LPS Regulates Endometrial Immune Homeostasis and Receptivity Through the TLR4/ERK Pathway in Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Collection of Uterine Tissues
2.3. Cell Culture and Drug Treatment
2.4. CCK-8 Assay
2.5. Western Blot
2.6. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
2.7. Immunofluorescence Microscopy Analysis
2.8. Statistical Analysis
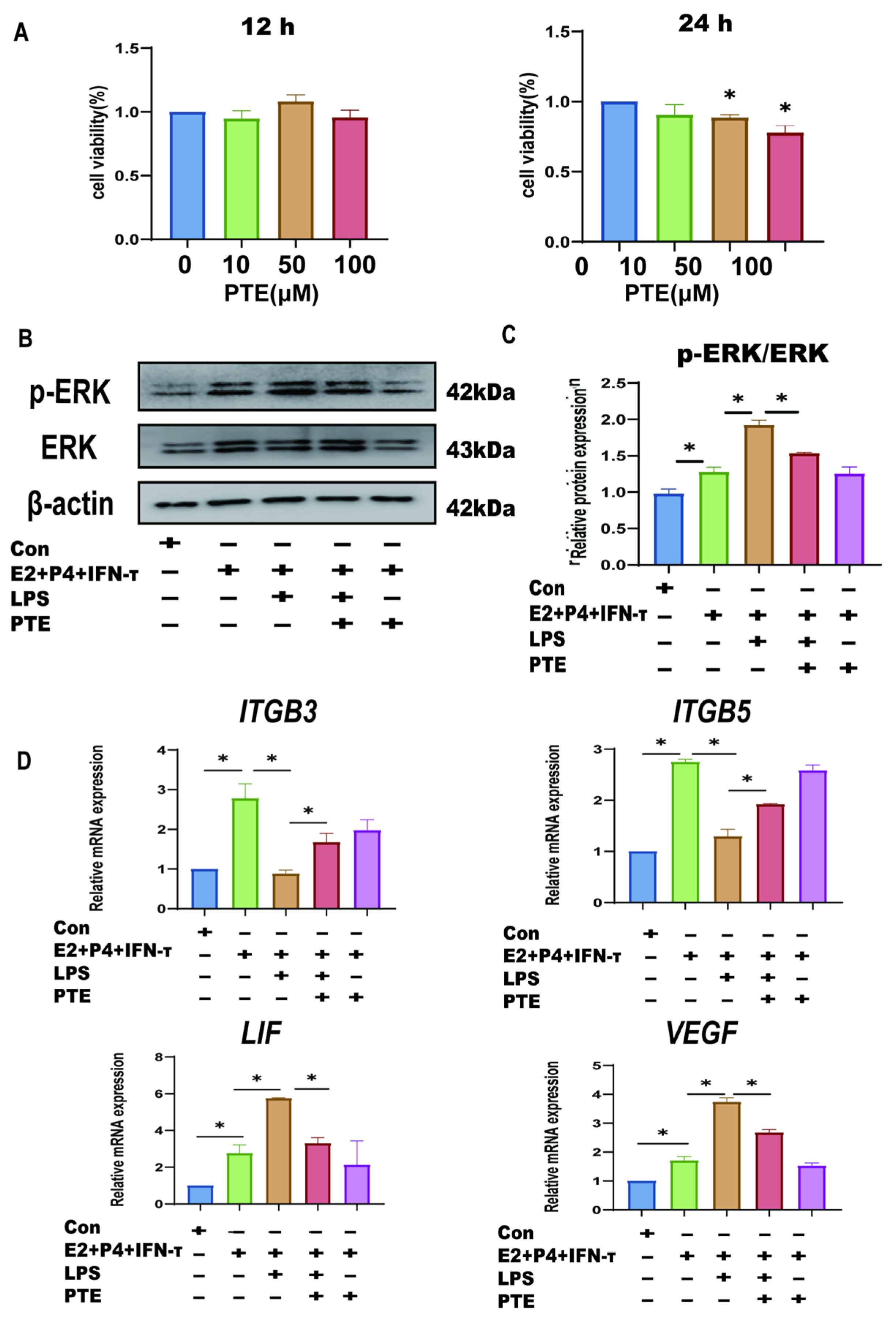
3. Results
3.1. Effect of LPS on the Expression Levels of Th1-Th2 Cytokines in the Endometrium of Sheep During Three Periods
3.2. Effect of LPS on the Expression of Endometrial Implantation Genes in Sheep at Three Periods
3.3. Effect of LPS on the TLR4/ERK Pathway in Sheep Endometrium Tissues
3.4. Establishment of a Model of Endometrial Epithelial Cell Inflammation in Sheep
3.5. LPS Affects Sheep Endometrium Through TLR4/ERK Pathway
3.6. PTE Alleviates LPS-Induced sEECs Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, J.; Liu, C.; Chen, H.; Xiang, M.; Hu, X.; Zhong, Z.; Liu, Q.; Wang, D.; Cheng, L. Transcriptomic analysis of bovine endometrial epithelial cells in response to interferon tau and hormone stimulation. Front. Vet. Sci. 2024, 11, 1344259. [Google Scholar] [CrossRef] [PubMed]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.; Burns, G.; Spencer, T.E. Conceptus elongation in ruminants: Roles of progesterone, prostaglandin, interferon tau and cortisol. J. Anim. Sci. Biotechnol. 2014, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Green, J.A.; Geisert, R.D.; Johnson, G.A.; Spencer, T.E. Implantation and Placentation in Ruminants. Adv. Anat. Embryol. Cell Biol. 2021, 234, 129–154. [Google Scholar] [CrossRef]
- Davenport, K.M.; Ortega, M.S.; Johnson, G.A.; Seo, H.; Spencer, T.E. Review: Implantation and placentation in ruminants. Animal 2023, 17 (Suppl. S1), 100796. [Google Scholar] [CrossRef]
- Spencer, T.E.; Johnson, G.A.; Bazer, F.W.; Burghardt, R.C. Implantation mechanisms: Insights from the sheep. Reproduction 2004, 128, 657–668. [Google Scholar] [CrossRef]
- Wang, W.; Sung, N.; Gilman-Sachs, A.; Kwak-Kim, J. T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front. Immunol. 2020, 11, 2025. [Google Scholar] [CrossRef]
- Guillomot, M.; Flechon, J.E.; Wintenberger-Torres, S. Conceptus attachment in the ewe: An ultrastructural study. Placenta 1981, 2, 169–182. [Google Scholar] [CrossRef]
- Mitchell, R.E.; Hassan, M.; Burton, B.R.; Britton, G.; Hill, E.V.; Verhagen, J.; Wraith, D.C. IL-4 enhances IL-10 production in Th1 cells: Implications for Th1 and Th2 regulation. Sci. Rep. 2017, 7, 11315. [Google Scholar] [CrossRef]
- Martinez, C.A.; Rubér, M.; Rodriguez-Martinez, H.; Alvarez-Rodriguez, M. Pig Pregnancies after Transfer of Allogeneic Embryos Show a Dysregulated Endometrial/Placental Cytokine Balance: A Novel Clue for Embryo Death? Biomolecules 2020, 10, 554. [Google Scholar] [CrossRef]
- Svensson, J.; Jenmalm, M.C.; Matussek, A.; Geffers, R.; Berg, G.; Ernerudh, J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J. Immunol. 2011, 187, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Sara, M.; Mili, M.; Goldsmith, L.T.; Kashani, B.N.; Ponzio, N.M. The maternal immune system during pregnancy and its influence on fetal development. Res. Rep. Biol. 2015, 6, 171–189. [Google Scholar]
- Koga, K.; Mor, G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am. J. Reprod. Immunol. 2010, 63, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, S.; Mahdavi, A.H.; Hajian, M.; Kowsar, R.; Varnosfaderani, S.R.; Nasr-Esfahani, M.H. The attenuation of the toxic effects of LPS on mouse pre-implantation development by alpha-lipoic acid. Theriogenology 2020, 143, 139–147. [Google Scholar] [CrossRef]
- Jackson, J.J.; Kropp, H. beta-Lactam antibiotic-induced release of free endotoxin: In vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP 3-specific ceftazidime. J. Infect. Dis. 1992, 165, 1033–1041. [Google Scholar] [CrossRef]
- Ahmed-Hassan, H.; Abdul-Cader, M.S.; Sabry, M.A.; Hamza, E.; Abdul-Careem, M.F. Toll-like receptor (TLR)4 signalling induces myeloid differentiation primary response gene (MYD) 88 independent pathway in avian species leading to type I interferon production and antiviral response. Virus Res. 2018, 256, 107–116. [Google Scholar] [CrossRef]
- Renaud, S.J.; Cotechini, T.; Quirt, J.S.; Macdonald-Goodfellow, S.K.; Othman, M.; Graham, C.H. Spontaneous pregnancy loss mediated by abnormal maternal inflammation in rats is linked to deficient uteroplacental perfusion. J. Immunol. 2011, 186, 1799–1808. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Hung, S.W.; Zhang, R.; Tan, Z.; Chung, J.P.W.; Zhang, T.; Wang, C.C. Pharmaceuticals targeting signaling pathways of endometriosis as potential new medical treatment: A review. Med. Res. Rev. 2021, 41, 2489–2564. [Google Scholar] [CrossRef]
- Hosseini, S.; Hosseini, S.; Salehi, M. Upregulation of Toll-like receptor 4 through anti-miR-Let-7a enhances blastocyst attachment to endometrial cells in mice. J. Cell. Physiol. 2020, 235, 9752–9762. [Google Scholar] [CrossRef]
- Lagana, A.S.; Garzon, S.; Gotte, M.; Vigano, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, A.; Kimura, F.; Kishi, Y.; Ishida, M.; Takahashi, A.; Yamanaka, A.; Takahashi, K.; Suginami, H.; Murakami, T. The association between endometriosis and chronic endometritis. PLoS ONE 2014, 9, e88354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bai, L.; Ren, Q.; Sun, G.; Si, Y. Protective effects of SIRT6 against lipopolysaccharide (LPS) are mediated by deacetylation of Ku70. Mol. Immunol. 2018, 101, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, Y.K.; Jaiswal, M.K.; Agrawal, V.; Chaturvedi, M.M. Bacterial endotoxin (LPS)-induced DNA damage in preimplanting embryonic and uterine cells inhibits implantation. Fertil. Steril. 2009, 91, 2095–2103. [Google Scholar] [CrossRef]
- Li, M.Z.; Wen, X.Y.; Liu, X.Q.; Wang, Y.Q.; Yan, L. LPS-Induced Activation of the cGAS-STING Pathway is Regulated by Mitochondrial Dysfunction and Mitochondrial DNA Leakage in Endometritis. J. Inflamm. Res. 2022, 15, 5707–5720. [Google Scholar] [CrossRef]
- Qi, M.Y.; Xu, L.Q.; Zhang, J.N.; Li, M.O.; Lu, M.H.; Yao, Y.C. Effect of the Booroola fecundity (FecB) gene on the reproductive performance of ewes under assisted reproduction. Theriogenology 2020, 142, 246–250. [Google Scholar] [CrossRef]
- Fan, X.; Wei, J.; Guo, Y.; Ma, J.; Qi, M.; Huang, H.; Zheng, P.; Jiang, W.; Yao, Y. LPS Disrupts Endometrial Receptivity by Inhibiting STAT1 Phosphorylation in Sheep. Int. J. Mol. Sci. 2024, 25, 13673. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, S.; Li, H.; Jiang, J.; Jia, Y.; Zhang, R.; Liu, H.; Wang, A.; Jin, Y.; Lin, P. USP18 promotes endometrial receptivity via the JAK/STAT1 and the ISGylation pathway. Theriogenology 2023, 202, 110–118. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, S.; Xue, Z.; Gao, K.; Sun, M.; Wang, A.; Lin, P.; Jin, Y. UFM1 inhibits the activation of the pyroptosis in LPS-induced goat endometritis. Theriogenology 2023, 196, 50–58. [Google Scholar] [CrossRef]
- Oh, H.K.; Choi, Y.S.; Yang, Y.I.; Kim, J.H.; Leung, P.C.; Choi, J.H. Leptin receptor is induced in endometriosis and leptin stimulates the growth of endometriotic epithelial cells through the JAK2/STAT3 and ERK pathways. Mol. Hum. Reprod. 2013, 19, 160–168. [Google Scholar] [CrossRef]
- Liu, P.; Tang, W.; Xiang, K.; Li, G. Pterostilbene in the treatment of inflammatory and oncological diseases. Front. Pharmacol. 2023, 14, 1323377. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, J.; Fang, M.; Yu, B. Pregnancy immune tolerance at the maternal-fetal interface. Int. Rev. Immunol. 2020, 39, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Li, S.; Zhu, M.; He, K.; Yao, X.; Zhang, L. Differential expression of interferon-gamma, IL-4 and IL-10 in peripheral blood mononuclear cells during early pregnancy of the bovine. Reprod. Biol. 2018, 18, 312–315. [Google Scholar] [CrossRef]
- Qian, Y.; Pei, Y.; Jiang, W.; Zheng, C. Astilbin improves pregnancy outcome in rats with recurrent spontaneous abortion by regulating Th1/Th2 balance. Immunopharmacol. Immunotoxicol. 2022, 44, 663–670. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.; Mi, H.; Liu, B.; Wang, B.; Yang, L. Modulation of Helper T Cytokines in Thymus during Early Pregnancy in Ewes. Animals 2019, 9, 245. [Google Scholar] [CrossRef]
- Ross, J.W.; Malayer, J.R.; Ritchey, J.W.; Geisert, R.D. Characterization of the interleukin-1beta system during porcine trophoblastic elongation and early placental attachment. Biol. Reprod. 2003, 69, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Collins, F.; Klonisch, T.; Sallenave, J.M.; Critchley, H.O.; Saunders, P.T. An additive interaction between the NFkappaB and estrogen receptor signalling pathways in human endometrial epithelial cells. Hum. Reprod. 2010, 25, 510–518. [Google Scholar] [CrossRef]
- Waclawik, A. Novel insights into the mechanisms of pregnancy establishment: Regulation of prostaglandin synthesis and signaling in the pig. Reproduction 2011, 142, 389–399. [Google Scholar] [CrossRef]
- Szostek, A.Z.; Adamowski, M.; Galvao, A.M.; Ferreira-Dias, G.M.; Skarzynski, D.J. Ovarian steroid-dependent tumor necrosis factor-alpha production and its action on the equine endometrium in vitro. Cytokine 2014, 67, 85–91. [Google Scholar] [CrossRef]
- Saini, V.; Arora, S.; Yadav, A.; Bhattacharjee, J. Cytokines in recurrent pregnancy loss. Clin. Chim. Acta 2011, 412, 702–708. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Wu, X.; He, B.; Cheng, Z.; Szenci, O.; Song, P.; Shao, D.; Zhang, S.; Yan, Z. Decreasing of S100A4 in bovine endometritis in vivo and in vitro. Theriogenology 2020, 153, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Mezera, M.A.; Li, W.; Liu, L.; Meidan, R.; Penagaricano, F.; Wiltbank, M.C. Effect of natural pre-luteolytic prostaglandin F2alpha pulses on the bovine luteal transcriptome during spontaneous luteal regressiondagger. Biol. Reprod. 2021, 105, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.A.; Burghardt, R.C.; Bazer, F.W. Osteopontin: A leading candidate adhesion molecule for implantation in pigs and sheep. J. Anim. Sci. Biotechnol. 2014, 5, 56. [Google Scholar] [CrossRef]
- Zhou, W.; Van Sinderen, M.; Rainczuk, K.; Menkhorst, E.; Sorby, K.; Osianlis, T.; Pangestu, M.; Santos, L.; Rombauts, L.; Rosello-Diez, A.; et al. Dysregulated miR-124-3p in endometrial epithelial cells reduces endometrial receptivity by altering polarity and adhesion. Proc. Natl. Acad. Sci. USA 2024, 121, e2401071121. [Google Scholar] [CrossRef]
- He, D.; Zeng, H.; Chen, J.; Xiao, L.; Zhao, Y.; Liu, N. H19 regulates trophoblastic spheroid adhesion by competitively binding to let-7. Reproduction 2019, 157, 423–430. [Google Scholar] [CrossRef]
- Choi, H.J.; Chung, T.W.; Park, M.J.; Kim, H.S.; You, S.; Lee, M.S.; Joo, B.S.; Lee, K.S.; Kim, K.J.; Wee, G.; et al. Benzoic Acid Enhances Embryo Implantation through LIF-Dependent Expression of Integrin αVβ3 and αVβ5. J. Microbiol. Biotechnol. 2017, 27, 668–677. [Google Scholar] [CrossRef]
- Vogiagis, D.; Fry, R.C.; Sandeman, R.M.; Salamonsen, L.A. Leukaemia inhibitory factor in endometrium during the oestrous cycle, early pregnancy and in ovariectomized steroid-treated ewes. J. Reprod. Fertil. 1997, 109, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Park, M.J.; Kim, H.S.; Choi, H.J.; Ha, K.T. Integrin alphaVbeta3 and alphaVbeta5 are required for leukemia inhibitory factor-mediated the adhesion of trophoblast cells to the endometrial cells. Biochem. Biophys. Res. Commun. 2016, 469, 936–940. [Google Scholar] [CrossRef]
- Fukui, Y.; Hirota, Y.; Saito-Fujita, T.; Aikawa, S.; Hiraoka, T.; Kaku, T.; Hirata, T.; Akaeda, S.; Matsuo, M.; Shimizu-Hirota, R.; et al. Uterine Epithelial LIF Receptors Contribute to Implantation Chamber Formation in Blastocyst Attachment. Endocrinology 2021, 162, bqab169. [Google Scholar] [CrossRef]
- Izvolskaia, M.; Sharova, V.; Zakharova, L. Prenatal Programming of Neuroendocrine System Development by Lipopolysaccharide: Long-Term Effects. Int. J. Mol. Sci. 2018, 19, 3695. [Google Scholar] [CrossRef]
- Sanchez-Lopez, J.A.; Caballero, I.; Montazeri, M.; Maslehat, N.; Elliott, S.; Fernandez-Gonzalez, R.; Calle, A.; Gutierrez-Adan, A.; Fazeli, A. Local activation of uterine Toll-like receptor 2 and 2/6 decreases embryo implantation and affects uterine receptivity in mice. Biol. Reprod. 2014, 90, 87. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ma, W.G.; Daikoku, T.; Zhao, X.; Paria, B.C.; Das, S.K.; Trzaskos, J.M.; Dey, S.K. Cyclooxygenase-2 differentially directs uterine angiogenesis during implantation in mice. J. Biol. Chem. 2002, 277, 29260–29267. [Google Scholar] [CrossRef]
- Gonzalez Lopez, R.; Contreras Caro Del Castillo, D.A.; Valdez Magana, G.; Sarmiento Silva, R.E.; Martinez Castaneda, F.E.; Trujillo Ortega, M.E. Expression and localization of vascular endothelial growth factor and its receptors in the pig uterus during peri-implantation and determination of the in vitro effect of the angiogenesis inhibitor SU5416 on VEGF system expression. Theriogenology 2023, 207, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Saito, Y.; Suzuki, T.; Imbaby, S.; Hattori, K.; Matsuda, N.; Hattori, Y. Vascular endothelial growth factor contributes to lung vascular hyperpermeability in sepsis-associated acute lung injury. Naunyn Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2365–2374. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, X.; Zeng, W.; Wu, F.; Wei, X.; Li, Q.; Lin, Y. DOCK1 deficiency drives placental trophoblast cell dysfunction by influencing inflammation and oxidative stress, hallmarks of preeclampsia. Hypertens. Res. 2024, 47, 3434–3446. [Google Scholar] [CrossRef]
- Rintala, S.E.; Savikko, J.; Rintala, J.M.; von Willebrand, E. Vascular endothelial growth factor (VEGF) ligand and receptor induction in rat renal allograft rejection. Transplant. Proc. 2006, 38, 3236–3238. [Google Scholar] [CrossRef] [PubMed]
- Ozden Tokalioglu, E.; Turgut, E.; Gulen Yildiz, E.; Ozturk Agaoglu, M.; Biriken, D.; Tanacan, A.; Yazihan, N.; Sahin, D. Comparison of VEGF-A levels in women with threatened abortion, early pregnancy loss and uncomplicated healthy pregnancies. Cytokine 2023, 170, 156343. [Google Scholar] [CrossRef]
- Lashkari, B.S.; Shahana, S.; Anumba, D.O. Toll-like receptor 2 and 4 expression in the pregnant and non-pregnant human uterine cervix. J. Reprod. Immunol. 2015, 107, 43–51. [Google Scholar] [CrossRef]
- Botero, T.M.; Shelburne, C.E.; Holland, G.R.; Hanks, C.T.; Nor, J.E. TLR4 mediates LPS-induced VEGF expression in odontoblasts. J. Endod. 2006, 32, 951–955. [Google Scholar] [CrossRef]
- Ghosh, C.; Bishayi, B. Characterization of Toll-like receptor-4 (TLR-4) in the spleen and thymus of Swiss albino mice and its modulation in experimental endotoxemia. J. Immunol. Res. 2015, 2015, 137981. [Google Scholar] [CrossRef]
- Dirandeh, E.; Sayyar, M.A.; Ansari-Pirsaraei, Z.; Deldar, H.; Thatcher, W.W. Peripheral leucocyte molecular indicators of inflammation and oxidative stress are altered in dairy cows with embryonic loss. Sci. Rep. 2021, 11, 12771. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lv, W.; Jia, J.; Wang, J.; Yang, J. Evaluation of serum concentrations of tumor necrosis factor (TNF)-α, interleukin (IL)-2, IL-10, and nitric oxide (NO) during the estrous cycle, early pregnancy and abortion in goats. Anim. Reprod. Sci. 2016, 174, 73–79. [Google Scholar] [CrossRef]
- Luan, X.; Zhai, J.; Li, S.; Du, Y. Downregulation of FHL2 suppressed trophoblast migration, invasion and epithelial-mesenchymal transition in recurrent miscarriage. Reprod. Biomed. Online 2024, 48, 103342. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wang, L.; Huang, Z.; Guan, H.; Leung, W.; Chen, X.; Xia, H.; Zhang, W. CD55 is upregulated by cAMP/PKA/AKT and modulates human decidualization via Src and ERK pathway and decidualization-related genes. Mol. Reprod. Dev. 2022, 89, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, Z.; Fei, F.; Jiang, Y.; Jiang, Y.; Guo, L.; Liu, K.; Cui, L.; Meng, X.; Li, J.; et al. Selenium Enhances the Growth of Bovine Endometrial Stromal Cells by PI3K/AKT/GSK-3beta and Wnt/beta-Catenin Pathways. Vet. Sci. 2024, 11, 674. [Google Scholar] [CrossRef]
- Chi, H.; Barry, S.P.; Roth, R.J.; Wu, J.J.; Jones, E.A.; Bennett, A.M.; Flavell, R.A. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 2274–2279. [Google Scholar] [CrossRef]
- Yang, N.; Wang, H.; Zhang, R.; Niu, Z.; Zheng, S.; Zhang, Z. C/EBP beta Mediates the Aberrant Inflammatory Response and Cell Cycle Arrest in Lps-stimulated Human Renal Tubular Epithelial Cells by Regulating NF-kappaB Pathway. Arch. Med. Res. 2021, 52, 603–610. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.M.; Li, X.; Lv, C.J.; Peng, L.Y.; Yu, X.F.; Song, Y.J.; Wang, C.J. Pterostilbene pre-treatment reduces LPS-induced acute lung injury through activating NR4A1. Pharm. Biol. 2022, 60, 394–403. [Google Scholar] [CrossRef]
- Su, C.M.; Lee, W.H.; Wu, A.T.; Lin, Y.K.; Wang, L.S.; Wu, C.H.; Yeh, C.T. Pterostilbene inhibits triple-negative breast cancer metastasis via inducing microRNA-205 expression and negatively modulates epithelial-to-mesenchymal transition. J. Nutr. Biochem. 2015, 26, 675–685. [Google Scholar] [CrossRef]
- Pei, H.L.; Mu, D.M.; Zhang, B. Anticancer Activity of Pterostilbene in Human Ovarian Cancer Cell Lines. Med. Sci. Monit. 2017, 23, 3192–3199. [Google Scholar] [CrossRef]
- Chavas, C.; Sapanidou, V.G.; Feidantsis, K.; Lavrentiadou, S.N.; Mavrogianni, D.; Zarogoulidou, I.; Fletouris, D.J.; Tsantarliotou, M.P. Treatment with Pterostilbene Ameliorates the Antioxidant Status of Bovine Spermatozoa and Modulates Cell Death Pathways. Antioxidants 2024, 13, 1437. [Google Scholar] [CrossRef] [PubMed]
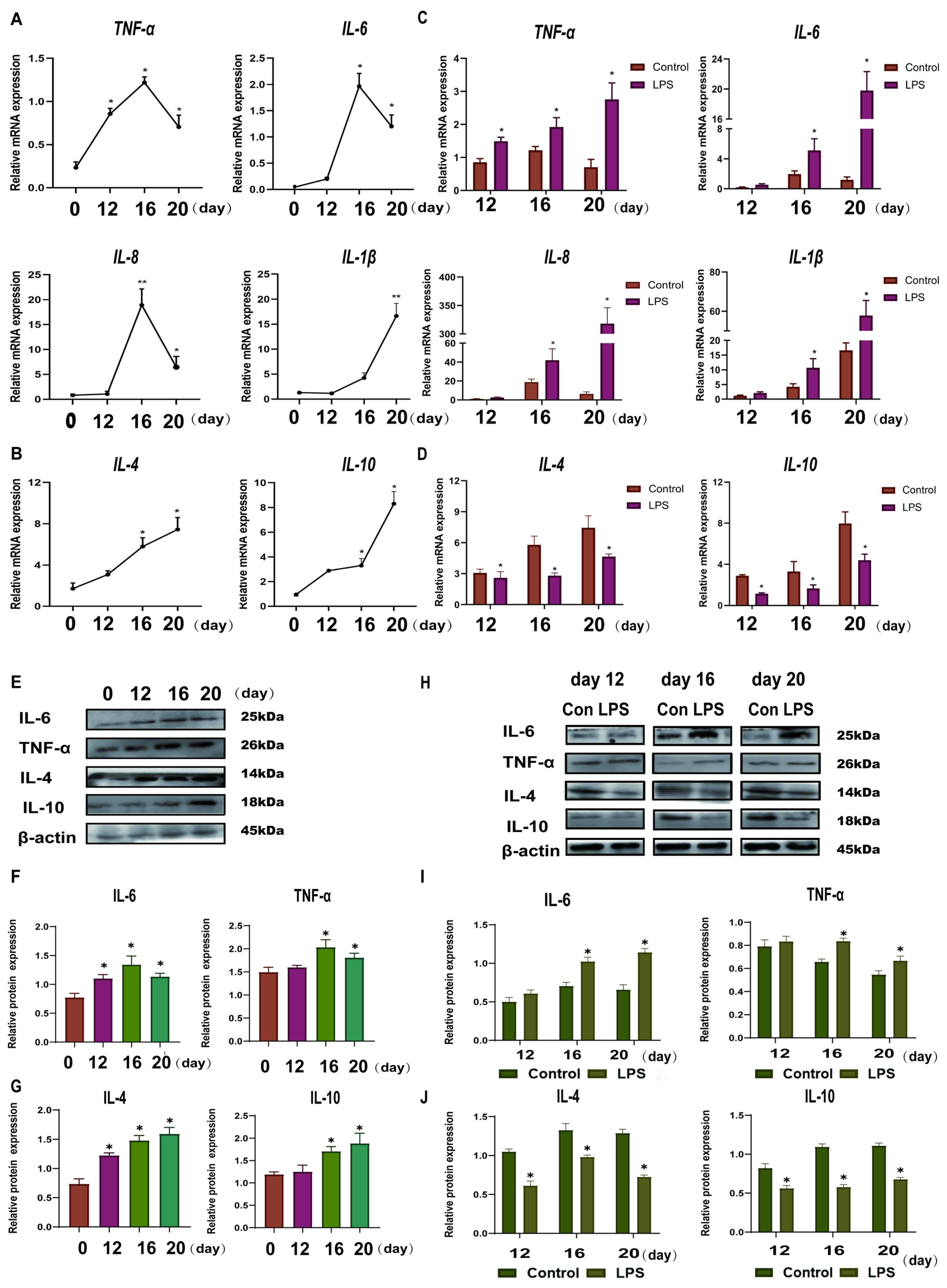
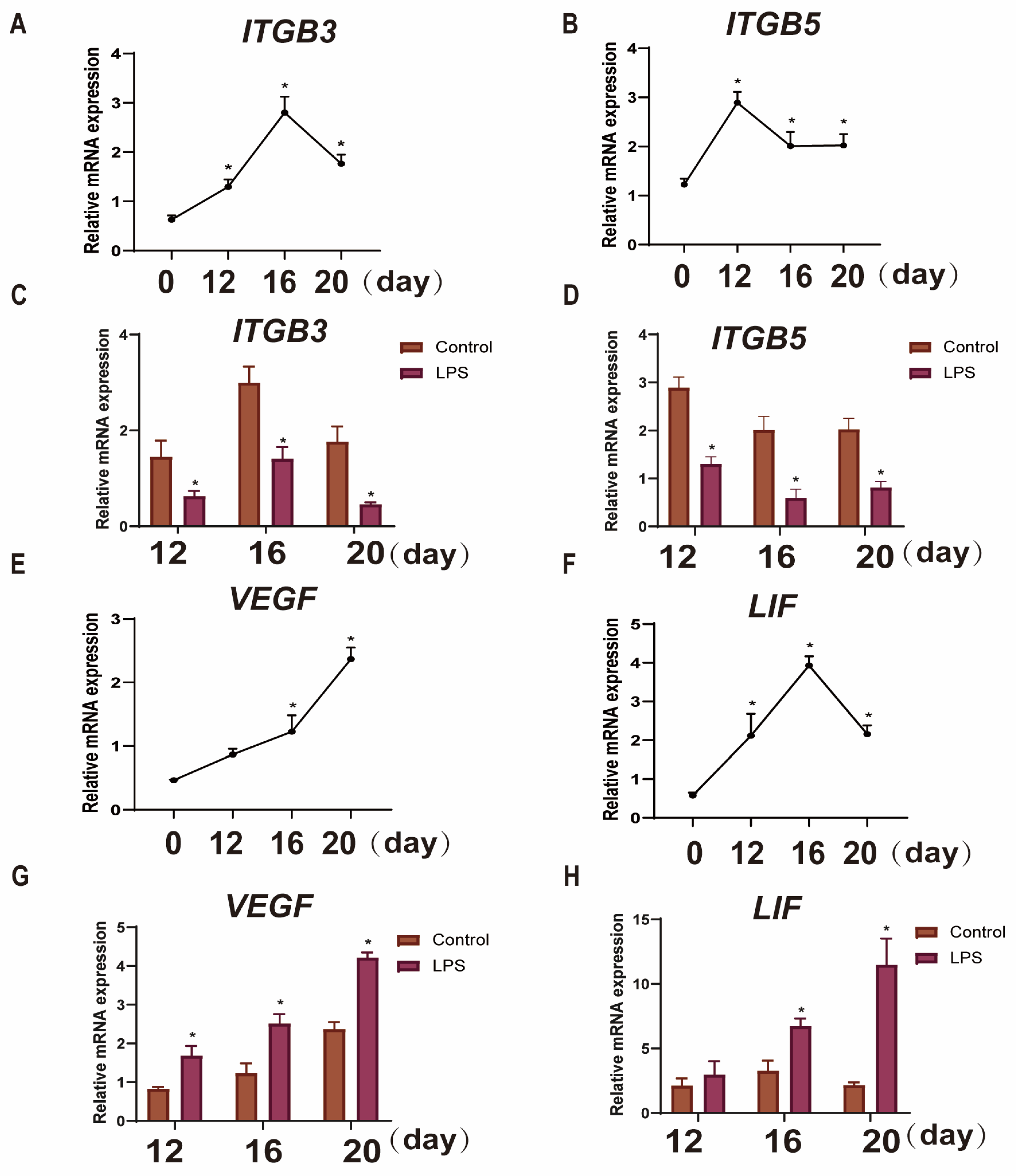
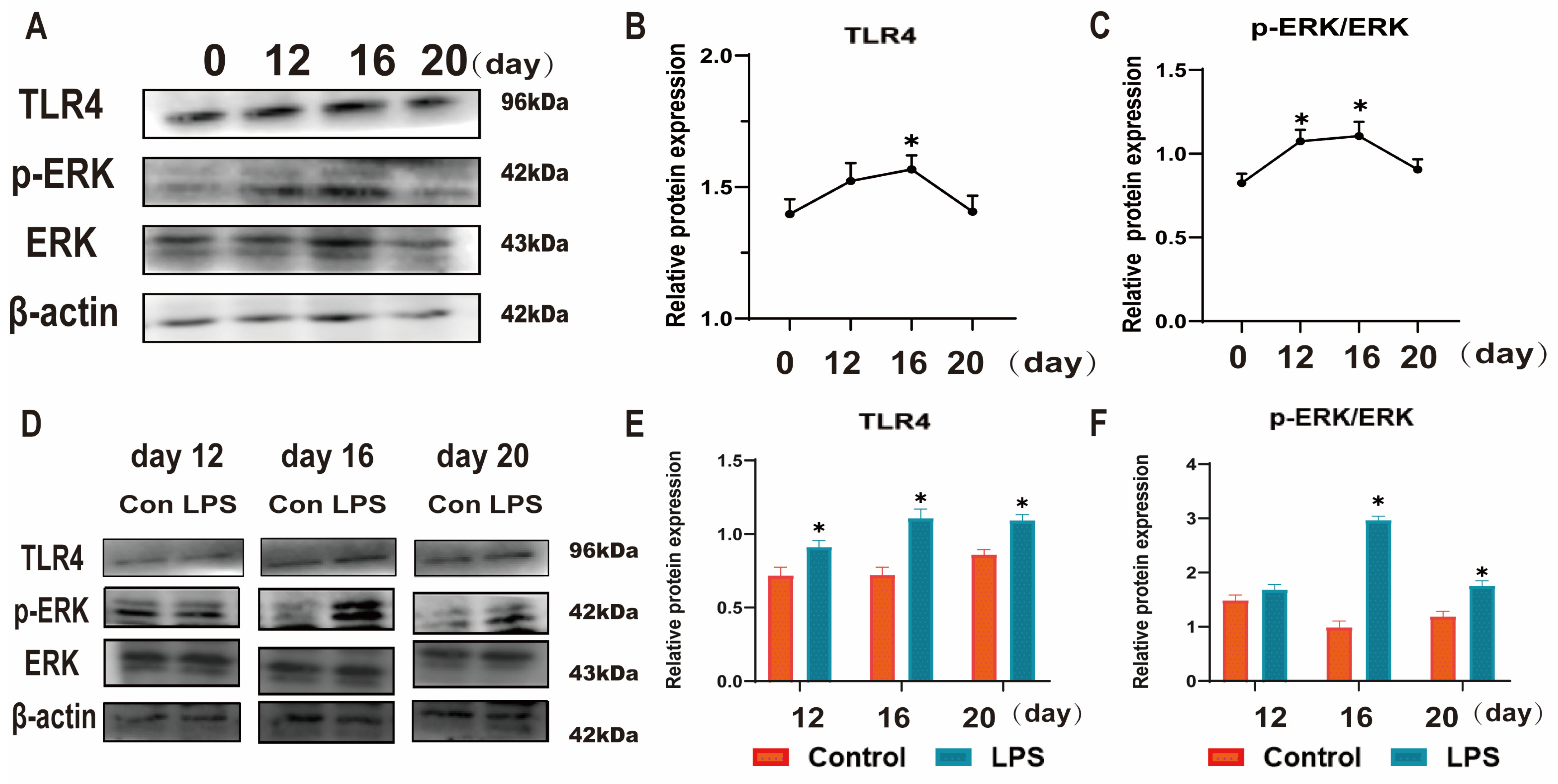


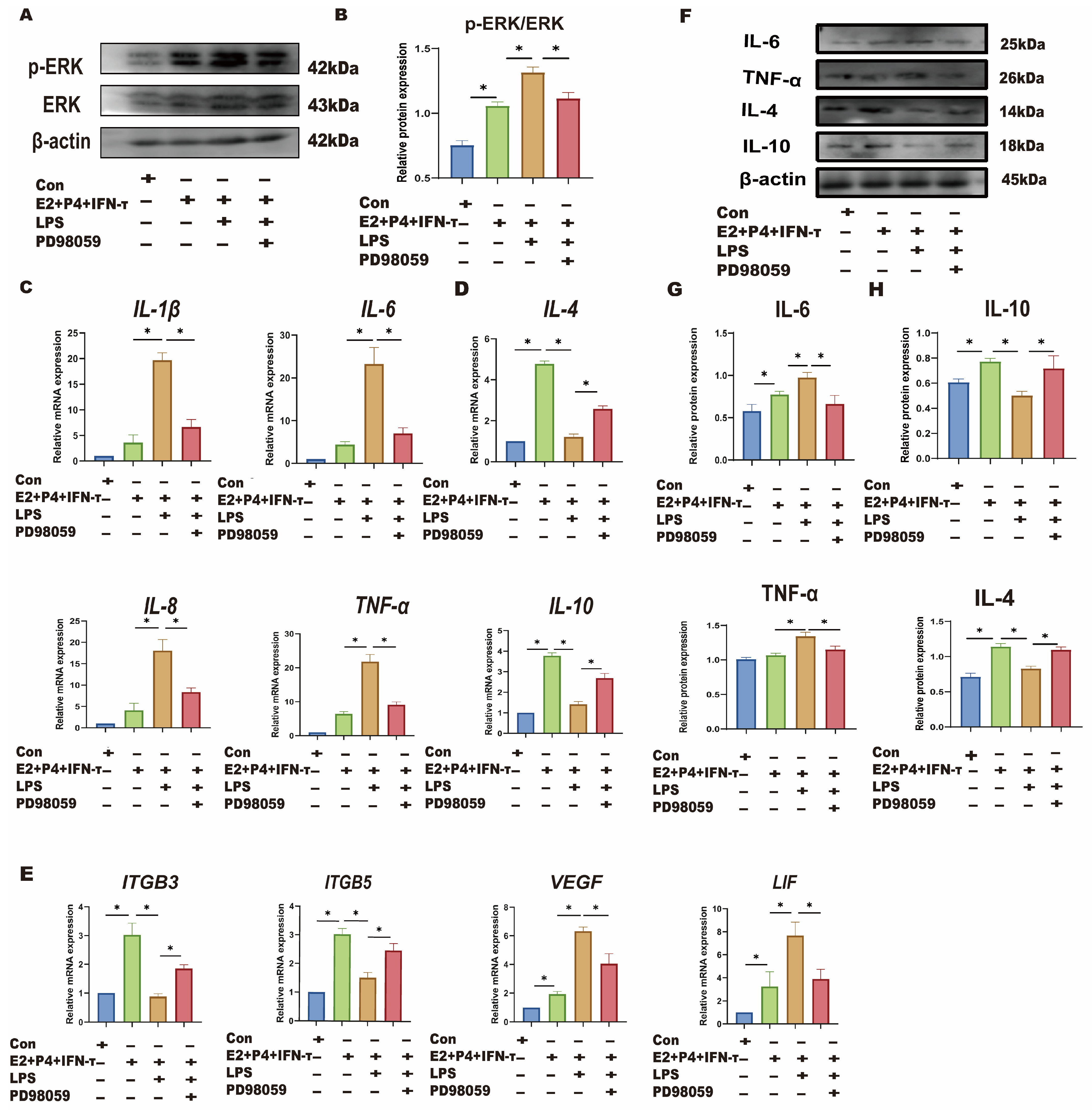

| Gene | Accession Number | Forward Primer Sequence (5′-3′) | Tm | Reverse Primer Sequence (5′-3′) | Tm | Product Length (bp) |
|---|---|---|---|---|---|---|
| IL-1β | NM_001009465.2 | F: AGCCGAGAAGTGGTGTTCTG | 59.97 | R:TGGCCACCTCTAAAACGTCC | 59.96 | 314 |
| TNF-α | NM_001024860.1 | F:AACAGGCCTCTGGTTCAGACA | 61.32 | R: CCATGAGGGCATTGGCATAC | 59.04 | 133 |
| IL-6 | NM_001009392.1 | F: GACACCACCCCAAGCAGACTA | 61.72 | R: TGCCAGTGTCTCCTTGCTGTT | 62.2 | 144 |
| IL-4 | NM_001009313.3 | F: ATCATCGGCATTTTGAACGAGG | 60.23 | R: TGCAGCTCCATGAGAACACTA | 61.67 | 129 |
| IL-8 | NM_001009401.2 | F: TCCTGCTCTCTGCAGCTCTGT | 63.24 | R: GGGTGGAAAGGTGTGGAATG | 58.74 | 100 |
| IL-10 | NM_001009327.1 | F: TGCTGGATGACTTTAAGGG | 56.97 | R: AGGGCAGAAAACGATGACA | 59.34 | 184 |
| ITGB3 | XM_060395753.1 | F: GGTGGTCTTGCTATCCGTGA | 59.46 | R: GTTGTTGGCAGTGTCCCATG | 59.96 | 151 |
| ITGB5 | XM_027956587.3 | F:GGGTCCGATGTCATCCAGC | 60.23 | R: GAACGTAGTCTTGTCACCGGG | 60.76 | 72 |
| LIF | XM_012098046.5 | F:TATCGCATCATCGCGTACCT | 59.92 | R: ACATGGCTCACGTGGTACTT | 59.31 | 176 |
| VEGF | NM_001025110.1 | F: CATGGATGTCTACCAGCGCA | 60.18 | R: TGGGCACACACTCCAGACTT | 61.34 | 161 |
| GAPDH | NM_001190390.1 | F: CAAGTTCCACGGCACAGTCA | 60.80 | R: TGGTTCACGCCCATCACAA | 59.85 | 249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Fan, X.; Zang, X.; Guo, Y.; Jiang, W.; Qi, M.; Han, H.; Yao, Y. LPS Regulates Endometrial Immune Homeostasis and Receptivity Through the TLR4/ERK Pathway in Sheep. Animals 2025, 15, 1712. https://doi.org/10.3390/ani15121712
Wei J, Fan X, Zang X, Guo Y, Jiang W, Qi M, Han H, Yao Y. LPS Regulates Endometrial Immune Homeostasis and Receptivity Through the TLR4/ERK Pathway in Sheep. Animals. 2025; 15(12):1712. https://doi.org/10.3390/ani15121712
Chicago/Turabian StyleWei, Jinzi, Xing Fan, Xiaorui Zang, Yu Guo, Wenjie Jiang, Meiyu Qi, Hongbing Han, and Yuchang Yao. 2025. "LPS Regulates Endometrial Immune Homeostasis and Receptivity Through the TLR4/ERK Pathway in Sheep" Animals 15, no. 12: 1712. https://doi.org/10.3390/ani15121712
APA StyleWei, J., Fan, X., Zang, X., Guo, Y., Jiang, W., Qi, M., Han, H., & Yao, Y. (2025). LPS Regulates Endometrial Immune Homeostasis and Receptivity Through the TLR4/ERK Pathway in Sheep. Animals, 15(12), 1712. https://doi.org/10.3390/ani15121712





