Preliminary Study of CCR9 and MAdCAM-1 Upregulation and Immune Imbalance in Canine Chronic Enteropathy: Findings Based on Histopathological Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Animal Selection
2.2. Sample Collection
2.3. Quantification of Serum Cytokines and Chemokines
2.4. Gene Expression Analysis in Intestinal Tissue
2.5. Statistical Analysis
3. Results
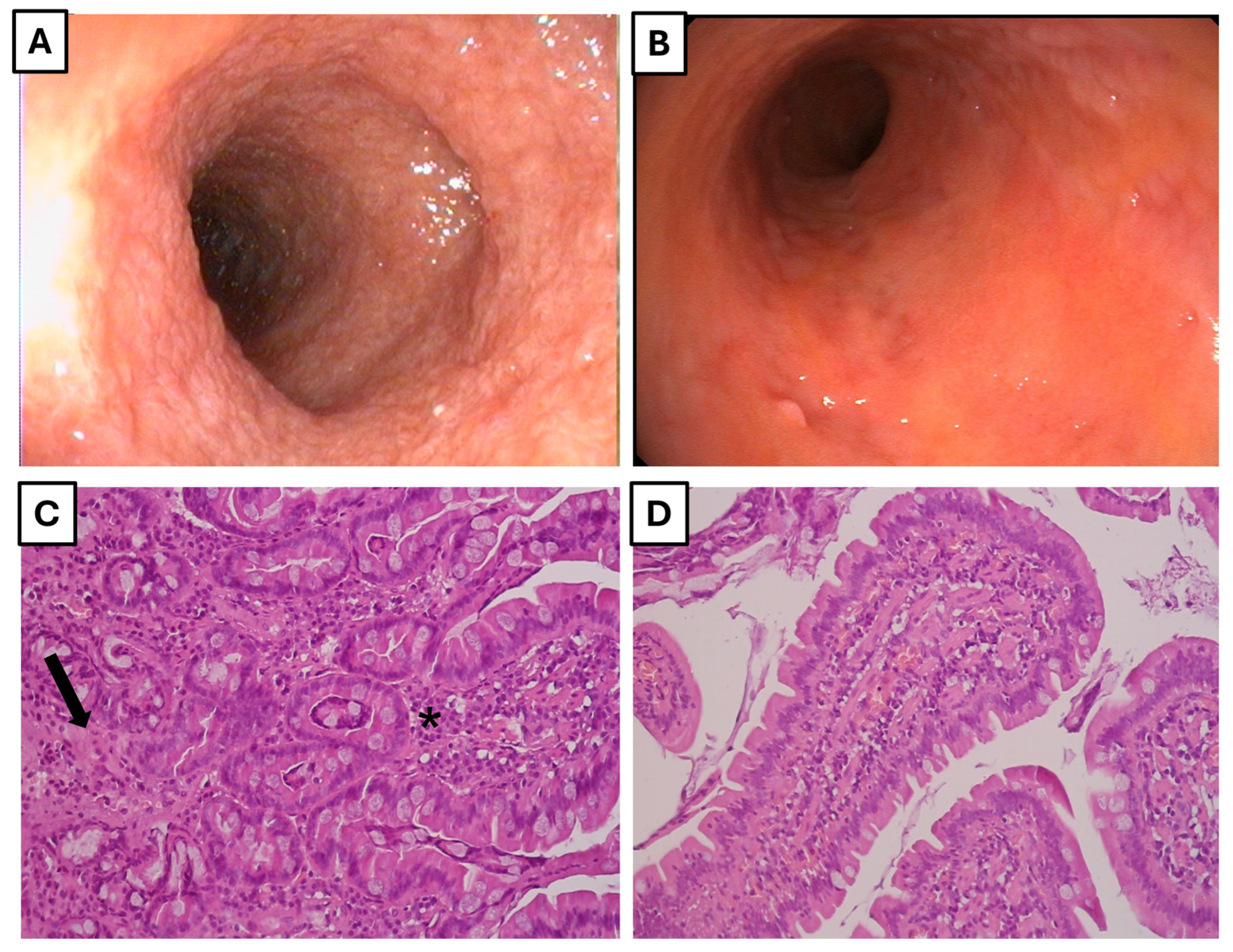
3.1. Study Population
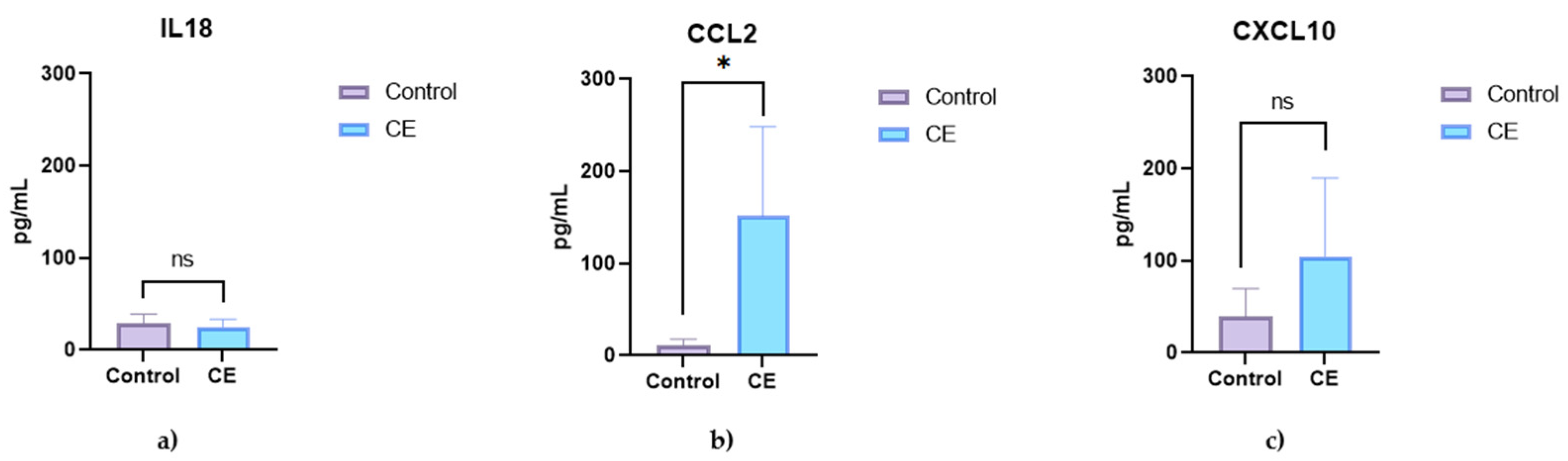
3.2. Serum Cytokine and Chemokine Concentrations
3.3. Intestinal Gene Expression of Immune Mediators
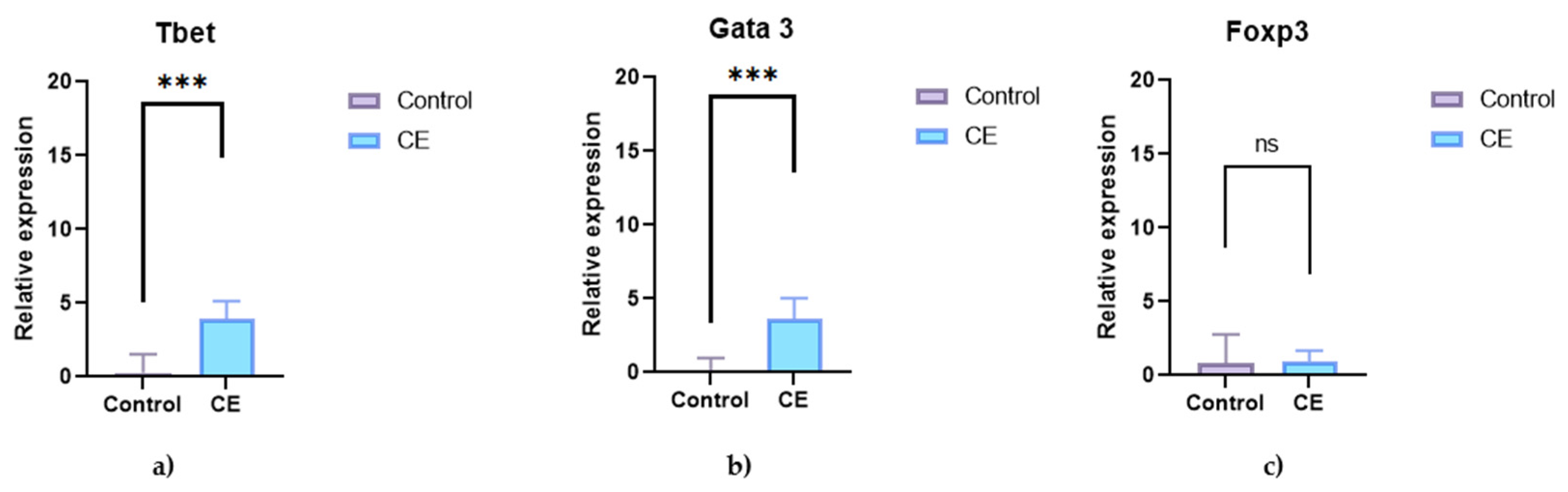
3.3.1. Transcription Factors in Intestinal Tissue
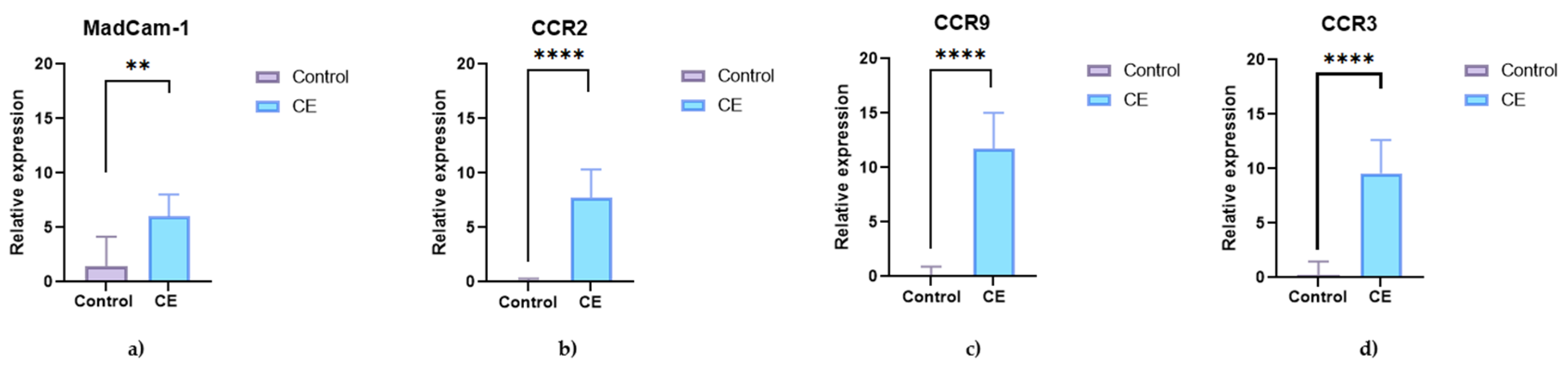
3.3.2. Expression of CCR9, CCR2, CCR3, and MAdCAM-1 in Duodenal Mucosa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| T Cell Response | mRNA Analyzed | Sequencing Primers |
|---|---|---|
| Th1 | Tbet F | AATCAGCACCAGACGGAGAT |
| Tbet R | GTCCACGAACATCCGGTAAT | |
| IFN-γ DogF1 | GCGCAAGGCGATAAATGAAC | |
| IFN-γ DogR1 | CTGACTCCTTTTCCGCTTCC | |
| Th2 | Gata 3 F | TACGTCCCCGAATACAGCTC |
| Gata 3 R | ACTCCCTGCCTTCTGTGCT | |
| Gata 3 F | CGAAGGCTGTCGGCAGCAAGAA | |
| Gata 3 R | ACGGGGTCTCCGTTGGCATT | |
| IL-4 DogF1 | TCACCAGCACCTTTGTCCAC | |
| IL-4 DogR1 | CGCTTGTGTTCTTTGGAGCA | |
| IL-17 DogF | GGAATCTGCACCGCAATGAGGAC | |
| IL-17 DogR | CGCAGAACCAGGATCTCTTGCTGG | |
| Treg | Foxp3 F | CAAATGGTGTCTGCAAGTGG |
| Foxp3 R | GTGCTCTGCCCTTCTCATCT | |
| IL-10 DogF1 | CGGGAGGGTGAAGACTTTCT | |
| IL-10 DogR1 | GGCATCACCTCCTCCAAGTA |
| Molecule | Sequence |
|---|---|
| α4β7 F receptor MAdCAM-1 F | CCCTACCAGCTCAGCAGAGGACA |
| α4β7 F receptor MAdCAM-1 R | ACCCGGGCTACACCCTCGTC |
| CCR9 F | CACTTCCTCCCACCCTTGTA |
| CCR9 R | TGGTCTTGACTCTGGTGCAG |
| CCR2 F | CGTGAAGCTCATTTTCGTGA |
| CCR2 R | GACTCCTGGAAGGTGCTCAG |
| CCR3 F | TCTTCCACGAGTCCCAAA AG |
| CCR3 R | GAGCAGAGGCAGAGCAAGAC |
| Animal Number | Breed | Age | Genre | Histopathological Severity |
|---|---|---|---|---|
| 1 | German shepherd | 3 years | Male | Severe |
| 2 | Mixed | 6 years | Female | Severe |
| 3 | French bulldog | 3 years | Male | Moderate |
| 4 | Labrador | 6 years | Female | Moderate |
| 5 | Mixed | 5 years | Male | No data |
| Animal Number | Breed | Age | Genre |
|---|---|---|---|
| 1 | Mixed | 7 years | Female |
| 2 | Mixed | 8 years | Female |
| 3 | Golden Retriever | 7 years | Male |
| 4 | Mixed | 5 years | Male |
| 5 | Mixed | 6 years | Male |
References
- Dandrieux, J.; Mansfield, C.S. Chronic Enteropathy in Canines: Prevalence, Impact and Management Strategies. Vet. Med. Res. Rep. 2019, 10, 203–214. [Google Scholar] [CrossRef]
- Junginger, J.; Lemensieck, F.; Moore, P.F.; Schwittlick, U.; Nolte, I.; Hewicker-Trautwein, M. Canine gut dendritic cells in the steady state and in inflammatory bowel disease. Innate Immun. 2014, 20, 145–160. [Google Scholar] [CrossRef]
- Procoli, F. Inflammatory Bowel Disease, Food-Responsive, Antibiotic-Responsive Diarrhoea, Protein Losing Enteropathy. Adv. Small Anim. Care 2020, 1, 127–414. [Google Scholar] [CrossRef]
- Dandrieux, J.R.S. Inflammatory Bowel Disease versus Chronic Enteropathy in Dogs: Are They One and the Same? J. Small Anim. Pract. 2016, 57, 589–599. [Google Scholar] [CrossRef]
- Cerquetella, M.; Rossi, G.; Suchodolski, J.S.; Schmitz, S.S.; Allenspach, K.; Rodríguez-Franco, F.; Furlanello, T.; Gavazza, A.; Marchegiani, A.; Unterer, S.; et al. Proposal for Rational Antibacterial Use in the Diagnosis and Treatment of Dogs with Chronic Diarrhoea. J. Small Anim. Pract. 2020, 61, 211–215. [Google Scholar] [CrossRef]
- Dupouy-Manescau, N.; Méric, T.; Sénécat, O.; Drut, A.; Valentin, S.; Leal, R.O.; Hernandez, J. Updating the Classification of Chronic Inflammatory Enteropathies in Dogs. Animals 2024, 14, 681. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef]
- Garzía-Sancho, M.; Rodríguez-Franco, F.; Sainz, A.; Mancho, C.; Rodríguez, A. Evaluation of Clinical, Macroscopic, and Histopathologic Response to Treatment in Nonhypoproteinemic Dogs with Lymphocytic-Plasmacytic Enteritis. J. Vet. Intern. Med. 2007, 21, 11–17. [Google Scholar] [CrossRef]
- German, A.J.; Helps, C.R.; Hall, E.J.; Day, M.J. Cytokine mRNA Expression in Mucosal Biopsies from German Shepherd Dogs with Small Intestinal Enteropathies. Dig. Dis. Sci. 2000, 45, 7–17. [Google Scholar] [CrossRef]
- Jergens, A.E. Inflammatory bowel disease in veterinary medicine. Front. Biosci. 2012, 4, 1404–1419. [Google Scholar] [CrossRef] [PubMed]
- Yogeshpriya, S.; Veeraselvam, M.; Krishnakumar, S.; Arulkumar, T.; Jayalakshmi, K.; Saravanan, M.; Ranjithkumar, M.; Sivakumar, M.; Selvaraj, P. Technical Review on Inflammatory Bowel Disease in dogs and cats. Int. J. Sci. Environ. Technol. 2017, 6, 1833–1842. [Google Scholar]
- Allenspach, K.; Wieland, B.; Gröne, A.; Gaschen, F. Chronic Enteropathies in Dogs: Evaluation of Risk Factors for Negative Outcome. Vet. Intern. Medicne 2007, 21, 700–708. [Google Scholar] [CrossRef]
- Jergens, A.E.; Heilmann, R.M. Canine Chronic Enteropathy—Current State-of-the-Art and Emerging Concepts. Front. Vet. Sci. 2022, 9, 923013. [Google Scholar] [CrossRef]
- Marchetti, V.; Gori, E.; Mariotti, V.; Gazzano, A.; Mariti, C. The Impact of Chronic Inflammatory Enteropathy on Dogs’ Quality of Life and Dog-Owner Relationship. Vet. Sci. 2021, 8, 166. [Google Scholar] [CrossRef]
- Allenspach, K.A.; Mochel, J.P.; Du, Y.; Priestnall, S.L.; Moore, F.; Slayter, M. Correlating Gastrointestinal Histopathologic Changes to Clinical Disease Activity in Dogs With Idiopathic Inflammatory Bowel Disease. Vet. Pathol. 2019, 56, 435–443. [Google Scholar] [CrossRef]
- Siel, D.; Beltrán, C.J.; Martínez, E.; Pino, M.; Vargas, N.; Salinas, A.; Pérez, O.; Pereira, I.; Ramírez-Toloza, G. Elucidating the Role of Innate and Adaptive Immune Responses in the Pathogenesis of Canine Chronic Inflammatory Enteropathy—A Search for Potential Biomarkers. Animals 2022, 12, 1645. [Google Scholar] [CrossRef]
- Tatiya-aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef]
- Jergens, A.E.; Sonea, I.M.; O’Connor, A.M.; Kauffman, L.K.; Grozdanic, S.D.; Ackermann, M.R.; Evans, R.B. Intestinal Cytokine mRNA Expression in Canine Inflammatory Bowel Disease: A Meta-Analysis with Critical Appraisal. Comp. Med. 2009, 59, 153–162. [Google Scholar]
- Heilmann, R.M.; Suchodolski, J.S. Is Inflammatory Bowel Disease in Dogs and Cats Associated with a Th1 or Th2 Polarization? Vet. Immunol. Immunopathol. 2015, 168, 131–134. [Google Scholar] [CrossRef]
- Ohta, H.; Takada, K.; Sunden, Y.; Tamura, Y.; Osuga, T.; Lim, S.Y.; Murakami, M.; Sasaki, N.; Wickramasekara Rajapakshage, B.K.; Nakamura, K.; et al. CD4+ T Cell Cytokine Gene and Protein Expression in Duodenal Mucosa of Dogs with Inflammatory Bowel Disease. J. Vet. Med. Sci. 2014, 76, 409–414. [Google Scholar] [CrossRef]
- Schmitz, S.; Garden, O.; Werling, D.; Allenspach, K. Gene Expression of Selected Signature Cytokines of T Cell Subsets in Duodenal Tissues of Dogs with and without Inflammatory Bowel Disease. Vet. Immunol. Immunopathol. 2012, 146, 87–91. [Google Scholar] [CrossRef]
- Tamura, Y.; Ohta, H.; Yokoyama, N.; Lim, S.Y.; Osuga, T.; Morishita, K.; Nakamura, K.; Yamasaki, M.; Takiguchi, M. Evaluation of Selected Cytokine Gene Expression in Colonic Mucosa from Dogs with Idiopathic Lymphocytic-Plasmacytic Colitis. J. Vet. Med. Sci. 2014, 76, 1407–1410. [Google Scholar] [CrossRef]
- Marafini, I.; Sedda, S.; Dinallo, V.; Monteleone, G. Inflammatory cytokines: From discoveries to therapies in IBD. Expert Opin. Biol. Ther. 2019, 19, 1207–1217. [Google Scholar] [CrossRef]
- Kaga, C.; Kakiyama, S.; Hokkyo, A.; Ogata, Y.; Shibata, J.; Nagahara, T.; Nakazawa, M.; Nakagawa, T.; Tsujimoto, H.; Chambers, J.K.; et al. Characterization of Faecal Microbiota and Serum Inflammatory Markers in Dogs Diagnosed with Chronic Enteropathy or Small-Cell Lymphoma: A Pilot Study. Sci. Rep. 2024, 14, 19387. [Google Scholar] [CrossRef]
- Mendes, V.; Galvão, I.; Vieira, A.T. Mechanisms by Which the Gut Microbiota Influences Cytokine Production and Modulates Host Inflammatory Responses. J. Interferon Cytokine Res. 2019, 39, 393–409. [Google Scholar] [CrossRef]
- Kulkarni, N.; Pathak, M.; Lal, G. Role of chemokine receptors and intestinal epithelial cells in the mucosal inflammation and tolerance. J. Leukoc. Biol. 2017, 101, 377–394. [Google Scholar] [CrossRef]
- Maeda, S.; Ohno, K.; Nakamura, K.; Uchida, K.; Nakashima, K.; Fukushima, K.; Tsukamoto, A.; Goto-Koshino, Y.; Fujino, Y.; Tsujimoto, H. Mucosal Imbalance of Interleukin-1β and Interleukin-1 Receptor Antagonist in Canine Inflammatory Bowel Disease. Vet. J. 2012, 194, 66–70. [Google Scholar] [CrossRef]
- Maeda, S.; Ohno, K.; Uchida, K.; Nakashima, K.; Fukushima, K.; Tsukamoto, A.; Nakajima, M.; Fujino, Y.; Tsujimoto, H. Decreased Immunoglobulin A Concentrations in Feces, Duodenum, and Peripheral Blood Mononuclear Cells of Dogs with Inflammatory Bowel Disease. Vet. Intern. Medicne 2013, 27, 47–55. [Google Scholar] [CrossRef]
- Silva, F.; Rodrigues, B.L.; Ayrizono, M.L.; Leal, R.F. The Immunological Basis of Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2016, 2016, 2097274. [Google Scholar] [CrossRef]
- Konstantinidis, A.O.; Adamama-Moraitou, K.K.; Pardali, D.; Dovas, C.I.; Brellou, G.D.; Papadopoulos, T.; Jergens, A.E.; Allenspach, K.; Rallis, T.S. Colonic Mucosal and Cytobrush Sample Cytokine mRNA Expression in Canine Inflammatory Bowel Disease and Their Correlation with Disease Activity, Endoscopic and Histopathologic Score. PLoS ONE 2021, 16, e0245713. [Google Scholar] [CrossRef] [PubMed]
- Agulla, B.; Villaescusa, A.; Sainz, Á.; Díaz-Regañón, D.; Rodríguez-Franco, F.; Calleja-Bueno, L.; Olmeda, P.; García-Sancho, M. Peripheral and Intestinal T Lymphocyte Subsets in Dogs with Chronic Inflammatory Enteropathy. Vet. Intern. Medicne 2024, 38, 1437–1448. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino, M.; Ramirez, G.; Beltrán, C.; Martinez, E.; Pereira, I.; Villegas, J.; Cifuentes, F.; Siel, D. Preliminary Study of CCR9 and MAdCAM-1 Upregulation and Immune Imbalance in Canine Chronic Enteropathy: Findings Based on Histopathological Analysis. Animals 2025, 15, 1710. https://doi.org/10.3390/ani15121710
Pino M, Ramirez G, Beltrán C, Martinez E, Pereira I, Villegas J, Cifuentes F, Siel D. Preliminary Study of CCR9 and MAdCAM-1 Upregulation and Immune Imbalance in Canine Chronic Enteropathy: Findings Based on Histopathological Analysis. Animals. 2025; 15(12):1710. https://doi.org/10.3390/ani15121710
Chicago/Turabian StylePino, Macarena, Galia Ramirez, Caroll Beltrán, Eduard Martinez, Ismael Pereira, Jaime Villegas, Federico Cifuentes, and Daniela Siel. 2025. "Preliminary Study of CCR9 and MAdCAM-1 Upregulation and Immune Imbalance in Canine Chronic Enteropathy: Findings Based on Histopathological Analysis" Animals 15, no. 12: 1710. https://doi.org/10.3390/ani15121710
APA StylePino, M., Ramirez, G., Beltrán, C., Martinez, E., Pereira, I., Villegas, J., Cifuentes, F., & Siel, D. (2025). Preliminary Study of CCR9 and MAdCAM-1 Upregulation and Immune Imbalance in Canine Chronic Enteropathy: Findings Based on Histopathological Analysis. Animals, 15(12), 1710. https://doi.org/10.3390/ani15121710








