Molecular Cardiac Changes in Feline Hyperthyroidism and Hypertrophic Cardiomyopathy: Focus on Desmin, Calreticulin, and Interleukin-10 Expression
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Pathomorphological Examination
2.3. Immunohistochemical Examination
2.4. Statistical Analysis
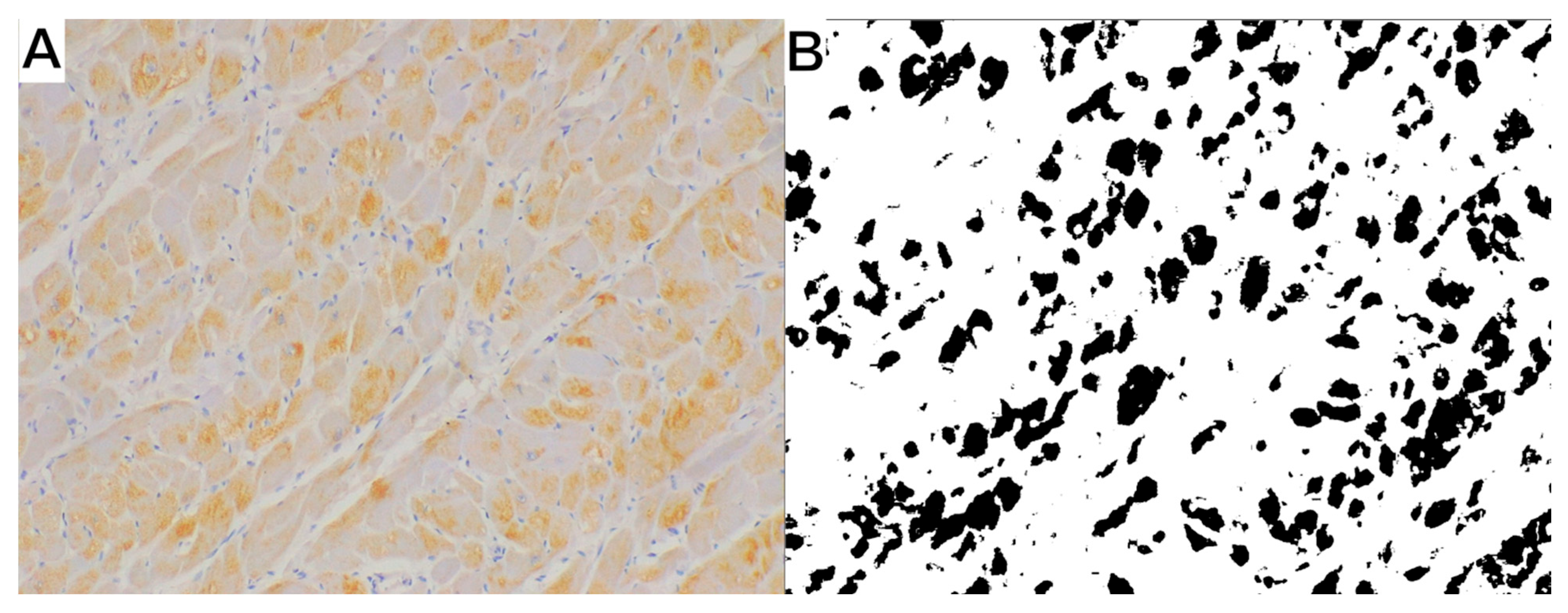
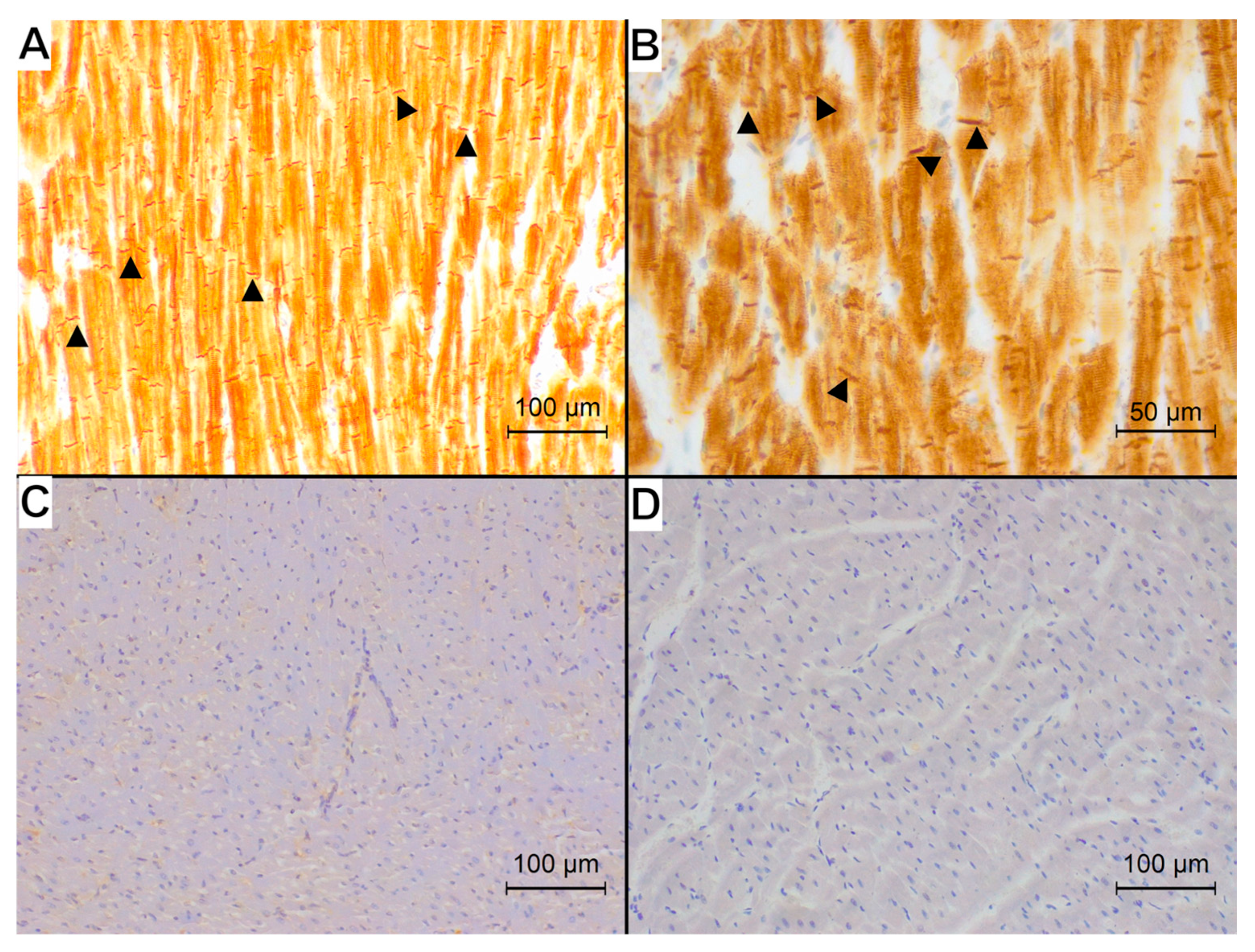
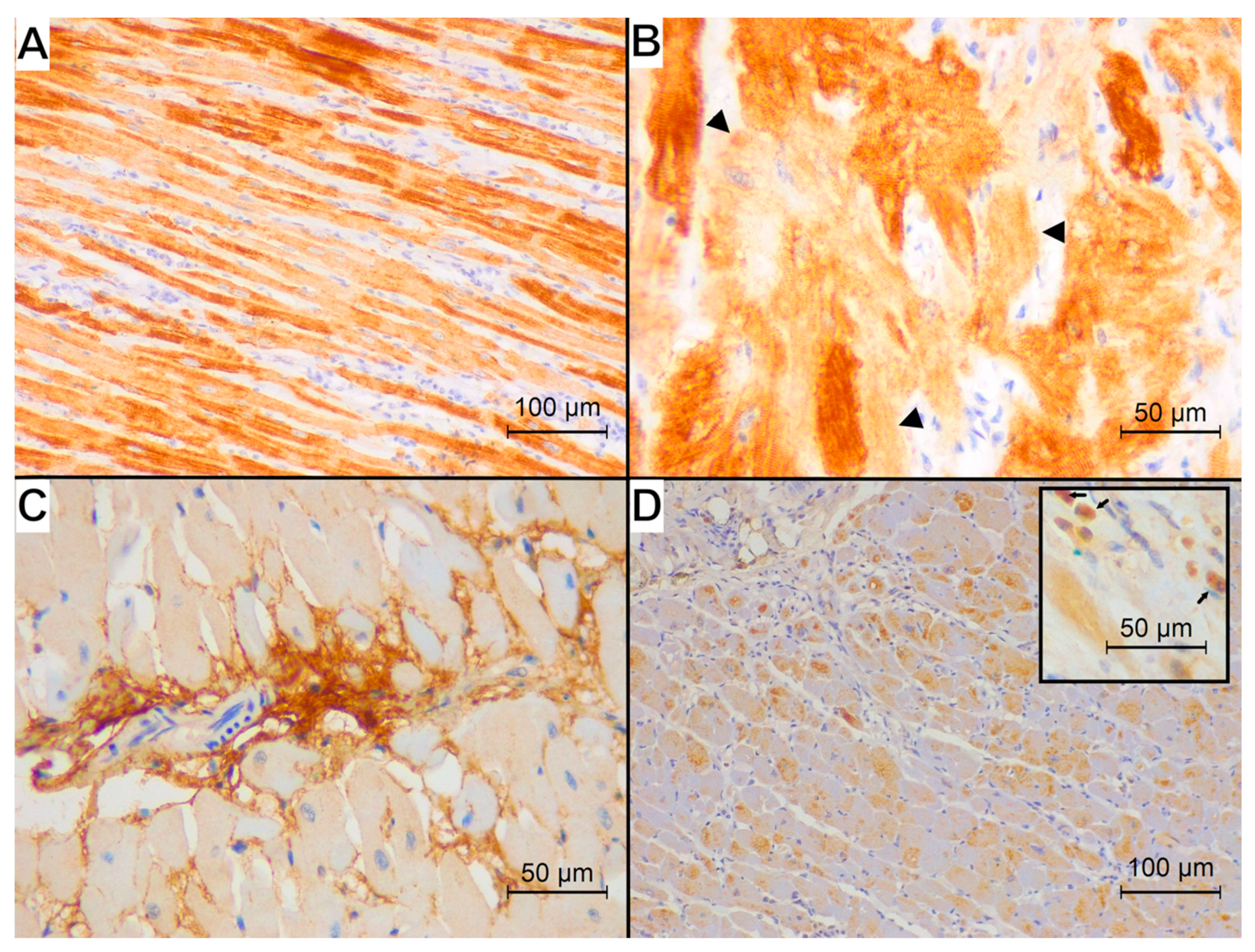
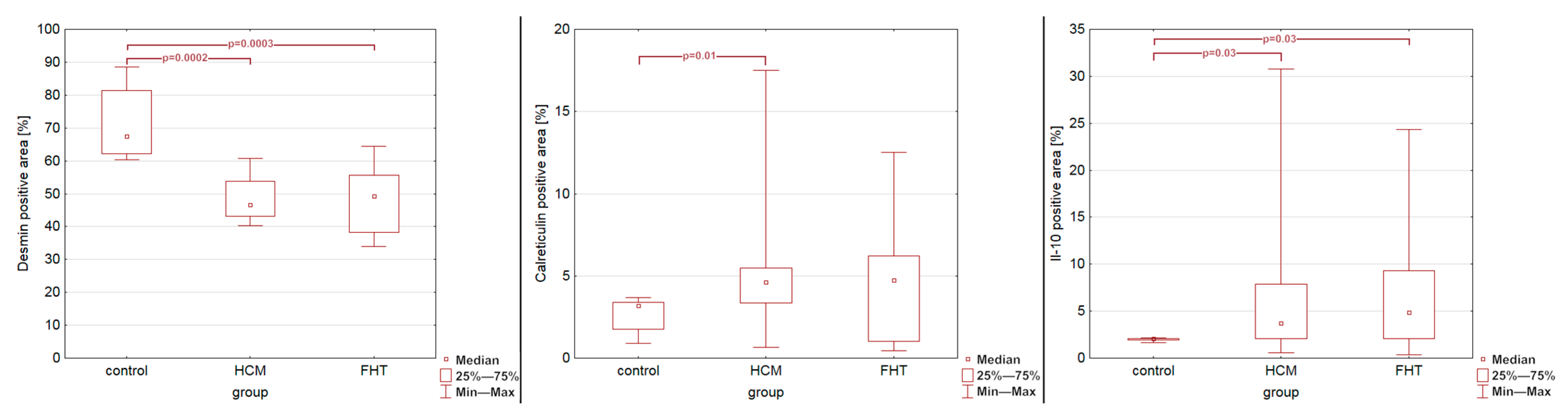
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLean, J.L.; Lobetti, R.G.; Mooney, C.T.; Thompson, P.N.; Schoeman, J.P. Prevalence of and Risk Factors for Feline Hyperthyroidism in South Africa. J. Feline Med. Surg. 2017, 19, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.J.; Neill, D.G.O.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Feline Hyperthyroidism Reported in Primary-care Veterinary Practices in England: Prevalence, Associated Factors and Spatial Distribution. Vet. Rec. 2014, 175, 458. [Google Scholar] [CrossRef] [PubMed]
- Gunn-Moore, D. Feline Endocrinopathies. Vet. Clin. North Am. Small Anim. Pract. 2005, 35, 171–210. [Google Scholar] [CrossRef]
- Mooney, C. Pathogenesis of Feline Hyperthyroidism. J. Feline Med. Surg. 2002, 4, 167–169. [Google Scholar] [CrossRef]
- Carney, H.C.; Ward, C.R.; Bailey, S.J.; Bruyette, D.; Dennis, S.; Ferguson, D.; Hinc, A.; Rucinsky, A.R. 2016 AAFP Guidelines for the Management of Feline Hyperthyroidism. J. Feline Med. Surg. 2016, 18, 400–416. [Google Scholar] [CrossRef]
- Luis Fuentes, V.; Abbott, J.; Chetboul, V.; Côté, E.; Fox, P.R.; Häggström, J.; Kittleson, M.D.; Schober, K.; Stern, J.A. ACVIM Consensus Statement Guidelines for the Classification, Diagnosis, and Management of Cardiomyopathies in Cats. J. Vet. Intern. Med. 2020, 34, 1062–1077. [Google Scholar] [CrossRef]
- Glaus, M.T.; Wess, G. Linksherzhypertrophie Bei Der Katze—«wenn Eine Hypertrophe Kardiomyopathie Keine Hypertrophe Kardiomyopathie Ist». Schweiz. Arch. Tierheilkd. 2010, 152, 325–330. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Sierra-Torres, C.H. Thyroid Hormones and the Heart. Horm. Mol. Biol. Clin. Investig. 2014, 18, 15–26. [Google Scholar] [CrossRef]
- Osuna, P.M.; Udovcic, M.; Sharma, M.D. Hypothyroidism and the Heart. Methodist Debakey Cardiovasc. J. 2017, 13, 60. [Google Scholar] [CrossRef]
- Janus, I.; Noszczyk-Nowak, A.; Bubak, J.; Tursi, M.; Vercelli, C.; Nowak, M. Comparative Cardiac Macroscopic and Microscopic Study in Cats with Hyperthyroidism vs. Cats with Hypertrophic Cardiomyopathy. Vet. Q. 2023, 43, 1–11. [Google Scholar] [CrossRef]
- Gofflot, S.; Kischel, P.; Thielen, C.; Radermacher, V.; Boniver, J.; de Leval, L. Characterization of an Antibody Panel for Immunohistochemical Analysis of Canine Muscle Cells. Vet. Immunol. Immunopathol. 2008, 125, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Hein, S.; Kostin, S.; Heling, A.; Maneo, Y.; Schaper, J. The Role of the Cytoskeleton in Heart Failure. Cardiovasc. Res. 2000, 45, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Gil, R.J. Desmin—An Important Structural Protein of a Cardiac Myocyte. Kardiol. Pol. 2007, 65, 303–309. [Google Scholar]
- Sharov, V.G.; Kostin, S.; Todor, A.; Schaper, J.; Sabbah, H.N. Expression of Cytoskeletal, Linkage and Extracellular Proteins in Failing Dog Myocardium. Heart Fail. Rev. 2005, 10, 297–303. [Google Scholar] [CrossRef]
- Michalak, M.; Groenendyk, J.; Szabo, E.; Gold, L.I.; Opas, M. Calreticulin, a Multi-Process Calcium-Buffering Chaperone of the Endoplasmic Reticulum. Biochem. J. 2009, 417, 651–666. [Google Scholar] [CrossRef]
- Mesaeli, N.; Nakamura, K.; Zvaritch, E.; Dickie, P.; Dziak, E.; Krause, K.-H.; Opas, M.; MacLennan, D.H.; Michalak, M. Calreticulin Is Essential for Cardiac Development. J. Cell Biol. 1999, 144, 857–868. [Google Scholar] [CrossRef]
- Groenendyk, J.; Wang, W.-A.; Robinson, A.; Michalak, M. Calreticulin and the Heart. Cells 2022, 11, 1722. [Google Scholar] [CrossRef]
- Michalak, M.; Lynch, J.; Groenendyk, J.; Guo, L.; Robert Parker, J.M.; Opas, M. Calreticulin in Cardiac Development and Pathology. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2002, 1600, 32–37. [Google Scholar] [CrossRef]
- Sánchez, D.; Gregor, P.; Čurila, K.; Hoffmanová, I.; Hábová, V.; Tučková, L.; Tlaskalová-Hogenová, H. Anti-Calreticulin Antibodies and Calreticulin in Sera of Patients Diagnosed with Dilated or Hypertrophic Cardiomyopathy. Autoimmunity 2016, 49, 554–562. [Google Scholar] [CrossRef]
- Papp, S.; Dziak, E.; Kabir, G.; Backx, P.; Clement, S.; Opas, M. Evidence for Calreticulin Attenuation of Cardiac Hypertrophy Induced by Pressure Overload and Soluble Agonists. Am. J. Pathol. 2010, 176, 1113–1121. [Google Scholar] [CrossRef]
- Fan, X.; Yao, Y.; Zhang, Y. Calreticulin Promotes Proliferation and Extracellular Matrix Expression through Notch Pathway in Cardiac Fibroblasts. Adv. Clin. Exp. Med. 2018, 27, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, J.; Liu, J.; Ye, J.; Xu, Y.; Wang, Z.; Yu, J.; Ye, D.; Zhao, M.; Feng, Y.; et al. The Role of Interleukin-10 Family Members in Cardiovascular Diseases. Int. Immunopharmacol. 2021, 94, 107475. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the Interleukin-10 Receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Kaur, K.; Dhingra, S.; Slezak, J.; Sharma, A.K.; Bajaj, A.; Singal, P.K. Biology of TNFα and IL-10, and Their Imbalance in Heart Failure. Heart Fail. Rev. 2009, 14, 113–123. [Google Scholar] [CrossRef]
- Yang, W.; Li, S.; Zhao, Y.; Sun, Y.; Huang, Y.; Cao, X.; Xing, C.; Yang, F.; Liu, W.; Zhao, X.; et al. Changes in the Expression of Interleukin-10 in Myocardial Infarction and Its Relationship with Macrophage Activation and Cell Apoptosis. Ann. Transl. Med. 2020, 8, 643. [Google Scholar] [CrossRef]
- Shao, M.; Wang, D.; Zhou, Y.; Du, K.; Liu, W. Interleukin-10 Delivered by Mesenchymal Stem Cells Attenuates Experimental Autoimmune Myocarditis. Int. Immunopharmacol. 2020, 81, 106212. [Google Scholar] [CrossRef]
- Blum, A.; Miller, H. Pathophysiological Role of Cytokines in Congestive Heart Failure. Annu. Rev. Med. 2001, 52, 15–27. [Google Scholar] [CrossRef]
- Verma, S.K.; Krishnamurthy, P.; Barefield, D.; Singh, N.; Gupta, R.; Lambers, E.; Thal, M.; Mackie, A.; Hoxha, E.; Ramirez, V.; et al. Interleukin-10 Treatment Attenuates Pressure Overload–Induced Hypertrophic Remodeling and Improves Heart Function via Signal Transducers and Activators of Transcription 3–Dependent Inhibition of Nuclear Factor-ΚB. Circulation 2012, 126, 418–429. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Mendoza, L.H.; Lindsey, M.L.; Ballantyne, C.M.; Michael, L.H.; Smith, C.W.; Entman, M.L. IL-10 Is Induced in the Reperfused Myocardium and May Modulate the Reaction to Injury. J. Immunol. 2000, 165, 2798–2808. [Google Scholar] [CrossRef]
- Yang, Z.; Zingarelli, B.; Szabó, C. Crucial Role of Endogenous Interleukin-10 Production in Myocardial Ischemia/Reperfusion Injury. Circulation 2000, 101, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Ma, Y.; Iyer, R.P.; DeLeon-Pennell, K.Y.; Yabluchanskiy, A.; Garrett, M.R.; Lindsey, M.L. IL-10 Improves Cardiac Remodeling after Myocardial Infarction by Stimulating M2 Macrophage Polarization and Fibroblast Activation. Basic. Res. Cardiol. 2017, 112, 33. [Google Scholar] [CrossRef] [PubMed]
- HAYMAN, R.; UNE, Y.; NOMURA, Y. Desmin as a Possible Immunohistochemical Marker for Feline Hypertrophic Cardiomyopathy. J. Vet. Med. Sci. 2000, 62, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Janus-Ziółkowska, I.; Bubak, J.; Ciaputa, R.; Kandefer-Gola, M.; Noszczyk-Nowak, A. The Pathological and Histopathological Findings in Cats with Clinically Recognised Hypertrophic Cardiomyopathy Are Related to the Severity of Clinical Signs and Disease Duration. Animals 2025, 15, 703. [Google Scholar] [CrossRef]
- Francalanci, P.; Gallo, P.; Bernucci, P.; Silver, M.D.; d’Amati, G. The Pattern of Desmin Filaments in Myocardial Disarray. Hum. Pathol. 1995, 26, 262–266. [Google Scholar] [CrossRef]
- Akoglu, H. User’s Guide to Correlation Coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Schaper, J.; Kostin, S.; Hein, S.; Elsässer, A.; Arnon, E.; Zimmermann, R. Structural Remodelling in Heart Failure. Exp. Clin. Cardiol. 2002, 7, 64–68. [Google Scholar]
- Janus, I.; Kandefer-Gola, M.; Ciaputa, R.; Noszczyk-Nowak, A.; Pasławska, U.; Tursi, M.; Nowak, M. The Immunohistochemical Evaluation of Selected Markers in the Left Atrium of Dogs with End-Stage Dilated Cardiomyopathy and Myxomatous Mitral Valve Disease—A Preliminary Study. Iran. Vet. J. 2016, 69, 18. [Google Scholar] [CrossRef]
- Biasato, I.; Francescone, L.; La Rosa, G.; Tursi, M. Anatomopathological Staging of Feline Hypertrophic Cardiomyopathy through Quantitative Evaluation Based on Morphometric and Histopathological Data. Res. Vet. Sci. 2015, 102, 136–141. [Google Scholar] [CrossRef]
- D’Amati, G.; Kahn, H.J.; Butany, J.; Silver, M.D. Altered Distribution of Desmin Filaments in Hypertrophic Cardiomyopathy: An Immunohistochemical Study. Mod. Pathol. 1992, 5, 165–168. [Google Scholar]
- Kypreou, K.P.; Kavvadas, P.; Karamessinis, P.; Peroulis, M.; Alberti, A.; Sideras, P.; Psarras, S.; Capetanaki, Y.; Politis, P.K.; Charonis, A.S. Altered Expression of Calreticulin during the Development of Fibrosis. Proteomics 2008, 8, 2407–2419. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Heymes, C.; Ohan, J.; Faggin, E.; Lesèche, G.; Tedgui, A. Expression of Interleukin-10 in Advanced Human Atherosclerotic Plaques. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Baban, B.; Liu, J.Y.; Mozaffari, M.S. Pressure Overload Regulates Expression of Cytokines, ΓH2AX, and Growth Arrest- and DNA-Damage Inducible Protein 153 via Glycogen Synthase Kinase-3β in Ischemic-Reperfused Hearts. Hypertension 2013, 61, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Baban, B.; Liu, J.Y.; Qin, X.; Weintraub, N.L.; Mozaffari, M.S. Upregulation of Programmed Death-1 and Its Ligand in Cardiac Injury Models: Interaction with GADD153. PLoS ONE 2015, 10, e0124059. [Google Scholar] [CrossRef]
- Cihakova, D. Interleukin-10 Stiffens the Heart. J. Exp. Med. 2018, 215, 379–381. [Google Scholar] [CrossRef]
- Thomas, T.P.; Grisanti, L.A. The Dynamic Interplay Between Cardiac Inflammation and Fibrosis. Front. Physiol. 2020, 11, 529075. [Google Scholar] [CrossRef]
- Kaur, K.; Sharma, A.K.; Dhingra, S.; Singal, P.K. Interplay of TNF-Alpha and IL-10 in Regulating Oxidative Stress in Isolated Adult Cardiac Myocytes. J. Mol. Cell. Cardiol. 2006, 41, 1023–1030. [Google Scholar] [CrossRef]
- Flora, Z.; Tang, P.K.; Smith, K.; Elliott, J. Cardiac Pathology Associated with Hypertension and Chronic Kidney Disease in Aged Cats. J. Comp. Pathol. 2025, 216, 40–49. [Google Scholar] [CrossRef]

| Group/Parameter | Control n = 10 | FHT n = 16 | HCM n = 12 |
|---|---|---|---|
| Heart weight [g] median (min–max) | 15 (13–15) | 22 (18–24) | 39 (30–45) |
| Heart height [mm] median (min–max) | 38.2 (30.3–41.2) | 40.4 (36.6–53.6) | 52.1 (40.3–63.5) |
| Left atrial appendage height [mm] median (min–max) | 10.9 (8.2–13.2) | 15.9 (10.0–29.8) | 26.6 (19.5–38) |
| Left atrial appendage width [mm] median (min–max) | 10.4 (8.4–11.8) | 15.9 (12.5–16.9) | 20.8 (12.8–34.2) |
| Interventricular septum thickness [mm] median (min–max) | 5.9 (4.9–6.8) | 6.0 (3.1–8.4) | 8.7 (6.3–9.6) |
| Left ventricular posterior wall thickness [mm] median (min–max) | 6.4 (6.0–7.9) | 6.5 (4.0–9.9) | 10.2 (7.9–13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janus-Ziółkowska, I.; Bubak, J.; Tursi, M.; Vercelli, C.; Ciaputa, R.; Kandefer-Gola, M.; Noszczyk-Nowak, A. Molecular Cardiac Changes in Feline Hyperthyroidism and Hypertrophic Cardiomyopathy: Focus on Desmin, Calreticulin, and Interleukin-10 Expression. Animals 2025, 15, 1719. https://doi.org/10.3390/ani15121719
Janus-Ziółkowska I, Bubak J, Tursi M, Vercelli C, Ciaputa R, Kandefer-Gola M, Noszczyk-Nowak A. Molecular Cardiac Changes in Feline Hyperthyroidism and Hypertrophic Cardiomyopathy: Focus on Desmin, Calreticulin, and Interleukin-10 Expression. Animals. 2025; 15(12):1719. https://doi.org/10.3390/ani15121719
Chicago/Turabian StyleJanus-Ziółkowska, Izabela, Joanna Bubak, Massimiliano Tursi, Cristina Vercelli, Rafał Ciaputa, Małgorzata Kandefer-Gola, and Agnieszka Noszczyk-Nowak. 2025. "Molecular Cardiac Changes in Feline Hyperthyroidism and Hypertrophic Cardiomyopathy: Focus on Desmin, Calreticulin, and Interleukin-10 Expression" Animals 15, no. 12: 1719. https://doi.org/10.3390/ani15121719
APA StyleJanus-Ziółkowska, I., Bubak, J., Tursi, M., Vercelli, C., Ciaputa, R., Kandefer-Gola, M., & Noszczyk-Nowak, A. (2025). Molecular Cardiac Changes in Feline Hyperthyroidism and Hypertrophic Cardiomyopathy: Focus on Desmin, Calreticulin, and Interleukin-10 Expression. Animals, 15(12), 1719. https://doi.org/10.3390/ani15121719










