Clinical and Clinico-Pathological Observations of the Erythrocyte Sedimentation Rate in Dogs Affected by Leishmaniosis and Monitored During Antileishmanial Treatment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Enrollment of Dogs
2.3. Laboratory Assays
2.4. Statistical Analysis
3. Results
3.1. Number of Dogs, Samples Collected and Type of Treatment
3.2. Number, Signalment, and Clinical Classification of Enrolled Dogs
3.3. Comparison of Measurements at Times T1 and T3
3.4. Comparison of Measurements at T1, T2, and T3
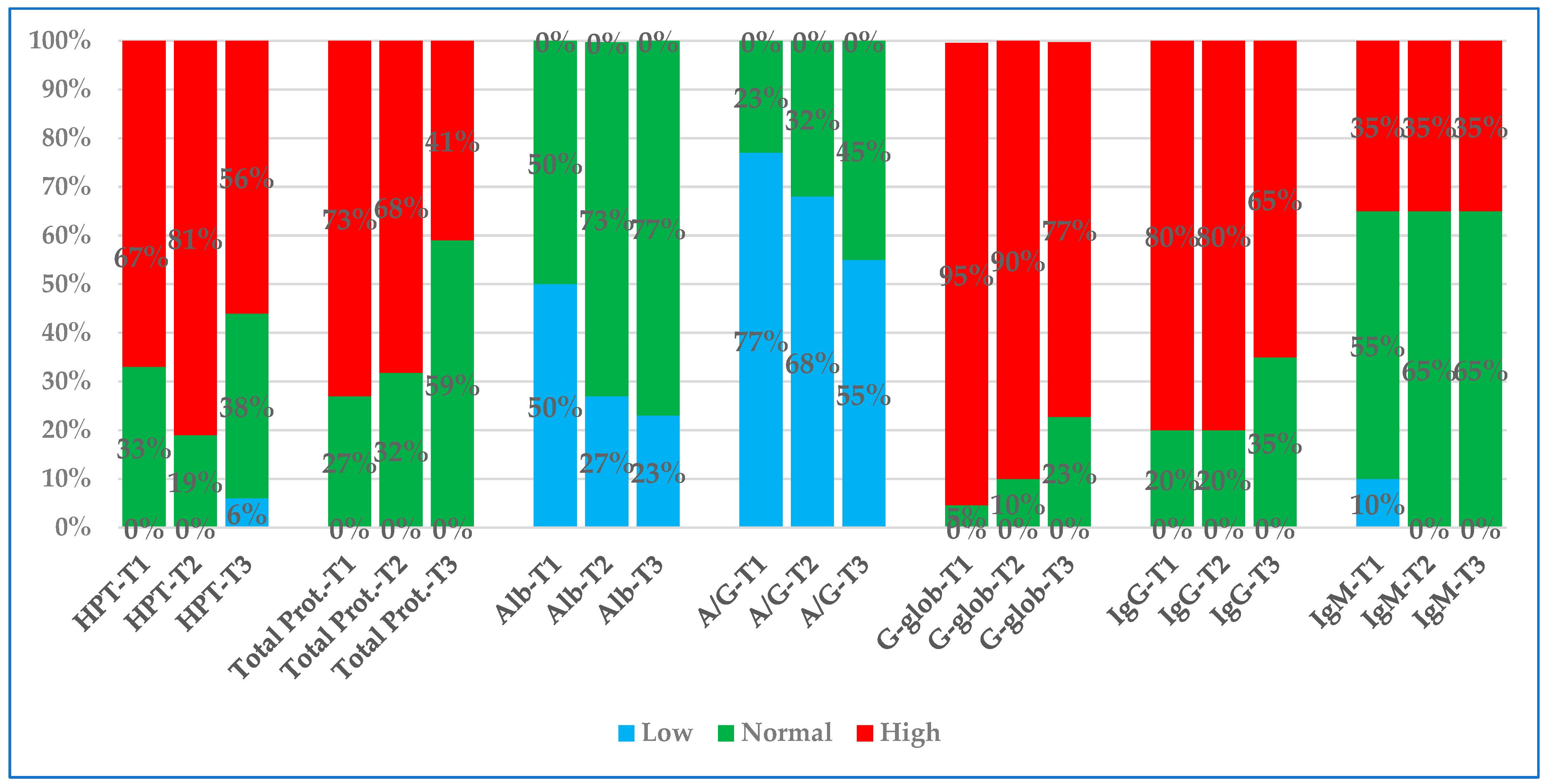
3.5. Comparison of Percentages of Values Outside the Reference Interval
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tishkowski, K.; Gupta, V. Erythrocyte Sedimentation Rate; StatPearls Publishing: Treasure Island, FL, USA, 2023; Volume 1. [Google Scholar]
- Brigden, M. The erythrocyte sedimentation rate: Still a helpful test when used judiciously. Postgrad. Med. 1998, 103, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Jou, J.M.; Lewis, S.M.; Briggs, C.; Lee, S.H.; De La Salle, B.; Mcfadden, S. ICSH review of the measurement of the erythocyte sedimentation rate. Int. J. Lab. Hematol. 2011, 33, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Bochen, K.; Krasowska, A.; Milaniuk, S.; Kulczynska, M.; Dzida, G. Erythrocyte sedimentation rate—An old marker with new applications. J. Pre-Clin. Clin. Res. 2011, 5, 50–55. [Google Scholar]
- Baskurt, O.K.; Meiselman, H.J. Erythrocyte aggregation: Basic aspects and clinical importance. Clin. Hemorheol. Microcirc. 2013, 53, 23–37. [Google Scholar] [CrossRef]
- Alende-Castro, V.; Alonso-Sampedro, M.; Vazquez-Temprano, N.; Tunez, C.; Rey, D.; Garcia-Iglesias, C.; Sopena, B.; Gude, F.; Gonzales-Quintela, A. Factors influencing erythrocyte sedimentation rate in adults. Medicine 2019, 98, e16816. [Google Scholar] [CrossRef]
- De Lourdes Chauffaille, M.; Takihi, I.Y.; Prieto, W.H.; de Sa Tavares Russo, P.; Sandres, A.F.; Perazzlo, A.B.; Silva, M.C.A.; Goncalves, M.V. New reference values for the old erythrocyte sedimentation rate. Int. J. Lab. Hematol. 2021, 43, O214–O217. [Google Scholar] [CrossRef]
- Kratz, A.; Plebani, M.; Peng, M.; Lee, Y.K.; McCafferty, R.; Machin, S.J. ICSH recommendations for modified and alternate methods measuring the erythrocyte sedimentation rate. Int. J. Lab. Hematol. 2017, 39, 448–457. [Google Scholar] [CrossRef]
- Tomassetti, F.; Calabrese, C.; Bertani, F.; Cennamo, M.; Diamanti, D.; Giovannelli, A.; Guerranti, R.; Leoncini, R.; Lorubbio, M.; Ognibene, A.; et al. Performance evaluation of automated Erytrocyte Sedimentation Rate (ESR) analyzers in a multicentric study. Diagnostics 2024, 14, 2011. [Google Scholar] [CrossRef]
- Diamanti, D.; Pieroni, C.; Pennisi, M.G.; Marchetti, V.; Gori, E.; Paltrinieri, S.; Lubas, G. The Erythrocyte Sedimentation Rate (ESR) in Veterinary Medicine: A Focused Review in Dogs and Cats. Animals 2025, 15, 246. [Google Scholar] [CrossRef]
- Militello, C.; Pasquini, A.; Medina Valentin, A.A.; Simčič, P.; De Feo, G.; Lubas, G. The Canine Erythrocyte Sedimentation Rate (ESR): Evaluation of a Point-of-Care Testing Device (MINIPET DIESSE). Vet. Med. Int. 2020, 6, 3146845. [Google Scholar] [CrossRef]
- Cavalera, M.A.; Gernone, F.; Uva, A.; Donghia, R.; Carelli, G.; Iatta, R.; Zatelli, A. Erythrocyte sedimentation rate in canine leishmaniosis diagnosis: A new resource. Front. Vet. Sci. 2022, 9, 949372. [Google Scholar] [CrossRef] [PubMed]
- Lubas, G.; Paltrinieri, S.; Papini, R.A.; Lensi, I.; Benali, S.L.; Cortadellas, O.; D’Anna, N.; Fonadti, A.; Roura, X.; Zini, E. Clinical and Clinico-Pathological Observations of the Erythrocyte Sedimentation Rate in Dogs Affected by Leishmaniosis and Other Inflammatory Diseases. Animals 2024, 14, 1013. [Google Scholar] [CrossRef] [PubMed]
- Cavalera, M.A.; Gusatoaia, O.; Uva, A.; Gernone, F.; Tarallo, V.D.; Donghia, R.; Silvestrino, M.; Zatelli, A. Erythrocyte sedimentation rate in heartworm naturally infected dogs “with or without” Leishmania infantum seropositivity: An observational prospective study. Front. Vet. Sci. 2024, 11, 1371690. [Google Scholar] [CrossRef]
- Uva, A.; Cavalera, M.A.; Gusatoaia, O.; Donghia, R.; Gernone, F.; Silvestrino, M.; Zatelli, A. Inflammatory status and chronic kidney disease in cats: Old and new inflammatory markers—A pilot prospective study. Animals 2023, 13, 3674. [Google Scholar] [CrossRef]
- Gori, E.; Pasquini, A.; Paltrinieri, S.; Lubas, G.; Militello, C.; Diamanti, D.; Carletti, C.; Pantoli, M.; Marchetti, M. Prospective Application of the Erythrocyte Sedimentation Rate (ESR) as a Possible Inflammatory Marker in Feline Patients. Vet. Med. Int. 2024, 8, 2313447. [Google Scholar] [CrossRef]
- De Melo Benvenutti, M.E.; de Miranda, J.A.L.; da Costa, Á.H.C.; Jorge, S.M.; de Melo Vaz, A.F. Diagnostic efficacy of erythrocyte sedimentation rate in horses: An integrative systematic review. Res. Soc. Dev. 2022, 11, e26911326416. [Google Scholar] [CrossRef]
- Pieroni, C.; Grassi, A.; Pantoli, M.; Berretti, M.; Messina, S.; Giovannini, C.; Lubas, G.; Diamanti, D. Analytical Validation of MINI-PET as Point-of-Care for Erythrocyte Sedimentation Rate Measure in Horses. Vet. Med. Int. 2023, 9, 9965095. [Google Scholar] [CrossRef]
- Oliva, G.; Roura, X.; Crotti, A.; Maroli, M.; Castagnaro, M.; Gradoni, L.; Lubas, G.; Paltrinieri, S.; Zatelli, A.; Zini, E. Guidelines for treatment of leishmaniasis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1192–1198. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Solano-Gallego, L.; Fondati, A.; Lubas, G.; Gradoni, L.; Castagnaro, M.; Crotti, A.; Maroli, M.; Oliva, G.; Roura, X.; et al. Guidelines for diagnosis and clinical classification of leishmaniasis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1184–1191. [Google Scholar] [CrossRef]
- Roura, X.; Fondati, A.; Lubas, G.; Gradoni, L.; Maroli, M.; Oliva, G.; Paltrinieri, S.; Zatelli, A.; Zini, E. Prognosis and monitoring of leishmaniasis in dogs: A working group report. Vet. J. 2013, 198, 43–47. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Ferrari, R.; Scavone, D.; Pieroni, C.; Diamanti, D.; Tagliasacchi, F. Increased Erythrocyte Sedimentation Rate in Dogs: Frequency in Routine Clinical Practice and Association with Hematological Changes. Animals 2024, 14, 1409. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Villanueva-Saz, S.; Carbonell, M.; Trotta, M.; Furlanello, T.; Natale, A. Serological diagnosis of canine leishmaniosis: Comparison of three commercial ELISA tests (Leiscan®, ID Screen® and Leishmania 96®), a rapid test (Speed Leish K®) and an in-house IFAT. Parasites Vectors 2014, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cortés, A.; Ojeda, A.; Todolí, F.; Alberola, J. Performance of commercially available serological diagnostic tests to detect Leishmania infantum infection on experimentally infected dogs. Vet. Parasitol. 2013, 191, 363–366. [Google Scholar] [CrossRef]
- Baxarias, M.; Mateu, C.; Miró, G.; Solano-Gallego, L. Serological survey of Leishmania infantum in apparently healthy dogs in different areas of Spain. Vet. Med. Sci. 2023, 9, 1980–1988. [Google Scholar] [CrossRef]
- Castelli, G.; Bruno, F.; Reale, S.; Catanzaro, S.; Valenza, V.; Vitale, F. Diagnosis of leishmaniasis: Quantification of parasite load by a real-time PCR assay with high sensitivity. Pathogens 2021, 10, 865. [Google Scholar] [CrossRef]
- Ceron, J.J.; Pardo-Marin, L.; Caldin, M.; Furlanello, T.; Solano-Gallego, L.; Tecles, F.; Bernal, L.; Baneth, G.; Martinez-Subiela, S. Use of acute phase proteins for the clinical assessment and management of canine leishmaniosis: General recommendations. BMC Vet. Res. 2018, 14, 196. [Google Scholar] [CrossRef]
- De Freitas, J.C.C.; Nunes-Pinheiro, D.C.S.; Neto, B.E.L.; Santos, G.J.L.; De Abreu, C.R.A.; Braga, R.R.; Campos, R.M.; De Oliveira, L.F. Alterações clínicas e laboratoriais em cães naturalmente infectados por Leishmania chagasi. Rev. Soc. Bras. Med. Trop. 2012, 45, 24–29. [Google Scholar] [CrossRef]
- Martinez-Subiela, S.; Strauss-Ayali, D.; Cerón, J.J.; Baneth, G. Acute phase protein response in experimental canine leishmaniosis. Vet. Parasitol. 2011, 180, 197–202. [Google Scholar] [CrossRef]
- Martinez-Subiela, S.; Cerón, J.J.; Strauss-Ayali, D.; Garcia-Martinez, J.D.; Tecles, F.; Tvarijonaviciute, A.; Caldin, M.; Baneth, G. Serum ferritin and paraoxonase-1 in canine leishmaniosis. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 23–29. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Gradoni, L.; Roura, X.; Zatelli, A.; Zini, E. Laboratory tests for diagnosing and monitoring canine leishmaniasis. Vet. Clin. Pathol. 2016, 45, 552–578. [Google Scholar] [CrossRef]
- Pardo-Marin, L.; Ceron, J.J.; Tecles, F.; Baneth, G.; Martínez-Subiela, S. Comparison of acute phase proteins in different clinical classification systems for canine leishmaniosis. Vet. Immunol. Immunopathol. 2020, 219, 109958. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, P.; Zoia, A.; Planellas, M.; Roura, X.; Pastor, J.; Ceron, J.J.; Caldin, M. Iron status and C-reactive protein in canine leishmaniasis. J. Small Anim. Pract. 2014, 55, 95–101. [Google Scholar] [CrossRef]
- Torrecilha, R.B.P.; Utsunomiya, Y.T.; Bosco, A.M.; Almeida, B.F.; Pereira, P.P.; Narciso, L.G.; Pereira, D.C.M.; Baptistiolli, L.; Calvo-Bado, L.; Courtenay, O.; et al. Correlations between peripheral parasite load and common clinical and laboratory alterations in dogs with visceral leishmaniasis. Prev. Vet. Med. 2016, 132, 83–87. [Google Scholar] [CrossRef]
- Daza González, M.A.; Fragío Arnold, C.; Fermín Rodríguez, M.; Checa, R.; Montoya, A.; Portero Fuentes, M.; Ruperez Noguer, C.; Martinez Subiela, S.; Ceron, J.J.; Miro, G. Effect of two treatments on changes in serum acute phase protein concentrations in dogs with clinical leishmaniosis. Vet. J. 2019, 245, 22–28. [Google Scholar] [CrossRef]
- Scott, M.; Stockham, S. Chapter 2 Leukocytes. In Fundamentals of Veterinary Clinical Pathology, 3rd ed.; Stockham, S., Scott, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2025; pp. 61–134. [Google Scholar]
- Friedrichs, K.R.; Scott, M.; Stockham, S. Chapter 7 Proteins. In Fundamentals of Veterinary Clinical Pathology, 3rd ed.; Stockham, S., Scott, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2025; pp. 471–536. [Google Scholar]
- Da Silva, K.R.; De Mendonça, V.R.R.; Silva, K.M.; Do Nascimento, L.F.M.; Mendes-Sousa, A.F.; De Pinho, F.A.; Barral-Netto, M.; Barral, A.M.P.; Pires e Cruz, M.d.S. Scoring clinical signs can help diagnose canine visceral leishmaniasis in a highly endemic area in Brazil. Mem. Inst. Oswaldo Cruz 2017, 112, 53–62. [Google Scholar] [CrossRef][Green Version]
- Riviere, J.E.; Papich, M.G. Veterinary Pharmacology and Therapeutics, 10th ed.; Wiley-Blackwell, J Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Budde, J.; McCluskey, D. Plumb’s Veterinary Drug Handbook., 10th ed.; Budde, J., McCluskey, D., Eds.; Wiley-Blackwell, J Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023. [Google Scholar]
- Bonavia, R.; Ferrer, L.; Roura, X. Use of corticosteroids to reverse acute renal failure due to canine leishmaniasis: Preliminary findings. In Proceedings of the 5th ECVIM Congress, Cambridge, UK, 31 August–2 September 1995; p. 58. [Google Scholar]
- Cortese, L.; Pelagalli, A.; Piantedosi, D.; Mastellone, V.; Di Loria, A.; Lombardi, P.; Ciaremella, P.; Avallone, L. The effects of prednisone on haemostasis in leishmaniotic dogs treated with meglumine antimoniate and allopurinol. Vet. J. 2008, 177, 405–540. [Google Scholar] [CrossRef]
- Monteiro, M.; Prata, S.; Cardoso, L.; Pereira da Fonseca, I.; Leal, R.O. Dogs with leishmaniosis: How are we managing proteinuria in daily practice? A Portuguese questionnaire-based study. Parasites Vectors 2022, 15, 125. [Google Scholar] [CrossRef]
- Segev, G.; Cowgill, L.D.; Heiene, R.; Labato, M.A.; Polzin, D.J. Consensus recommendations for immunosuppressive treatment of dogs with glomerular disease based on established pathology. J. Vet. Intern. Med. 2013, 27, 60–66. [Google Scholar] [CrossRef]
- Waters, C.B.; Adams, L.G.; Scott-Moncrieff, J.C.; De Nocila, D.D.; Snyder, P.W.; White, R.M.; Gasparini, M. Effects of glucocorticoid therapy on urine protein-to-creatinine ratios and renal morphology in dogs. J. Vet. Intern. Med. 1997, 11, 172–177. [Google Scholar] [CrossRef]
- Blavier, A.; Keroack, S.; Denerolle, P.; Goy-Thollot, I.; Chabanne, L.; Cadoré, J.L.; Bourdoiseau, G. Atypical forms of canine leishmaniosis. Vet. J. 2001, 162, 108–120. [Google Scholar] [CrossRef]
- Adamama-Moraitou, K.K.; Saridomichelakis, M.N.; Polizopoulou, Z.; Kritsepi, M.; Tsompanakou, A.; Koutinas, A.F. Short-term exogenous glucocorticosteroidal effect on iron and copper status in canine leismaniasis (Leihmania infantum). Can. J. Vet. Res. 2005, 69, 287–292. [Google Scholar] [PubMed]
- Goldstein, R.E.; Brovida, C.; Fernandez-del-Palacio, M.J.; Littman, M.P.; Polzin, D.J.; Zatelli, A.; Cowgill, L.D. Consensus Recommendations for Treatment for Dogs with Serology Positive Glomerular Disease. J. Vet. Intern. Med. 2013, 27, S60–S66. [Google Scholar] [CrossRef] [PubMed]
- Roura, X.; Cortadellas, O.; Day, M.J.; Benali, S.L.; Canine Leishmaniosis Working Group; Zatelli, A. Canine leishmaniosis and kidney disease: Q&A for an overall management in clinical practice. J. Small Anim. Pract. 2021, 62, E1–E19. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.; Abramo, F.; Borio, S.; Albanese, F.; Noli, C.; Dedola, C.; Leone, F. Pustular dermatitis in dogs affected by leishmaniosis: 22 cases. Vet. Dermatol. 2016, 27, 9-e4. [Google Scholar] [CrossRef]
- Bardagí, M.; Monaco, M.; Fondevila, D. Sterile or nonantibiotic-responsive pustular dermatitis and canine leishmaniosis: A 14 case series description and a statistical association study on 2420 cases. Vet. Dermatol. 2020, 31, 197-e41. [Google Scholar] [CrossRef]
- Peña, M.T.; Roura, X.; Davidson, M.G. Ocular and periocular manifestations of leishmaniasis in dogs: 105 Cases (1993–1998). Vet. Ophthalmol. 2000, 3, 35–41. [Google Scholar] [CrossRef]
- Digiaro, S.; Recchia, A.; Colella, A.; Cucciniello, S.; Greco, B.; Buonfrate, D.; Paradies, P. Treatment of Canine Leishmaniasis with Meglumine Antimoniate: A Clinical Study of Tolerability and Efficacy. Animals 2024, 14, 2244. [Google Scholar] [CrossRef]
- Ikeda-Garcia, F.A.; Lopes, R.S.; Marques, F.J.; Felix de Lima, V.M.; Morinishi, C.K.; Bonello, F.L.; Zenette, M.F.; Venturoli Perri, S.H.; Feitosa, M.M. Clinical and parasitological evaluation of dogs naturally infected by Leishmania (Leishmania) chagasi submitted to treatment with meglumine antimoniate. Vet. Parasitol. 2007, 143, 254–259. [Google Scholar] [CrossRef]
- Mateo, M.; Maynard, L.; Vischer, C.; Bianciardi, P.; Miró, G. Comparative study on the short term efficacy and adverse effects of miltefosine and meglumine antimoniate in dogs with natural leishmaniosis. Parasitol. Res. 2009, 105, 155–162. [Google Scholar] [CrossRef]
- Miró, G.; Oliva, G.; Cruz, I.; Canavate, C.; Mortarino, M.; Vischer, C.; Bianciardi, P. Multicentric, controlled clinical study to evaluate effectiveness and safety of miltefosine and allopurinol for canine leishmaniosis. Vet. Dermatol. 2009, 20, 397–404. [Google Scholar] [CrossRef]
- Santos, M.F.; Alexandre-Pires, G.; Pereira, M.A.; Marques, C.S.; Gomes, J.; Correia, J.; Duarte, A.; Gomes, L.; Rodrigues, A.V.; Basso, A.; et al. Meglumine antimoniate and miltefosine combined with allopurinol sustain pro-inflammatory immune environments during canine leishmaniosis treatment. Front. Vet. Sci. 2019, 6, 352. [Google Scholar] [CrossRef] [PubMed]
- Vineta, C.; Castro, J.; Lopez, M.C.; Frau, M.; Costas, A.; Arenas, C.; Roura, X. Is pancreatitis associated with meglumine antimoniate treatment for canine leishmaniosis? A multicentric prospective study. Parasites Vectors 2024, 17, 532. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.; West, C. Prednisone-induced Increases in Serum Alpha-2-Globulin and Haptoglobin Concentrations in Dogs. Vet. Pathol. 1987, 24, 90–92. [Google Scholar] [CrossRef]
- Martinez-Subiela, S.; Ginel, P.J.; Ceron, J.J. Effects of different glucocorticoids treament on serum acute phase proteins in dogs. Vet. Rec. 2004, 154, 814–817. [Google Scholar] [CrossRef]
- Harvey, J.; Levin, D.E.; Chen, C.L. Potential effects of glucocorticoids on serum iron concentration in dogs. Vet. Clin. Pathol. 1987, 16, 46–50. [Google Scholar] [CrossRef]
- Manna, L.; Corso, R.; Galiero, G.; Cerrone, A.; Muzj, P.; Gravino, A.E. Long-term follow-up of dogs with leishmaniosis treated with meglumine antimoniate plus allopurinol versus miltefosine plus allopurinol. Parasites Vectors 2015, 8, 289. [Google Scholar] [CrossRef]
- Gillum, R. A racial difference in erythrocyte sedimentation. J. Natl. Med. Assoc. 1993, 85, 47–50. [Google Scholar]
- Siemons, L.; Ten Klooster, P.M.; Vonkenam, H.; Van Riel, P.L.C.M.; Glas, C.A.W.; Van de Laar, M.A.F.J. How age and sex affect the erythrocyte sedimentation rate and C-reactive protein in early rheumatoid arthritis. BMC Musculoskelet. Disord. 2014, 15, 368. [Google Scholar] [CrossRef]
- Pawlotsky, Y.; Goasguen, J.; Guggenbuhl, P.; Veillard, E.; Jard, C.; Pouchard, M.; Perdriger, A.; Meadeb, J.; Chales, G. Σ Esr An Erythrocyte Sedimentation Rate Adjusted for the Hematocrit and Hemoglobin Concentration. Am. J. Clin. Pathol. 2004, 122, 802–810. [Google Scholar] [CrossRef]
- Frank, D.W. The Influence of Selected Factors on ESR in the Dog and Cat. Master’s Thesis, Kansas State University, Manhattan, KS, USA, 1966. [Google Scholar]
- Briend-Marchal, A.; Chapellier, P.; Perret, D.; Braun, J.P.; Guelfi, J.F. Comparaison de l’hémogramme, de la vitesse de sédimentation, de la fibrinogénémie et des protéines sériques de chiens âgés (≥10 ans) et de chiens adultes (1 à 8 ans) en bonne santé. Rev. Med. Vet. 2003, 154, 629–632. [Google Scholar]
- Khan, S.A.; Epstein, J.H.; Olival, K.J.; Hassan, M.M.; Hossain, M.B.; Rahman, K.B.M.A.; Elahi, M.F.; Mamun, M.A.; Haider, N.; Yasin, G.; et al. Hematology and serum chemistry reference values of stray dogs in Bangladesh. Open Vet. J. 2011, 1, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.R.; Da Silva, S.M.; de Oliveira Fulgêncio, G.; Michalick, M.S.M.; Frézard, F.J.G. Relationship between clinical and pathological signs and severity of canine leishmaniasis. Rev. Bras. Parasitol. Vet. 2013, 22, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Segarra, S.; Mirò, G.; Montoya, A.; Pardo-Marin, L.; Boque, N.; Ferrer, L.; Ceron, J. Randomized, allopurinol-controlled trial of the effects of dietary nucleotides and active hexose correlated compound in the treatment of canine leishmaniosis. Vet. Parasitol. 2017, 239, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Proverbio, D.; Perego, R.; Spada, E. The Use of Two Clinical Staging Systems of Canine Leishmaniasis in A Clinical Setting: A Critical Evaluation. J. Vet. Clin. Pract. Pet Care 2016, 103, 1–7. [Google Scholar] [CrossRef]
- Fabry, T.L. Mechanism of Erythrocyte Aggregation and Sedimentation. Blood 1987, 70, 1572–1576. [Google Scholar] [CrossRef]
- Fuster, Ó.; Vayá, A.; Giménez, C.; Todolí, J.; Hernández, J.L.; Laiz, B. Is erythrocyte sedimentation rate a useful inflammatory marker independently of the hematocrit? Comparison results with plasma viscosity. Clin. Hemorheol. Microcirc. 2014, 58, 381–384. [Google Scholar] [CrossRef]
- Borawski, J.; Mysliwiec, M. The Hematocrit-Corrected Erythrocyte Sedimentation Rate Can Be Useful in Diagnosing Inflammation in Hemodialysis Patients. Nephron 2001, 89, 381–383. [Google Scholar] [CrossRef]

| Breed | N | Age | Sex | N |
|---|---|---|---|---|
| Mixed | 19 | Median 4 years | Males intact | 26 |
| English setter | 5 | |||
| French Bouledogue | 4 | Range 2–14 years | Males castrated | 4 |
| Boxer | 3 | |||
| Chihuahua, Siberian husky, and Pug (two each) | 6 | Females intact | 4 | |
| American Staffordshire, Corso, Espagneul Breton, Dobermann, Poodle, Yorkshire terrier (1 each) | 6 | Females spayed | 9 |
| Main Clinical Problem/s or Sign/s | N | |
|---|---|---|
| Weight loss and skin disease | 7 | |
| Skin disease and lymphadenopathy | 5 | CLWG |
| Weight loss and epistaxis | 4 | Stage D |
| Polyarthritis and lymphadenopathy and weight loss and enteropathy (two each) | 4 | N = 23 |
| Skin disease, weight loss, and weight loss and enteropathy (one each) | 3 | |
| Weight loss | 9 | CLWG |
| Weight loss and enteropathy | 4 | Stage C |
| Weight loss and skin disease | 3 | N = 20 |
| Enteropathy, enteropathy and lymphadenopathy, polyarthritis, and skin disease (one each) | 4 |
| Parameter (Units) | Reference Interval | N | Before Treatment (T1) Median (I–III IQR) | End Treatment (T3) Median (I–III IQR) | p * |
|---|---|---|---|---|---|
| ESR (mm/h) | <10 | 43 | 45.0 (21.3–55.8) | 15.0 (12.0–38.0) | <0.0001 |
| Hematocrit (%) | 37.3–61.7 | 43 | 35.2 (27.8–40.5) | 40.9 (35.8–45.3) | <0.0001 |
| WBC (×10³/µL) | 5.05–16.76 | 43 | 8.70 (6.25–11.60) | 8.32 (6.81–11.65) | ns |
| CRP (mg/L) | 0–0.15 | 43 | 8.4 (1.8–23.4) | 1.2 (0.1–7.2) | <0.0001 |
| Fibrinogen (mg/dL) | 104–342 | 34 | 353 (258–512) | 242 (196–317) | <0.0001 |
| Iron (µg/dL) | 70–270 | 43 | 80 (59–100) | 99 (84–127) | 0.001 |
| Ferritin (ng/mL) | 95–287 | 43 | 569 (327–708) | 327 (201–392) | <0.0001 |
| HPT (mg/dL) | 18–117 | 34 | 203 (108–302) | 138 (76–211) | 0.016 |
| Total proteins (g/dL) | 5.5–7.6 | 43 | 7.82 (7.07–9.22) | 7.02 (6.71–7.83) | <0.0001 |
| Albumin (g/dL) | 2.4–3.8 | 43 | 2.37 (1.91–2.69) | 2.65 (2.28–2.82) | <0.0002 |
| A/G ratio | 0.6–1.3 | 43 | 0.41 (0.29–0.63) | 0.62 (0.46–0.71) | <0.0001 |
| G-glob (%) | 5–15 | 43 | 30.0 (19.5–42.6) | 19.5 (13.7–31.6) | <0.0001 |
| IgG (mg/dL) | 307–787 | 40 | 1494 (789–2274) | 849 (526–1366) | <0.0001 |
| IgM (mg/dL) | 64–176 | 40 | 194 (126–260) | 172 (115–234) | 0.027 |
| Parameter (Units) § | N | Before Treat. T1 Median (I–III IQR) | Middle Treat. T2 Median (I–III IQR) | End Treat. T3 Median (I–III IQR) | p | p * T1 vs. T2 | p * T1 vs. T3 | p * T2 vs. T3 |
|---|---|---|---|---|---|---|---|---|
| ESR (mm/h) | 22 | 42.5 (20.7–55.5) | 39.0 (17.8–47.2) | 15.0 (11.0–36.2) | 0.001 | ns | 0.0004 | 0.003 |
| Hematocrit (%) | 22 | 33.0 (24.7–37.9) | 36.8 (31.8–39.6) | 38.1 (34.0–42.5) | 0.0004 | 0.007 | 0.0002 | 0.012 |
| WBC (K/µL) | 22 | 9.05 (6.99–13.74) | 9.19 (7.45–12.50) | 9.13 (6.98–12.12) | ns | -- | -- | -- |
| CRP (mg/L) | 22 | 4.3 (1.1–10.5) | 1.7 (0.2–9.8) | 1.1 (0.4–2.6) | 0.035 | ns | 0.0007 | ns |
| Fibrinogen (mg/dL) | 15 | 265 (245–418) | 318 (245–374) | 215 (191–270) | 0.015 | ns | 0.006 | 0.011 |
| Iron (µg/dL) | 22 | 78 (59–109) | 95 (71–119) | 107 (88–130) | 0.042 | ns | 0.042 | ns |
| Ferritin (ng/mL) | 22 | 586 (471–715) | 347 (287–628) | 341 (200–415) | <0.0001 | 0.009 | <0.0001 | 0.005 |
| Haptoglobin (mg/dL) | 15 | 251 (114–306) | 282 (190–327) | 179 (83–292) | 0.002 | ns | ns | 0.001 |
| Total Proteins (g/dL) | 22 | 8.93 (7.39–10.53) | 8.40 (7.11–9.63) | 7.24 (6.23–8.45) | <0.0001 | ns | <0.0001 | <0.0001 |
| Albumin (g/dL) | 22 | 2.40 (1.96–2.64) | 2.59 (2.38–2.83) | 2.65 (2.41–2.78) | 0.004 | 0.002 | 0.0004 | ns |
| A/G | 22 | 0.39 (0.26–0.58) | 0.50 (0.33–0.60) | 0.58 (0.46–0.79) | <0.0001 | 0.012 | <0.0001 | <0.0001 |
| G-glob (%) | 21 | 41.8 (25.8–50.9) | 30.7 (19.4–44.6) | 20.5 (16.4–35.6) | <0.0001 | 0.0003 | <0.0001 | <0.0001 |
| IgG (mg/dL) | 18 | 2136 (791–2790) | 1563 (857–2161) | 1128 (476–1843) | <0.0001 | 0.003 | <0.0001 | 0.008 |
| IgM (mg/dL) | 18 | 131 (80–191) | 145 (108–194) | 151 (108–179) | ns | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubas, G.; Paltrinieri, S.; Papini, R.A.; Lensi, I.; Benali, S.; Cortadellas, O.; Fondati, A.; Roura, X.; Zini, E. Clinical and Clinico-Pathological Observations of the Erythrocyte Sedimentation Rate in Dogs Affected by Leishmaniosis and Monitored During Antileishmanial Treatment. Animals 2025, 15, 1716. https://doi.org/10.3390/ani15121716
Lubas G, Paltrinieri S, Papini RA, Lensi I, Benali S, Cortadellas O, Fondati A, Roura X, Zini E. Clinical and Clinico-Pathological Observations of the Erythrocyte Sedimentation Rate in Dogs Affected by Leishmaniosis and Monitored During Antileishmanial Treatment. Animals. 2025; 15(12):1716. https://doi.org/10.3390/ani15121716
Chicago/Turabian StyleLubas, George, Saverio Paltrinieri, Roberto Amerigo Papini, Ilaria Lensi, Silvia Benali, Oscar Cortadellas, Alessandra Fondati, Xavier Roura, and Eric Zini. 2025. "Clinical and Clinico-Pathological Observations of the Erythrocyte Sedimentation Rate in Dogs Affected by Leishmaniosis and Monitored During Antileishmanial Treatment" Animals 15, no. 12: 1716. https://doi.org/10.3390/ani15121716
APA StyleLubas, G., Paltrinieri, S., Papini, R. A., Lensi, I., Benali, S., Cortadellas, O., Fondati, A., Roura, X., & Zini, E. (2025). Clinical and Clinico-Pathological Observations of the Erythrocyte Sedimentation Rate in Dogs Affected by Leishmaniosis and Monitored During Antileishmanial Treatment. Animals, 15(12), 1716. https://doi.org/10.3390/ani15121716






