate, given early in postnatal life, on both growth and various bone parameters in pigs. J. Anim. Physiol. Anim. Nutr. 2008, 92, 519–528. [Google Scholar] [CrossRef]
- Tekwe, C.D.; Yao, K.; Lei, J.; Li, X.; Gupta, A.; Luan, Y.; Meininger, C.J.; Bazer, F.W.; Wu, G. Oral administration of α-ketoglutarate enhances nitric oxide synthesis by endothelial cells and whole-body insulin sensitivity in diet-induced obese rats. Exp. Biol. Med. 2019, 244, 1081–1088. [Google Scholar] [CrossRef]
- Roe, D.S.; Roe, C.R.; Brivet, M.; Sweetman, L. Evidence for a short-chain carnitine–acylcarnitine translocase in mitochondria specifically related to the metabolism of branched-chain amino acids. Mol. Genet. Metab. 2000, 69, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.; Kristal, B.S. Multiple roles of glutathione in the central nervous system. Biol. Chem. Hoppe Seyler. 1997, 378, 793–802. [Google Scholar]
- Wang, L.; Hou, Y.; Yi, D.; Li, Y.; Ding, B.; Zhu, H.; Liu, J.; Xiao, H.; Wu, G. Dietary supplementation with glutamate precursor α-ketoglutarate attenuates lipopolysaccharide-induced liver injury in young pigs. Amino Acids 2015, 47, 1309–1318. [Google Scholar] [CrossRef]
- Filip, R.; Pierzynowski, S.G. The role of glutamine and alfa-ketoglutarate in gut metabolism and the potential application in medicine and nutrition. J. Pre-Clin. Clin. Res. 2007, 1, 9–15. [Google Scholar]
- He, L.; Li, H.; Huang, N.; Tian, J.; Liu, Z.; Zhou, X.; Yao, K.; Li, T.; Yin, Y. Effects of alpha-ketoglutarate on glutamine metabolism in piglet enterocytes in vivo and in vitro. J. Agric. Food Chem. 2016, 64, 2668–2673. [Google Scholar] [CrossRef]
- Tian, J.; Yang, F.; Bao, X.; Jiang, Q.; Li, Y.; Yao, K.; Yin, Y. Dietary alpha-ketoglutarate supplementation improves bone growth, phosphorus digestion, and growth performance in piglets. Animals 2023, 13, 569. [Google Scholar] [CrossRef]
- He, L.; Zhou, X.; Huang, N.; Li, H.; Cui, Z.; Tian, J.; Jiang, Q.; Liu, S.; Wu, J.; Li, T. Administration of alpha-ketoglutarate improves epithelial restitution under stress injury in early-weaning piglets. Oncotarget 2017, 8, 91965. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, H.; Long, L.; Zhao, Y.; Jiang, Q.; Wu, F.; Kang, B.; Liu, S.; Adebowale, T.O.; Fu, C. The effects of dietary supplementation with α-ketoglutarate on the intestinal microbiota, metabolic profiles, and ammonia levels in growing pigs. Anim. Feed. Sci. Technol. 2017, 234, 321–328. [Google Scholar] [CrossRef]
- Zhou, B.; Magana, L.; Hong, Z.; Huang, L.S.; Chakraborty, S.; Tsukasaki, Y.; Huang, C.; Wang, L.; Di, A.; Ganesh, B. The angiocrine Rspondin3 instructs interstitial macrophage transition via metabolic–epigenetic reprogramming and resolves inflammatory injury. Nat. Immunol. 2020, 21, 1430–1443. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.; Wang, S.; Zhou, H.; Feng, D.; Wei, J.; Shi, X.; Wu, L.; Zhang, P.; Yang, H. α-Ketoglutarate modulates macrophage polarization through regulation of PPARγ transcription and mTORC1/p70S6K pathway to ameliorate ALI/ARDS. Shock 2020, 53, 103–113. [Google Scholar] [CrossRef]
- Council, N.R.; Earth, D.o.; Studies, L.; Committee on Nutrient Requirements of Swine; Division on Earth and Life Studies; National Research Council. Nutrient Requirements of Swine; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Pub AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Newkirk, R.; Classen, H.; Scott, T.; Edney, M. The digestibility and content of amino acids in toasted and non-toasted canola meals. Can. J. Anim. Sci. 2003, 83, 131–139. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, Z.; Jia, S.; Wu, G. Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by HPLC with fluorescence detection. J. Chromatogr. B 2014, 964, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; James, P.; Smith, M.; Bourne, F. Effect of weaning on the capacity of pig intestinal villi to digest and absorb nutrients. J. Agric. Sci. 1986, 107, 579–590. [Google Scholar] [CrossRef]
- Nabuurs, M.; Hoogendoorn, A.; Van Der Molen, E.; Van Osta, A. Villus height and crypt depth in weaned and unweaned pigs, reared under various circumstances in the Netherlands. Res. Vet. Sci. 1993, 55, 78–84. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Huang, N.; Li, H.; Tian, J.; Zhou, X.; Li, T.; Yao, K.; Wu, G.; Yin, Y. AMPK/α-ketoglutarate axis regulates intestinal water and ion homeostasis in young pigs. J. Agric. Food Chem. 2017, 65, 2287–2298. [Google Scholar] [CrossRef]
- Chen, J.; Wu, F.; Yang, H.; Li, F.; Jiang, Q.; Liu, S.; Kang, B.; Li, S.; Adebowale, T.; Huang, N. Growth performance, nitrogen balance, and metabolism of calcium and phosphorus in growing pigs fed diets supplemented with alpha-ketoglutarate. Anim. Feed. Sci. Technol. 2017, 226, 21–28. [Google Scholar] [CrossRef]
- Wang, L.; Fan, Z.; Wu, D.; Li, J.; Xu, Q.; Miao, L.; Ge, X.; Cao, D.; Zheng, X. Effects of dietary α-ketoglutarate on the growth performance, digestive enzymes, tor signaling pathway and intestinal microbiota of juvenile mirror carp (Cyprinus carpio) fed low phosphorus diets. Aquaculture 2023, 574, 739736. [Google Scholar] [CrossRef]
- Chen, L.; Li, P.; Wang, J.; Li, X.; Gao, H.; Yin, Y.; Hou, Y.; Wu, G. Catabolism of nutritionally essential amino acids in developing porcine enterocytes. Amino Acids 2009, 37, 143–152. [Google Scholar] [CrossRef]
- Jones, C.; Palmer, T.A.; Griffiths, R. Randomized clinical outcome study of critically ill patients given glutamine-supplemented enteral nutrition. Nutrition 1999, 15, 108–115. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, H.; Sun, H. Effects of low-protein diet supplementation with alpha-ketoglutarate on growth performance, nitrogen metabolism and mTOR signalling pathway of skeletal muscle in piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 300–309. [Google Scholar] [CrossRef]
- Ai, F.; Wang, L.; Li, J.; Xu, Q. Effects of a-ketoglutarate (AKG) supplementation in low phosphorous diets on the growth, phosphorus metabolism and skeletal development of juvenile mirror carp (Cyprinus carpio). Aquaculture 2019, 507, 393–401. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Huang, N.; Zhou, X.; Tian, J.; Li, T.; Wu, J.; Tian, Y.; Yin, Y.; Yao, K. Alpha-ketoglutarate suppresses the NF-κB-mediated inflammatory pathway and enhances the PXR-regulated detoxification pathway. Oncotarget 2017, 8, 102974. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.; Gotto, A.M.; LaRosa, J.C.; Maroni, J.; Szarek, M.; Grundy, S.M.; Kastelein, J.J.; Bittner, V.; Fruchart, J.-C. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 2007, 357, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Radzki, R.P.; Bieńko, M.; Pierzynowski, S.G. Effect of dietary alpha-ketoglutarate on blood lipid profile during hypercholesterolaemia in rats. Scand. J. Clin. Lab. Investig. 2009, 69, 175–180. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhu, C.; Wang, Y.; Sun, J.; Feng, J.; Ma, Z.; Li, P.; Peng, W.; Yin, C.; Xu, G. α-Ketoglutaric acid ameliorates hyperglycemia in diabetes by inhibiting hepatic gluconeogenesis via serpina1e signaling. Sci. Adv. 2022, 8, eabn2879. [Google Scholar] [CrossRef]
- Wu, G. Intestinal mucosal amino acid catabolism. J. Nutr. 1998, 128, 1249–1252. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).