Transcriptomic Profiling Reveals Lysine-Mediated Proliferative Mechanisms in Mongolian Horse Myogenic Satellite Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Source and Preservation
2.2. Primary Culture and Preservation of Equine Muscle Satellite Cells
2.3. Cell Thawing
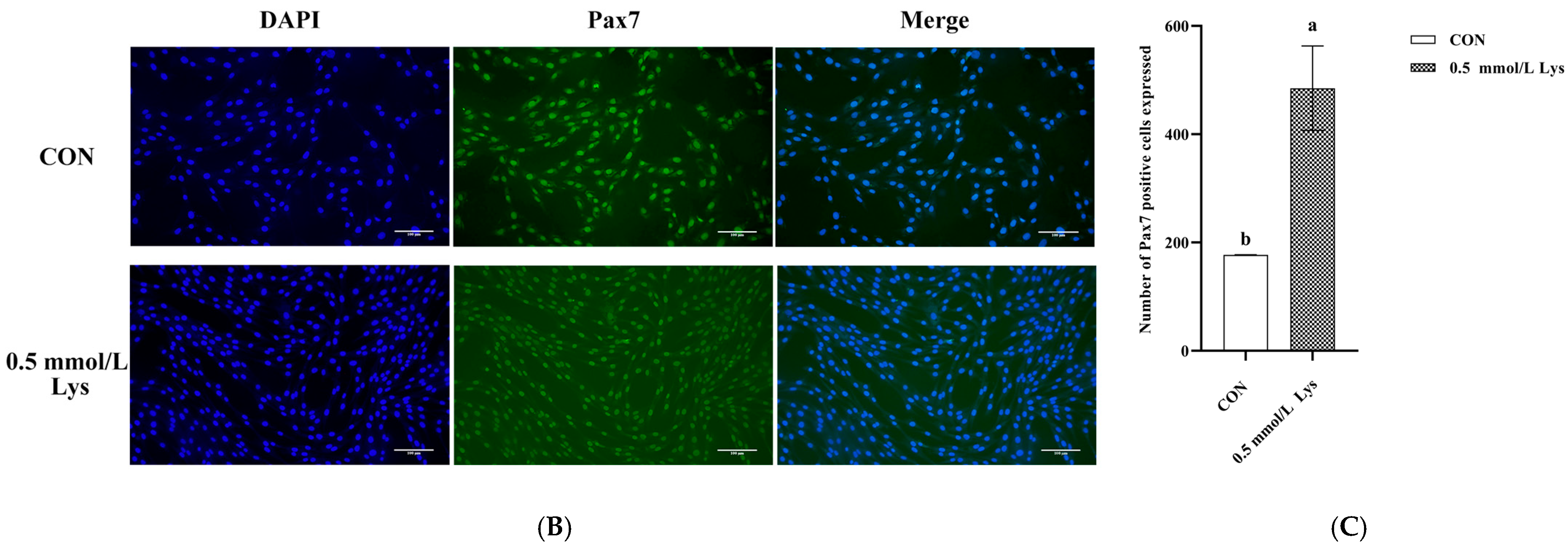
2.4. Immunofluorescence
2.5. Experimental Design
2.6. RNA Extraction
2.7. Library Construction
2.8. Real-Time Fluorescence Quantitative PCR
2.9. GO and KEGG Enrichment Analysis of Dif-mRNA, Dif-lncRNA, Dif-circRNA, and Dif-miRNAs
2.10. Competing Endogenous RNA (ceRNA) Network Analysis
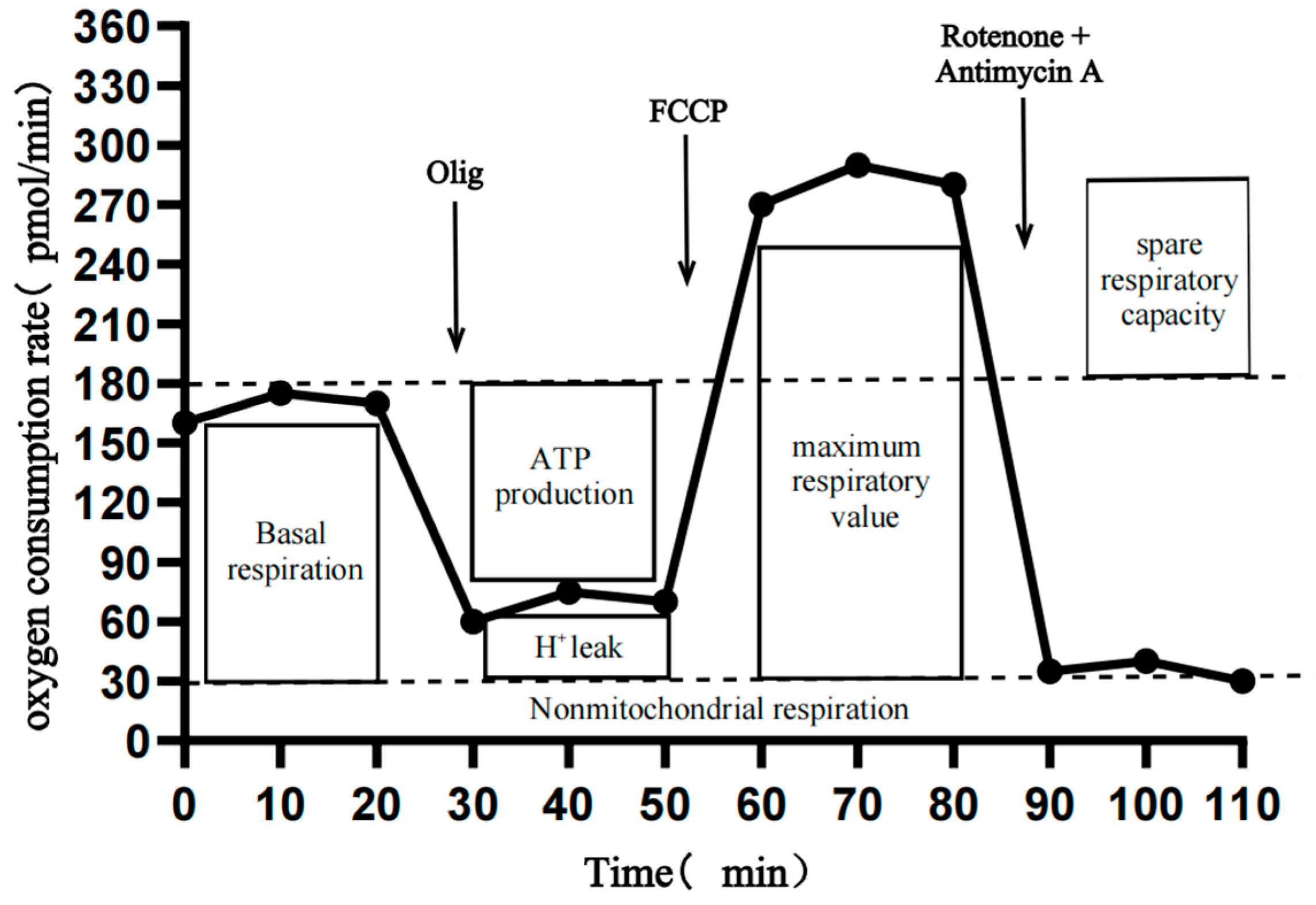
2.11. Mitochondrial Stress Detection
3. Results
3.1. Effect of Lysine on the Viability of Mongolian Horse Myosatellite Cells
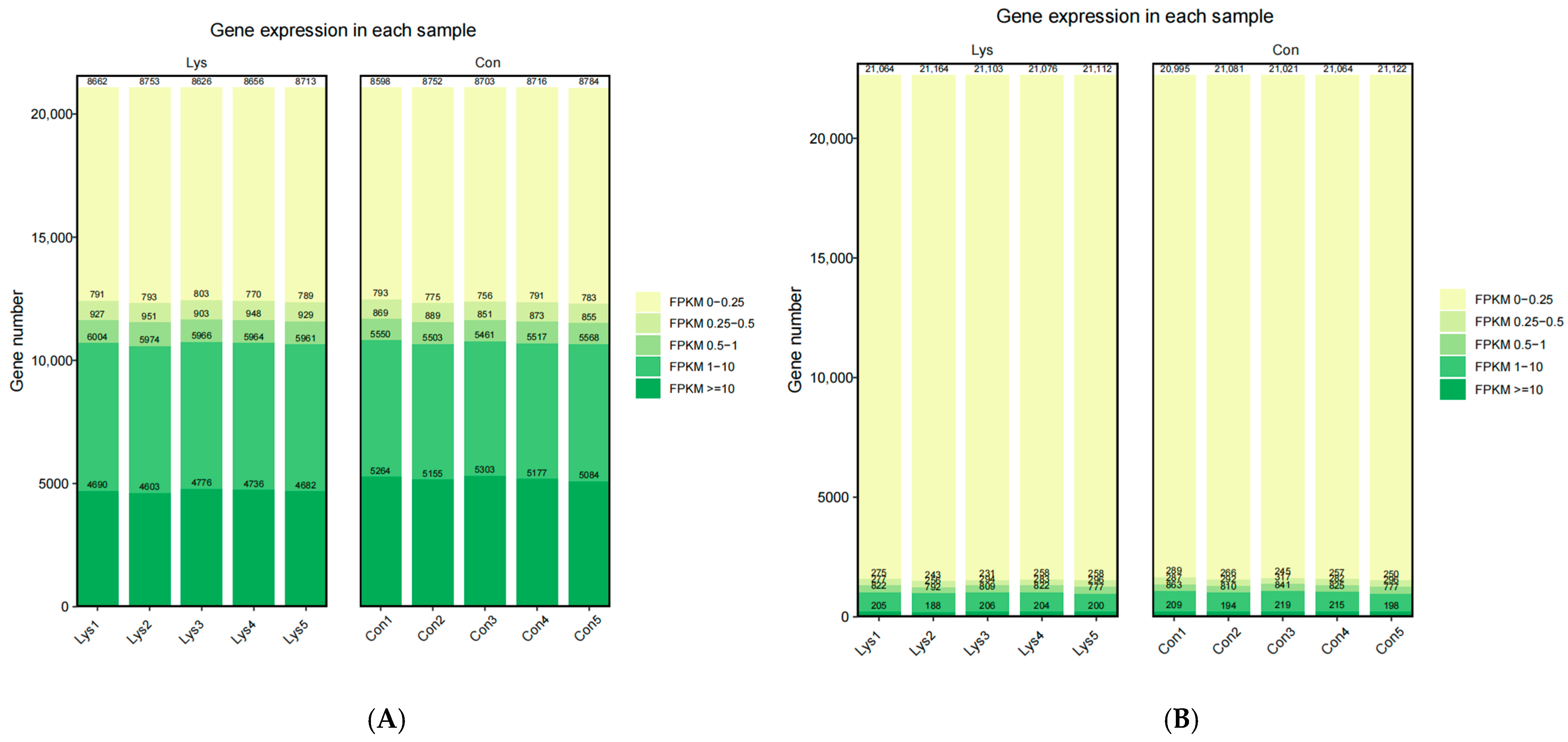
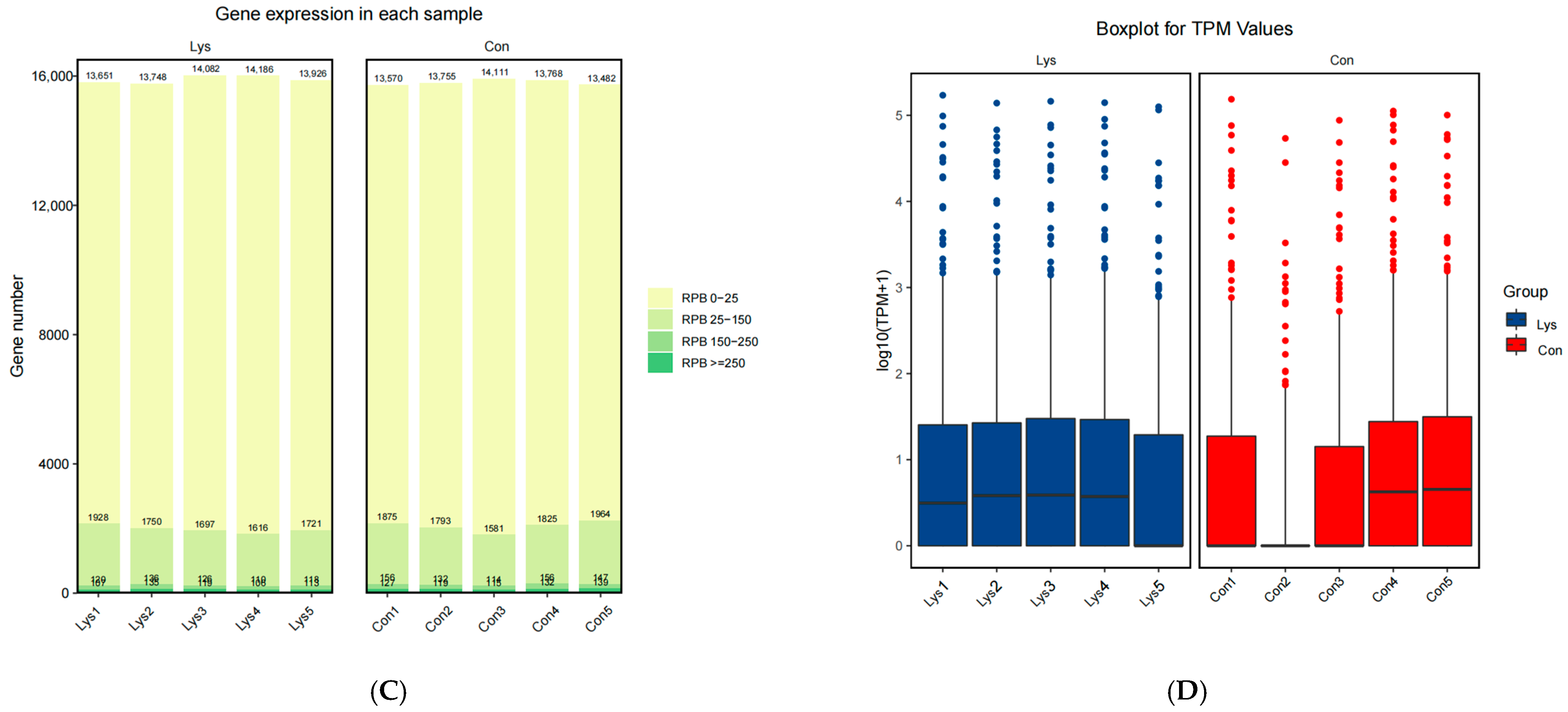
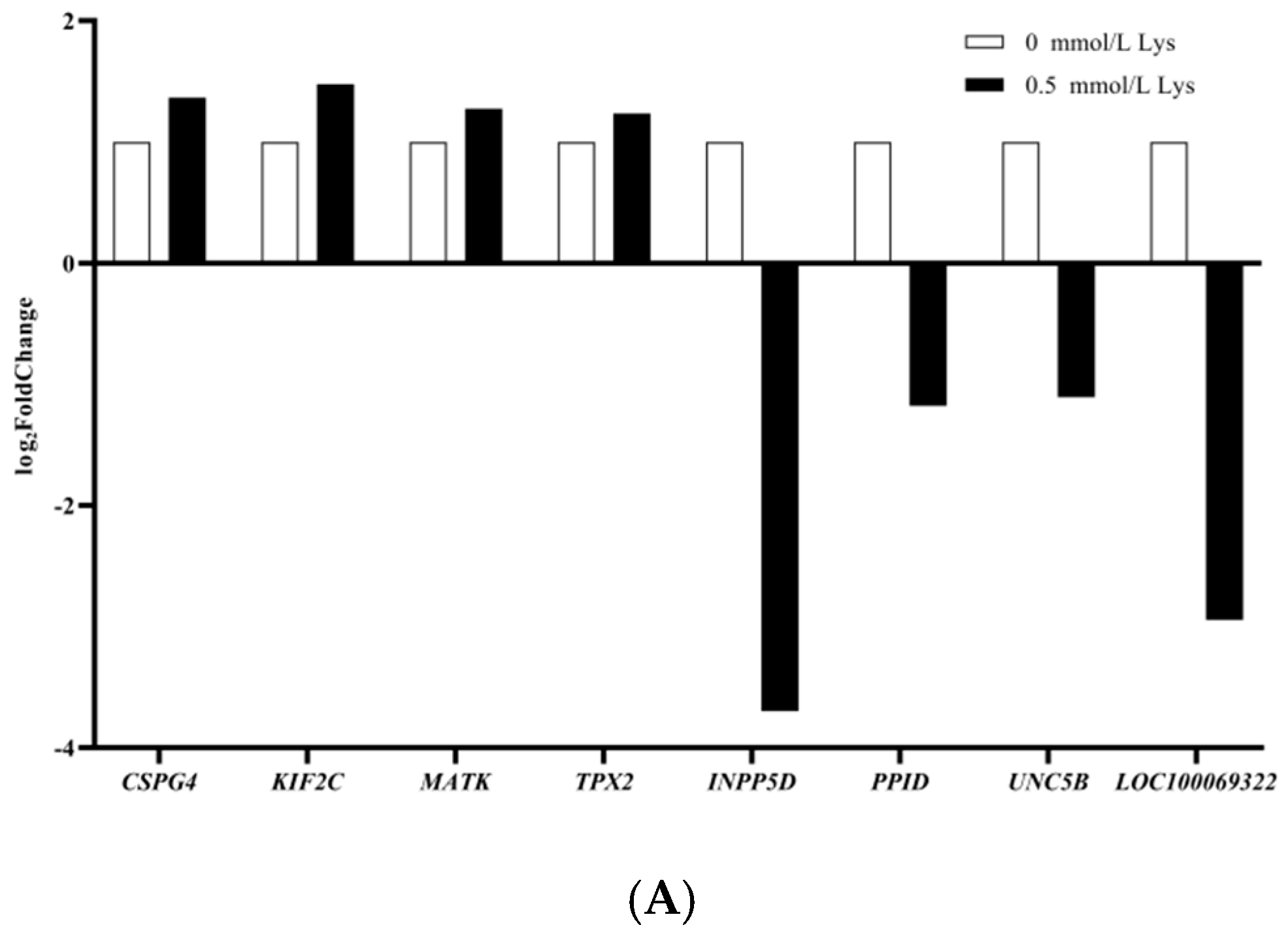
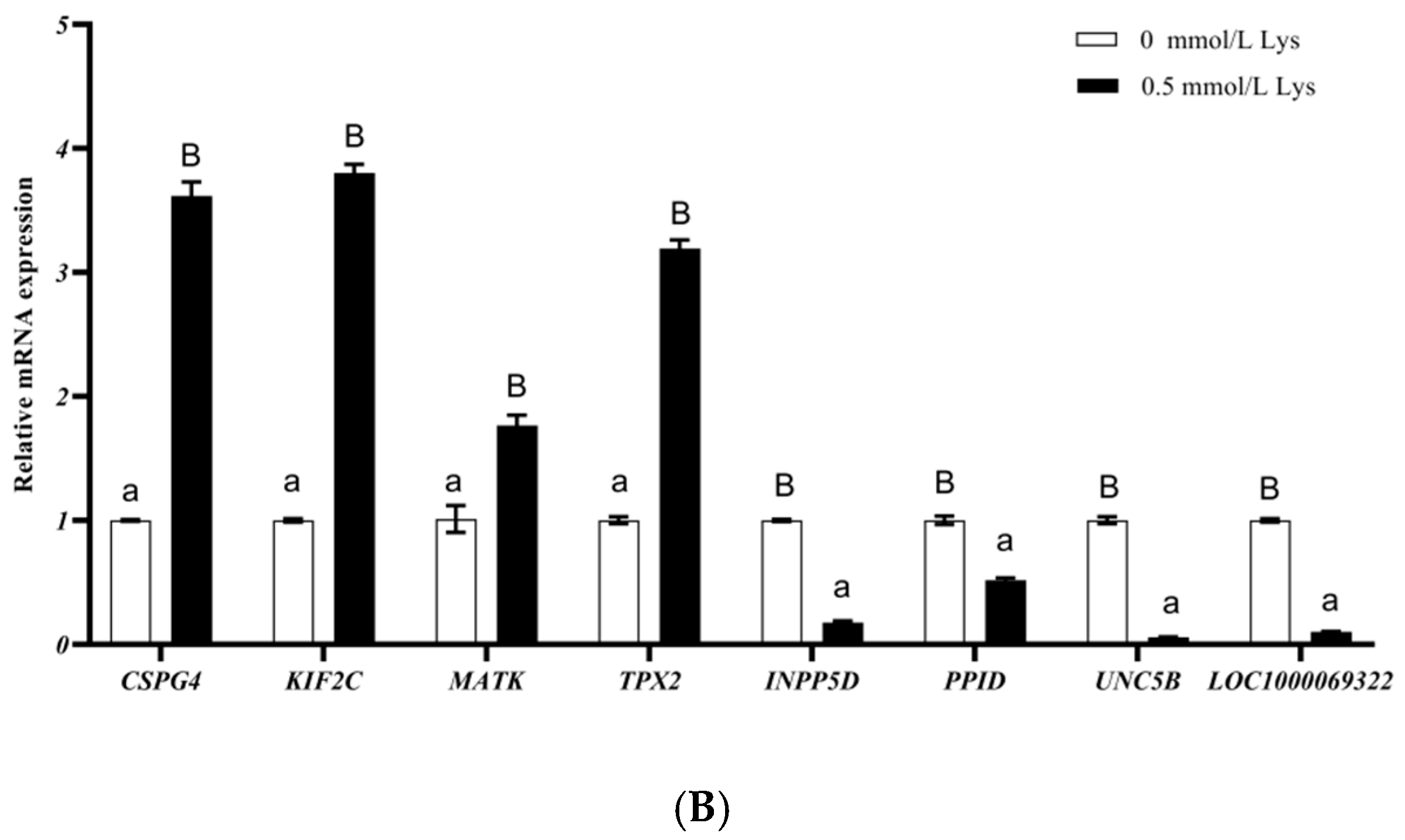
3.2. Lysine Mediates Differential Gene Expression in Mongolian Horse Myosatellite Cells
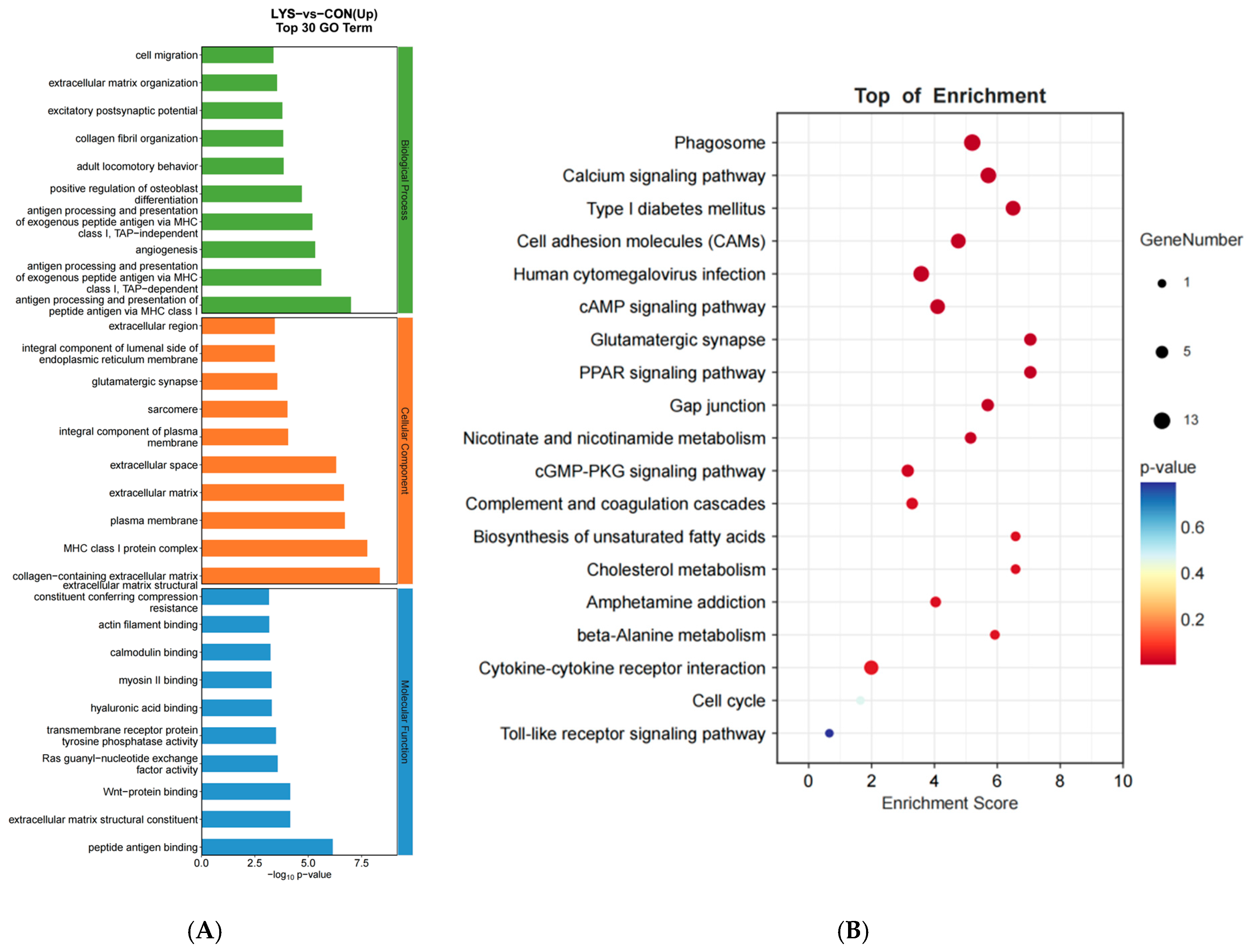
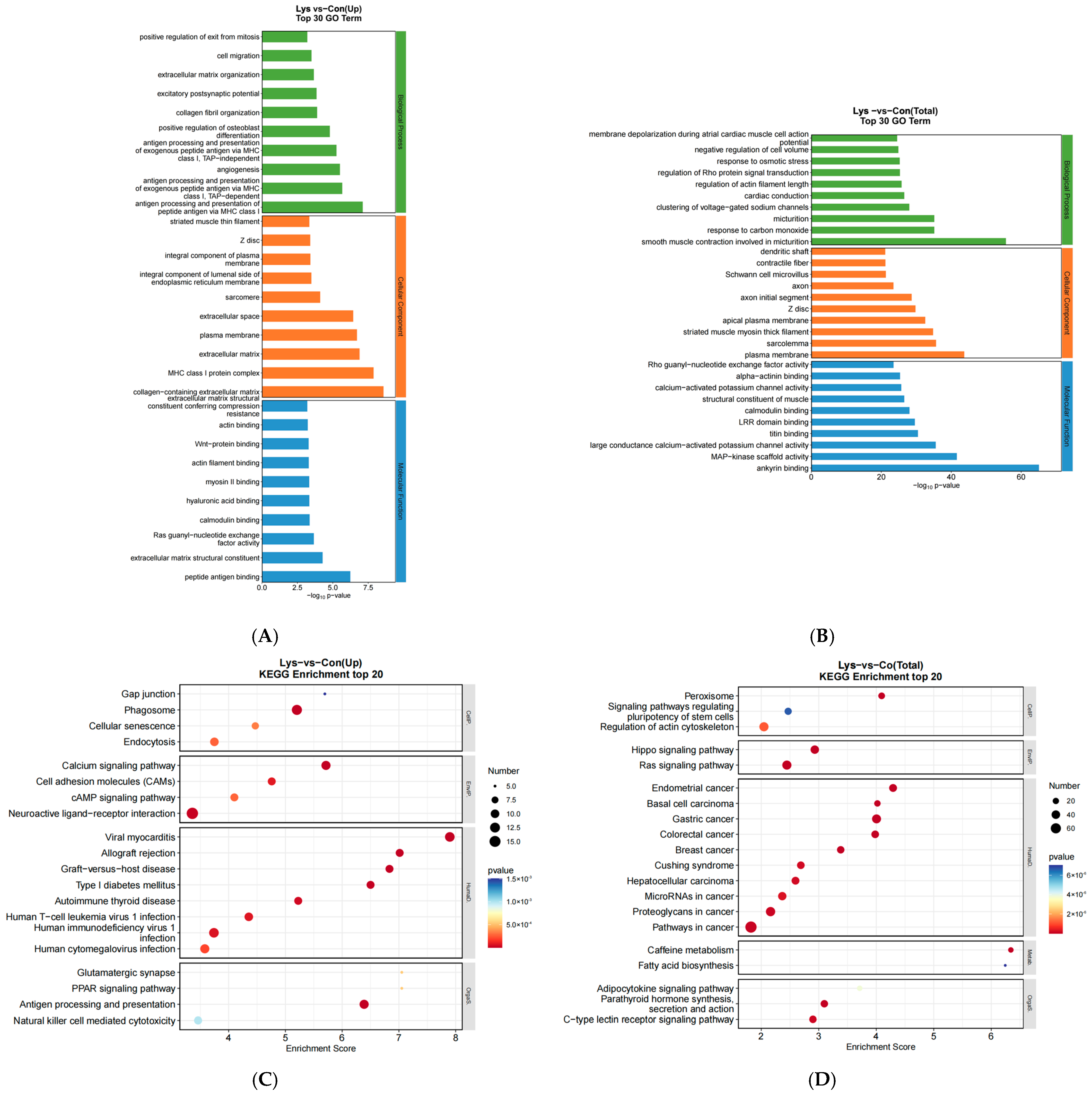
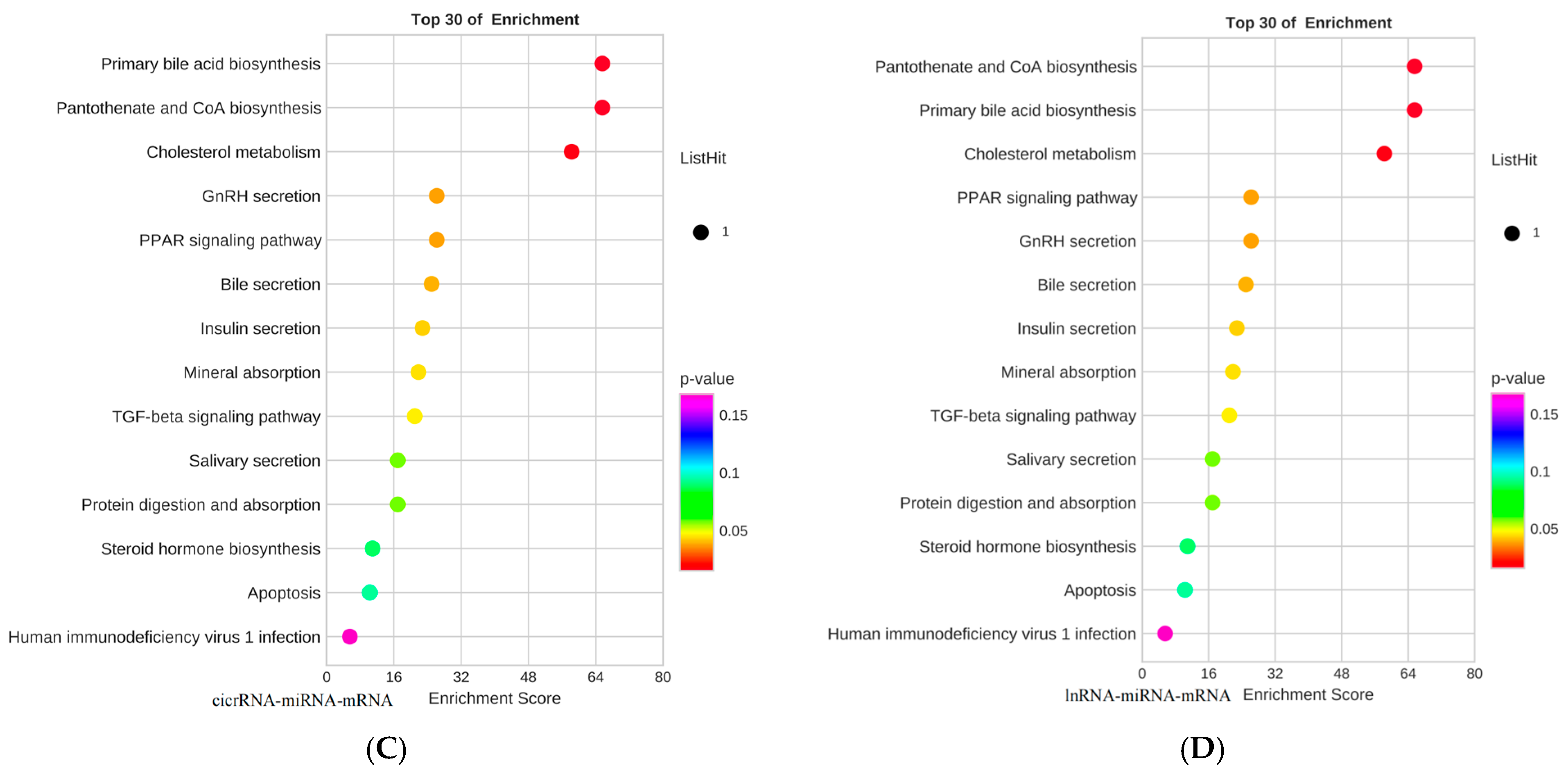
3.3. GO Functional Analysis and KEGG Pathway Analysis of Lysine-Induced Mongolian Horse Myosatellite Cell Differential mRNAs
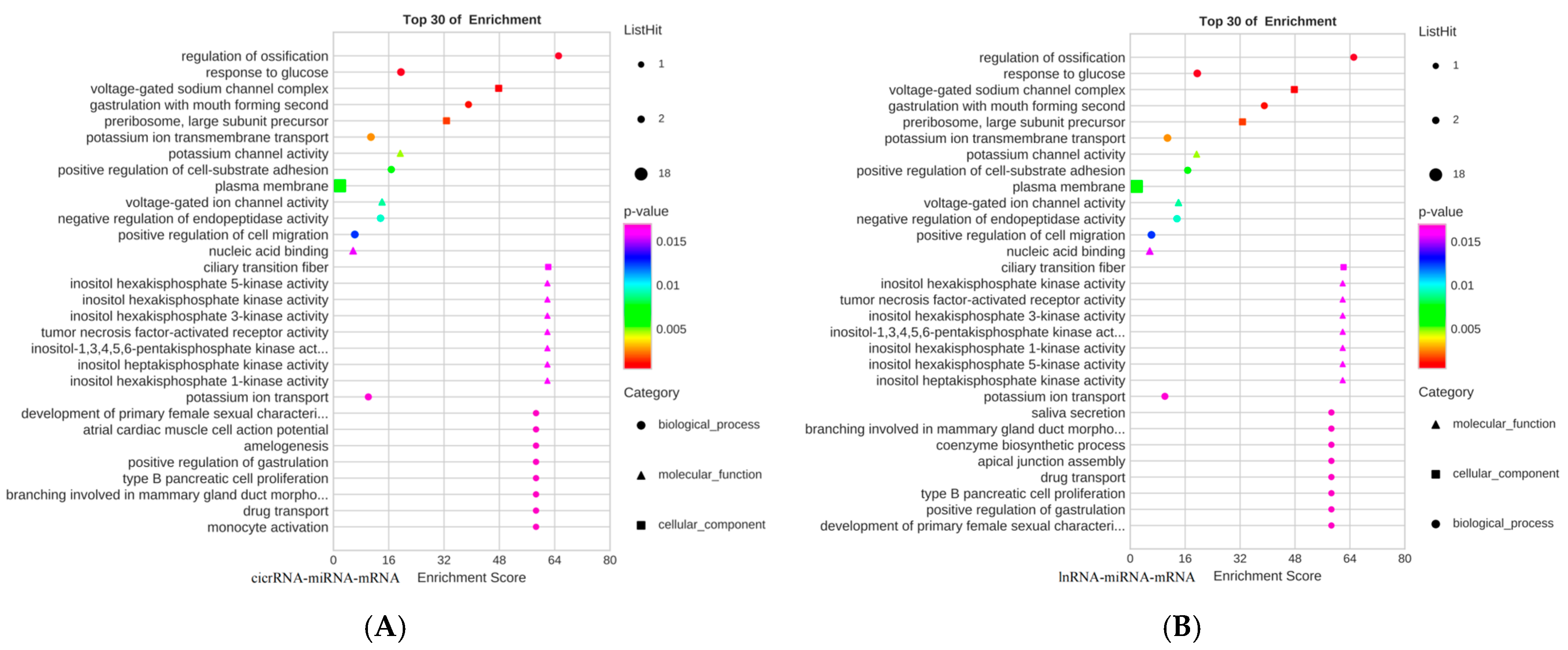
3.4. GO Functional Analysis and KEGG Pathway Analysis of Lysine-Induced Differential lncRNAs and miRNAs in Mongolian Horse Myosatellite Cells
3.5. ceRNA Network Construction
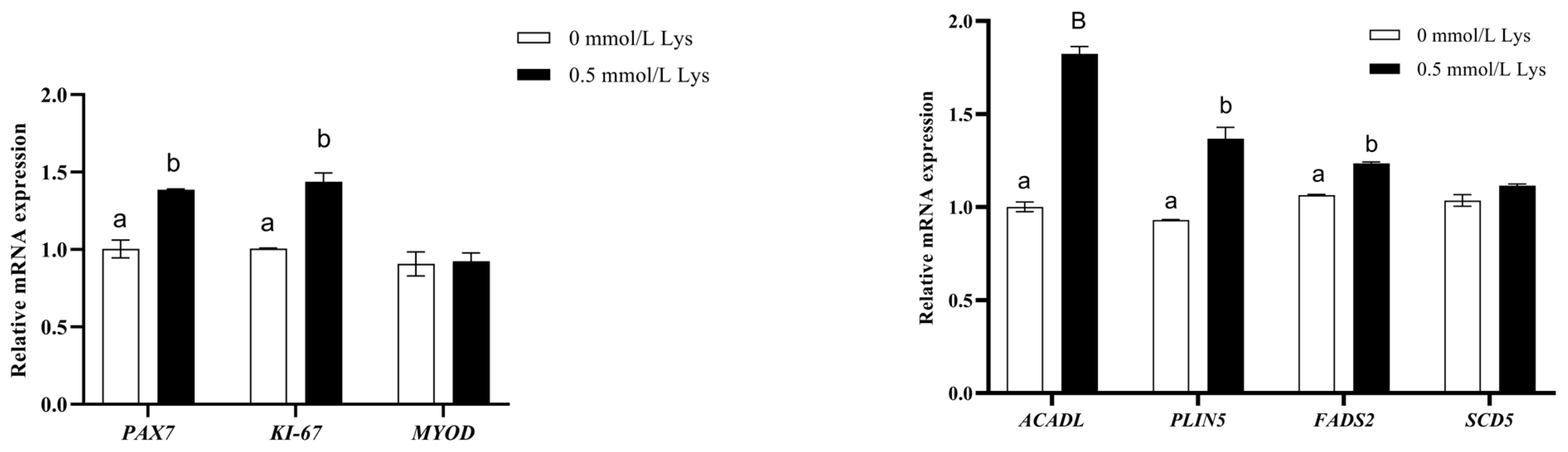
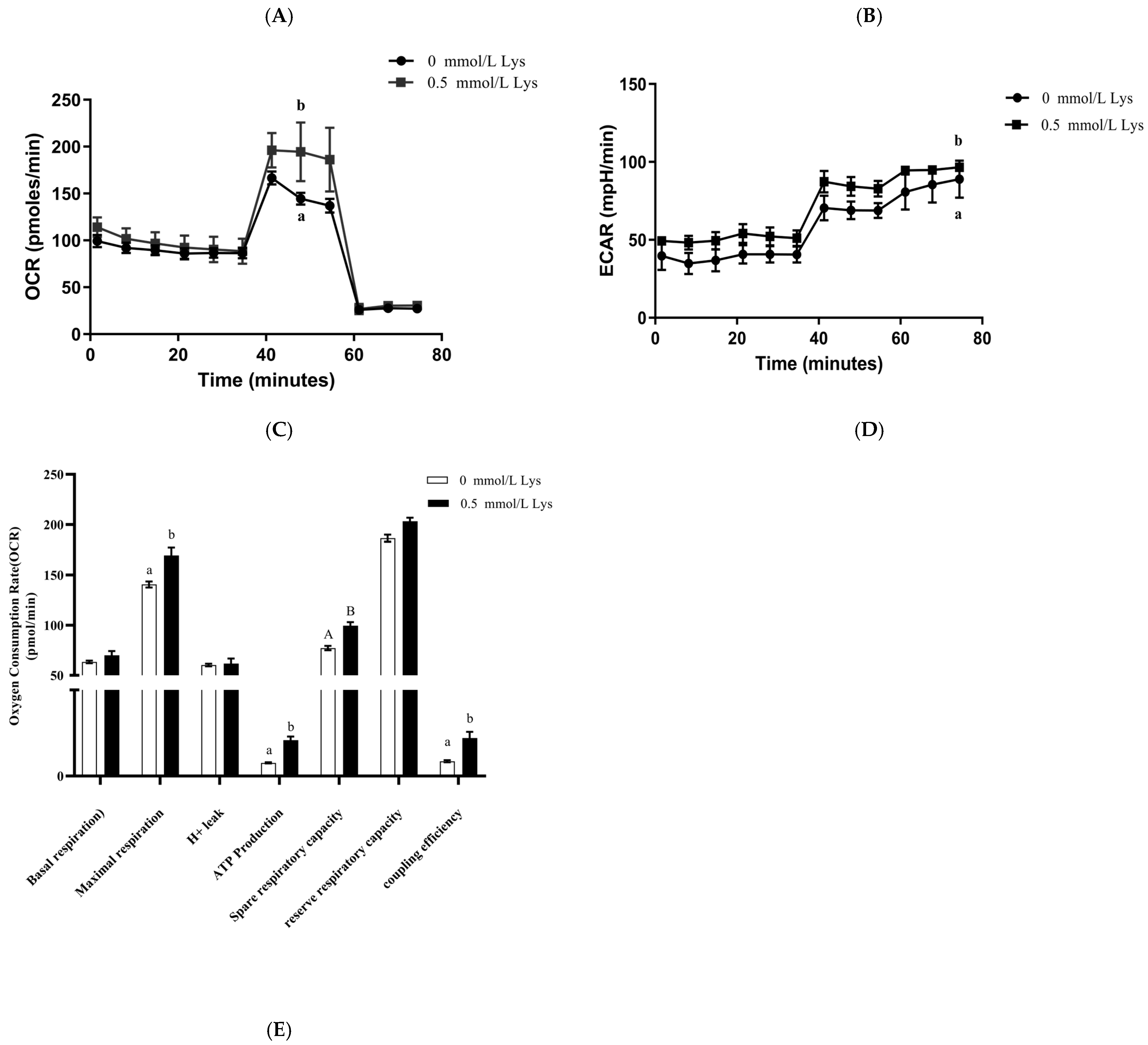
3.6. Lysine Regulates the PPAR Pathway to Provide Energy for the Proliferation of Mongolian Horse Myosatellite Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Culture Solution Name | Culture Fluid Composition |
|---|---|
| Culture fluid | 89% DMEM, 10% FBS, 1% dual antibody |
| 100 mmol/L Lysine culture fluid | 0.183 g Lysine powder, 10 mL aseptic and enzyme-free water |
| Lysine-free DMEN culture solution | 84 mg/L Arginine culture fluid, Lysine and arginine free DMEM |
| Different concentrations of lysine proliferating cultures (0.0, 0.2, 0.5, 1.0, 2.0 mmol/L) | Lysine-free DMEM culture medium, 10%FBS, 1% Dual Antibody, Different volumes (0, 20, 50, 100, and 200 μL) of 100 mmol/L lysine culture medium |
| 84 mg/L arginine culture solution | 0.084 g arginine powder, aseptic and enzyme-free water |
| Gene | Primer Sequence (5′→3′) | Product Length (bp) | Annealing Temperatur (°C) |
|---|---|---|---|
| ACADL | F:AGGAGTAAGAACAAATGCCAGAAAGG | 107 | 58.58 |
| R:CCGCCACTACGATCACAACATC | |||
| PLIN5 | F:CCCACTTCCTGCCCATGACC | 80 | 61.9 |
| R:TTCCACCGAGCCCACTTCAG | |||
| SCD5 | F:CGTGGTGCTCATGTGCTTCC | 115 | 59.85 |
| R:GTTGAGTGAGATGGTATAGCGAAGG | |||
| FADS2 | F:ATGTGTTCGTCTTGGGTGAATGG | 81 | 58.54 |
| R:CATGCTGGTGGTTGTAAGGAAGG | |||
| PAX7 | F:AGAAAGCCAAGCACAGCATCG | 98 | 58.71 |
| R:CTTCAGAGGGAGGTCGGGTTC | |||
| KI-67 | F:ACTGTTCTTCATTCTTCAGGACTTCC | 120 | 57.76 |
| R:CTTCACTTCTCCATTACGGCTCAC | |||
| MYOD | F:CCAGCGGGCACCACCAAG | 83 | 63.73 |
| R:GCGGCGGTCGGCGTTAG | |||
| GAPDH | F:GCATCCTGGGCTACACTGAG | 111 | 63.73 |
| R:AGTGGTCGTTGAGGGCAAT | |||
| CSPG4 | F:CTTGAGGAGGACGAGTACGAGGAG | 144 | 60.71 |
| R:GGTGAAGTTGGCGAAGACAGGAG | |||
| KIF2C | F:CGGTCTCGCCATCAAGATCCAAC | 111 | 60.79 |
| R:GCACCTCCTTCTGTCCATTCCAC | |||
| MATK | F:CCGAGGCTCTCAAACAAGGGAAG | 124 | 60.6 |
| R:GACACCTCCTTCAGCGACATCTTG | |||
| TPX2 | F:CCAGGCAGCAGGAGGAAGAG | 123 | 59.19 |
| R:ATACGGGCACAGTCAGAGGATG | |||
| INPP5D | F:ACTCACCGCTTCACCCACCTC | 108 | 62.69 |
| R:GTACTGCTGCTGCCTGATCTTCTG | |||
| PPID | F:GCAGGCCGCAATACAAATGGTTC | 120 | 59.87 |
| R:CAGTATCCTTGCCACACCCATTCC | |||
| UNC5B | F:CCATCGACCACAACCTCATCATCC | 136 | 60.21 |
| R:GACCAGCCGCCATTCACATAGAC | |||
| LOC100069322 | F:GCACTCGTCTAGCCCTCATCATC | 119 | 59.37 |
| R:CACACTGTAGTCCAGGTCCTCAAG |
References
- Sprayberry, K.A.; Slovis, N.M. Sales performance and athletic outcome in young Thoroughbreds with pericarditis. Equine Vet. J. 2017, 49, 729–733. [Google Scholar] [PubMed]
- Valberg, S.J. Muscle Conditions Affecting Sport Horses. Vet. Clin. N. Am. Equine Pract. 2018, 34, 253–276. [Google Scholar]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, C.; Tang, H.; Gong, M.; Yue, Z.; Zhao, M.; Liu, L.; Li, F. Dietary lysine supplementation improves growth performance and skeletal muscle development in rabbits fed a low protein diet. J. Anim. Physiol. Anim. Nutr. 2022, 106, 1118–1129. [Google Scholar]
- Wolf, P.; Pinna, W.; Boatto, G.; Pudda, F.; Cappai, M.G.; Accioni, F. Variation of Hematochemical Profile and Vitamin E Status in Feral Giara Horses From Free Grazing in the Wild to Hay Feeding During Captivity. J. Equine Vet. Sci. 2020, 94, 103220. [Google Scholar]
- National Research Council. Nutrient Requirements of Horses, 6th ed.; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Ott, E.A.; Asquith, R.L.; Feaster, J.P.; Martin, F.G. Influence of Protein Level and Quality on the Growth and Development of Yearling Foals3. J. Anim. Sci. 1979, 49, 620–628. [Google Scholar]
- Malesky, S.; Turner, J.; Browne-Silva, J.; Chen, L.; Löest, C. Nitrogen Retention and Plasma Amino Acid Responses in Mature Geldings Fed Three Dietary Concentrations of Lysine. J. Equine Vet. Sci. 2013, 33, 733–738. [Google Scholar]
- Song, Z.W.; Jin, C.L.; Ye, M.; Gao, C.; Yan, H.; Wang, X. Lysine inhibits apoptosis in satellite cells to govern skeletal muscle growth via the JAK2-STAT3 pathway. Food Funct. 2020, 11, 3941–3951. [Google Scholar]
- Wang, X.; Zong, X.; Ye, M.; Jin, C.; Xu, T.; Yang, J.; Gao, C.; Wang, X.; Yan, H. Lysine Distinctively Manipulates Myogenic Regulatory Factors and Wnt/Ca2+ Pathway in Slow and Fast Muscles, and Their Satellite Cells of Postnatal Piglets. Cells 2024, 13, 650. [Google Scholar]
- Jin, C.L.; Ye, J.L.; Yang, J.; Gao, C.; Yan, H.; Li, H.; Wang, X. mTORC1 Mediates Lysine-Induced Satellite Cell Activation to Promote Skeletal Muscle Growth. Cells 2019, 8, 1549. [Google Scholar]
- Wang, T.; Feugang, J.M.; Crenshaw, M.A.; Naresh Regmi, N.; Blanton, J.R.; Liao, S.F. A Systems Biology Approach Using Transcriptomic Data Reveals Genes and Pathways in Porcine Skeletal Muscle Affected by Dietary Lysine. Int. J. Mol. Sci. 2017, 18, 885. [Google Scholar] [PubMed]
- Sato, T.; Ito, Y.; Nagasawa, T. L-Lysine suppresses myofibrillar protein degradation and autophagy in skeletal muscles of senescence-accelerated mouse prone 8. Biogerontology 2017, 18, 85–95. [Google Scholar] [PubMed]
- Palma-Granados, P.; Haro, A.; Seiquer, I.; Lara, L.; Aguilera, J.F.; Nieto, R. Similar effects of lysine deficiency in muscle biochemical characteristics of fatty and lean piglets. J. Anim. Sci. 2017, 95, 3025–3036. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Pallafacchina, G.; Blaauw, B.; Schiaffino, S. Role of satellite cells in muscle growth and maintenance of muscle mass. Nutr. Metab. Cardiovasc. Dis. 2013, 23 (Suppl. 1), S12–S18. [Google Scholar] [CrossRef]
- Venema, R.C.; Caprara, M.G.; Hatzoglou, M.; Majumder, M.; Wang, C.; Snider, M.D.; Gaccioli, F.; Komar, A.A.; Yaman, I.; Zeenko, V.V. The hnRNA-binding proteins hnRNP L and PTB are required for efficient translation of the Cat-1 arginine/lysine transporter mRNA during amino acid starvation. Mol. Cell Biol. 2009, 29, 2899–2912. [Google Scholar]
- Morales, A.; García, H.; Arce, N.; Cota, M.; Zijlstra, R.T.; Araiza, B.A.; Cervantes, M. Effect of L-lysine on expression of selected genes, serum concentration of amino acids, muscle growth and performance of growing pigs. J. Anim. Physiol. Anim. Nutr. 2015, 99, 701–709. [Google Scholar]
- Fogl, C.; Puckey, L.; Hinssen, U.; Zaleska, M.; El-Mezgueldi, M.; Croasdale, R.; Bowman, A.; Matsukawa, A.; Samani, N.J.; Savva, R.; et al. A structural and functional dissection of the cardiac stress response factor MS1. Proteins 2012, 80, 398–409. [Google Scholar] [CrossRef]
- Olson, E.N.; Bassel-Duby, R.; Richardson, J.A.; Frey, N.; Bezprozvannaya, S.; Barrientos, T.; Kuwahara, K.; Pipes, G.C.T.; Frank, D.; Katus, H.A. Two novel members of the ABLIM protein family, ABLIM-2 and -3, associate with STARS and directly bind F-actin. J. Biol. Chem. 2007, 282, 8393–8403. [Google Scholar]
- Arai, A.; Spencer, J.A.; Olson, E.N. STARS, a striated muscle activator of Rho signaling and serum response factor-dependent transcription. J. Biol. Chem. 2002, 277, 24453–24459. [Google Scholar]
- Lamon, S.; Russell, A.P.; Wallace, M.A. The regulation and function of the striated muscle activator of rho signaling (STARS) protein. Front. Physiol. 2012, 3, 469. [Google Scholar]
- Taniguchi, K.; Tominaga, R.; Takeya, R.; Kan-O, M.; Narusawa, M.; Shiose, A.; Sumimoto, H.; Suetsugu, S. Mammalian Formin Fhod3 Regulates Actin Assembly and Sarcomere Organization in Striated Muscles. J. Biol. Chem. 2009, 284, 29873–29881. [Google Scholar] [PubMed]
- Frey, N.; Olson, E.N. Calsarcin-3, a novel skeletal muscle-specific member of the calsarcin family, interacts with multiple Z-disc proteins. J. Biol. Chem. 2002, 277, 13998–14004. [Google Scholar] [PubMed]
- Suila, H.; Zara, I.; Belgrano, A.; Carpen, O.; Faulkner, G.; von Nandelstadh, P.; Valle, G.; Gardin, C.; Ismail, M. A class III PDZ binding motif in the myotilin and FATZ families binds enigma family proteins: A common link for Z-disc myopathies. Mol. Cell. Biol. 2009, 29, 822–834. [Google Scholar]
- Park, J.; Moon, S.S.; Song, S.; Cheng, H.; Im, C.; Du, L.; Kim, G.-D. Comparative review of muscle fiber characteristics between porcine skeletal muscles. J. Anim. Sci. Technol. 2024, 66, 251–265. [Google Scholar]
- Yamano, S.; Eto, D.; Hiraga, A.; Miyata, H. Recruitment pattern of muscle fibre type during high intensity exercise (60–100% VO2max) in thoroughbred horses. Res. Vet. Sci. 2006, 80, 109–115. [Google Scholar]
- Chan, S.; Seto, J.T.; MacArthur, D.G.; Yang, N.; North, K.N.; Head, S.I. A gene for speed: Contractile properties of isolated whole EDL muscle from an alpha-actinin-3 knockout mouse. Am. J. Physiol. Cell Physiol. 2008, 295, C897–C904. [Google Scholar]
- Payne, R.C.; Crook, T.C.; Cruickshank, S.E.; Stubbs, N.; Wilson, A.M.; McGowan, C.M.; Hodson-Tole, E. A comparison of the moment arms of pelvic limb muscles in horses bred for acceleration (Quarter Horse) and endurance (Arab). J. Anat. 2010, 217, 26–37. [Google Scholar]
- Willoughby, D. Organization of cAMP signalling microdomains for optimal regulation by Ca2+ entry. Biochem. Soc. Trans. 2012, 40, 246–250. [Google Scholar]
- Clarke, K.; Branco, C.; Ashmore, T.; West, J.A.; Morash, A.J.; Murfitt, S.A.; Kotwica, A.O.; Finnerty, J.; Griffin, J.L.; Johnson, R.S.; et al. Nitrate enhances skeletal muscle fatty acid oxidation via a nitric oxide-cGMP-PPAR-mediated mechanism. BMC Biol. 2015, 13, 110. [Google Scholar]
- Wu, J.; Luo, J.; Xia, Y.; An, X.; Guo, P.; He, Q.; Tian, H.; Hu, Q.; Li, C.; Wang, H. Goat FADS2 controlling fatty acid metabolism is directly regulated by SREBP1 in mammary epithelial cells. J. Anim. Sci. 2023, 101, skad030. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, W.; Brown, K.M.; Baker, E.; van der Merwe, M.; Sharma, S.; Puppa, M.J. Delta-6-desaturase (FADS2) inhibition and omega-3 fatty acids in skeletal muscle protein turnover. Biochem. Biophys. Rep. 2019, 18, 100622. [Google Scholar]
- Parkington, H.C.; Bayliss, J.; Suturin, V.M.; Bruce, C.R.; Montgomery, M.K.; Mokhtar, R.; Watt, M.J. Perilipin 5 Deletion Unmasks an Endoplasmic Reticulum Stress-Fibroblast Growth Factor 21 Axis in Skeletal Muscle. Diabetes 2018, 67, 594–606. [Google Scholar]
- Ni, H.Y.; Yu, L.; Zhao, X.; Wang, L.; Zhao, C.; Huang, H.; Zhu, H.; Efferth, T.; Gu, C.; Fu, Y. Seed oil of Rosa roxburghii Tratt against non-alcoholic fatty liver disease in vivo and in vitro through PPARα/PGC-1α-mediated mitochondrial oxidative metabolism. Phytomedicine 2022, 98, 153919. [Google Scholar]
- Hesselink, M.; Sparks, L.; Jorgensen, J.; Kersten, S.; Schrauwen, P.; Bosma, M.; Hooiveld, G.; Houten, S. Overexpression of PLIN5 in skeletal muscle promotes oxidative gene expression and intramyocellular lipid content without compromising insulin sensitivity. Biochim. Biophys. Acta 2013, 1831, 844–852. [Google Scholar]
- Wu, T.; Wang, S.; Wang, L.; Zhang, W.; Chen, W.; Lv, X.; Li, Y.; Hussain, Z.; Sun, W. Long Noncoding RNA (lncRNA) CTTN-IT1 Elevates Skeletal Muscle Satellite Cell Proliferation and Differentiation by Acting as ceRNA for YAP1 Through Absorbing miR-29a in Hu Sheep. Front. Genet. 2020, 11, 843. [Google Scholar]
- Ge, M.X.; Shao, R.G.; He, H. Advances in understanding the regulatory mechanism of cholesterol 7α-hydroxylase. Biochem. Pharmacol. 2019, 164, 152–164. [Google Scholar]
- Patel, D.D.; Knight, B.L.; Soutar, A.K.; Gibbons, G.F.; Wade, D.P. The effect of peroxisome-proliferator-activated receptor-alpha on the activity of the cholesterol 7 alpha-hydroxylase gene. Biochem. J. 2000, 351 Pt 3, 747–753. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, Y.; Bai, D.; Dugarjaviin, M.; Zhang, X. Transcriptomic Profiling Reveals Lysine-Mediated Proliferative Mechanisms in Mongolian Horse Myogenic Satellite Cells. Animals 2025, 15, 1711. https://doi.org/10.3390/ani15121711
Liu Y, Liu Y, Bai D, Dugarjaviin M, Zhang X. Transcriptomic Profiling Reveals Lysine-Mediated Proliferative Mechanisms in Mongolian Horse Myogenic Satellite Cells. Animals. 2025; 15(12):1711. https://doi.org/10.3390/ani15121711
Chicago/Turabian StyleLiu, Yumeng, Yuanyi Liu, Dongyi Bai, Manglai Dugarjaviin, and Xinzhuang Zhang. 2025. "Transcriptomic Profiling Reveals Lysine-Mediated Proliferative Mechanisms in Mongolian Horse Myogenic Satellite Cells" Animals 15, no. 12: 1711. https://doi.org/10.3390/ani15121711
APA StyleLiu, Y., Liu, Y., Bai, D., Dugarjaviin, M., & Zhang, X. (2025). Transcriptomic Profiling Reveals Lysine-Mediated Proliferative Mechanisms in Mongolian Horse Myogenic Satellite Cells. Animals, 15(12), 1711. https://doi.org/10.3390/ani15121711





