Effects of Cholinergic and Opioid Antagonists on In Vitro Release of Met-Enkephalin, Somatostatin and Insulin-like Growth Factor-1 by and PENK Expression in Crop, Proventriculus and Duodenum of Newly Hatched Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
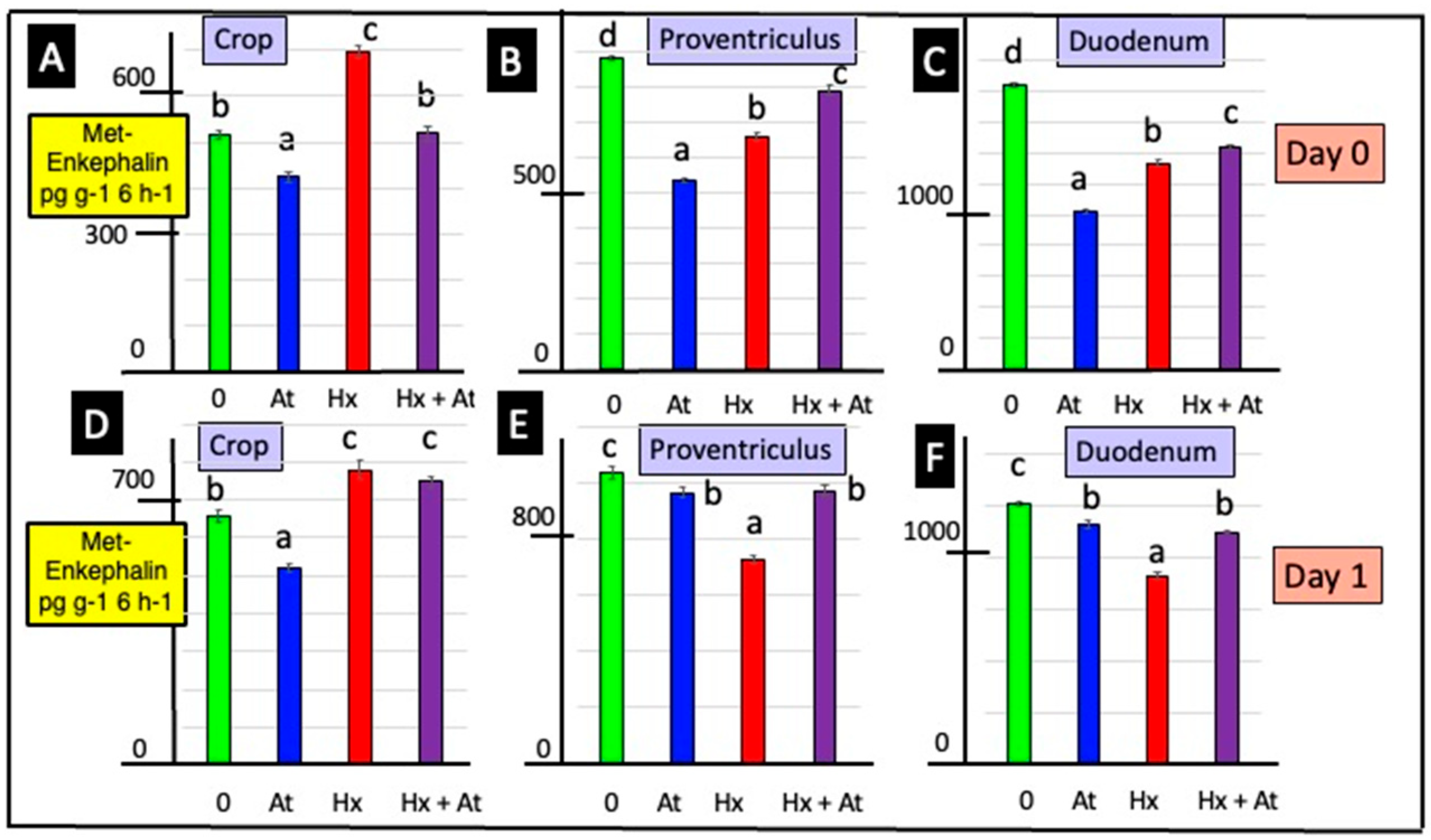
3.1. Release of Met-Enkephalin
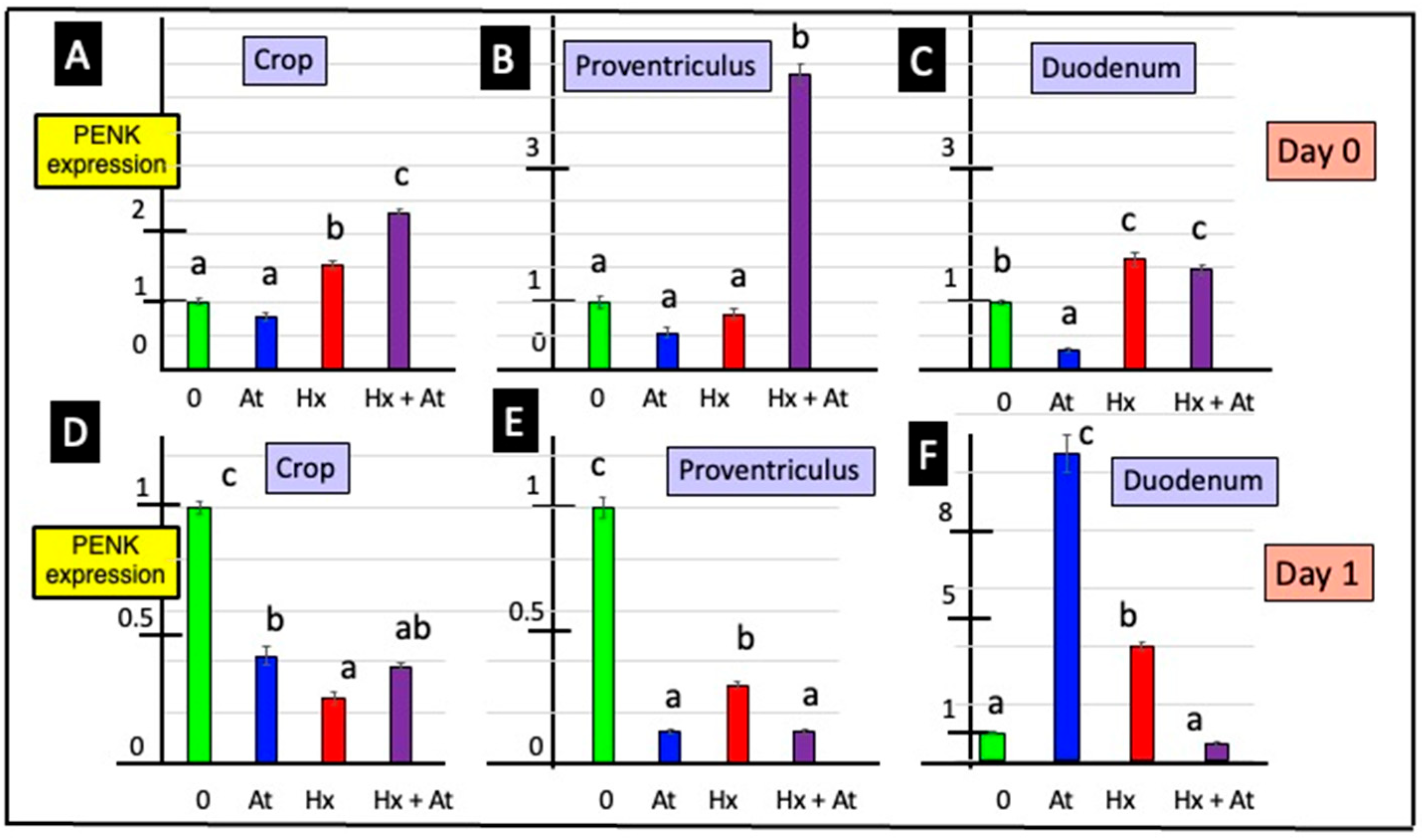
3.2. Expression of the PENK Gene
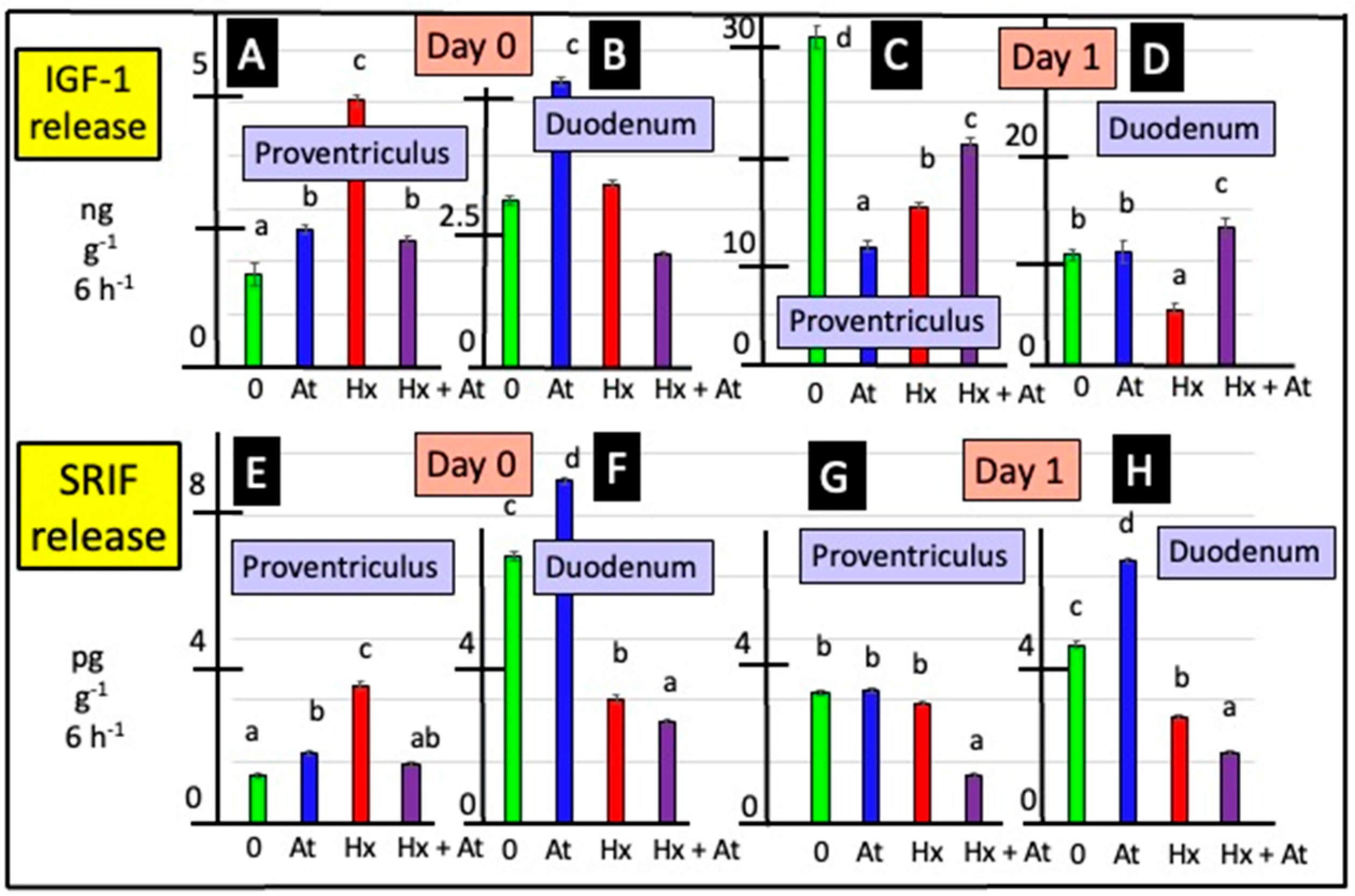
3.3. Release of SRIF
3.4. Release of IGF-1
3.5. Relationships Between Release of Met-Enkephalin, IGF-1 and SRIF
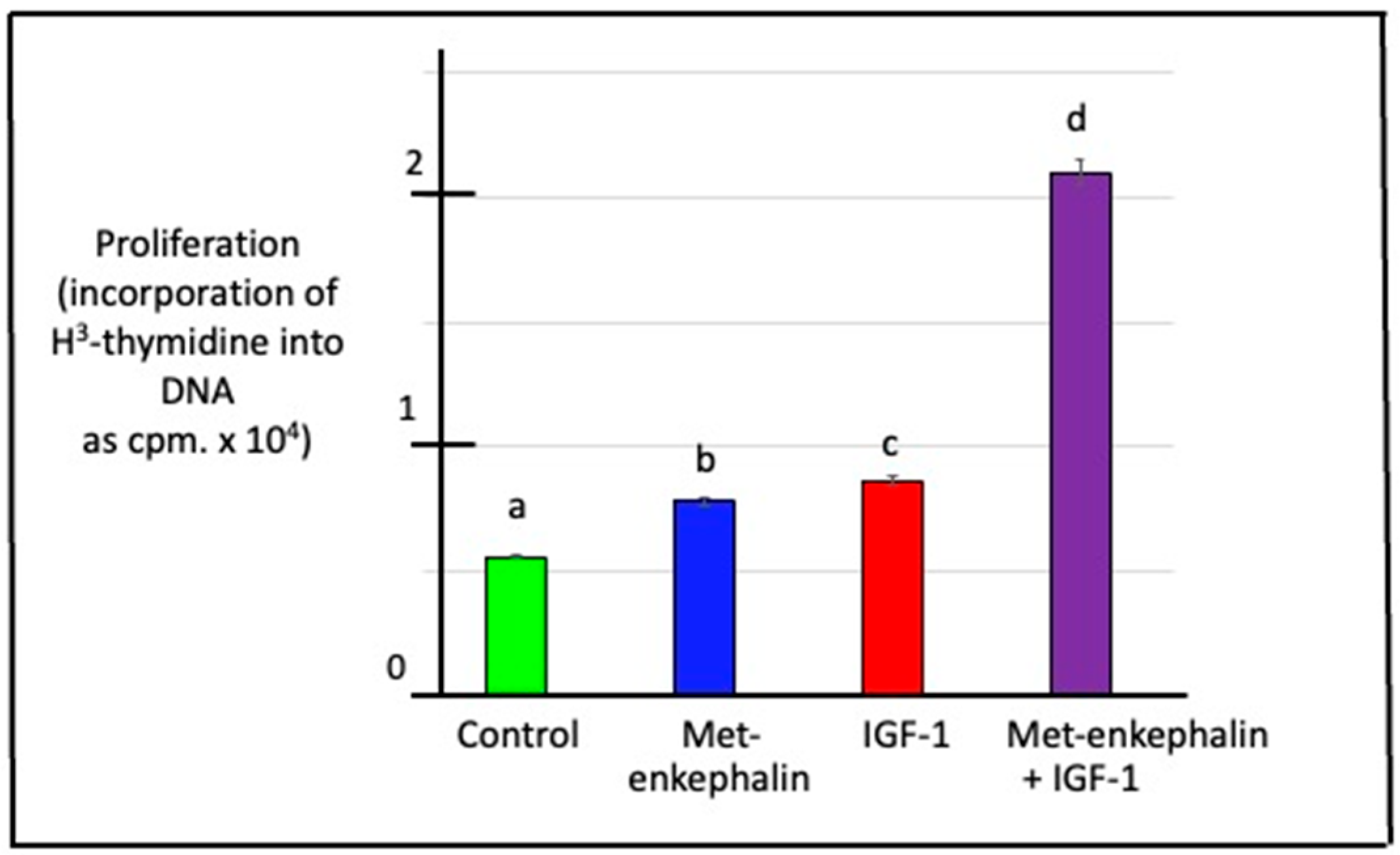
3.6. Effects of Met-Enkephalin and/or IGF-1 on DNA Synthesis (Cell Proliferation) in Duodenal Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polak, J.M.; Bloom, S.R.; Sullivan, S.N.; Facer, P.; Pearse, A.G.E. Enkephalin-like immunoreactivity in the human gastrointestinal tract. Lancet 1977, 309, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Ferri, G.L.; Botti, P.; Biliotti, G.; Rebecchi, L.; Bloom, S.R.; Tonelli, L.; Labò, G.; Polak, J.M. VIP-, substance P- and met-enkephalin-immunoreactive innervation of the human gastroduodenal mucosa and Brunner’s glands. Gut 1984, 25, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Costa, M.; Miller, R.J. Distribution and projections of nerves with enkephalin-like immunoreactivity. Neuroscience 1983, 8, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.I.; Stengaard-Pendersen, K. Immunocytochemical and ultrastructural differentiation between Met-enkephalin-, Leu-enkephalin-, and Met/Leu-enkephalin-immunoreactive neurons of feline gut. J. Neurosci. 1982, 2, 861–878. [Google Scholar] [CrossRef]
- Daniel, E.E.; Costa, M.; Furness, J.B.; Kent, J.R. Peptide neurons in the canine small intestine. J. Comp. Neurol. 1985, 237, 227–238. [Google Scholar] [CrossRef]
- Kokrashvili, Z.; Rodriguez, D.; Yevshayeva, V.; Zhou, H.; Margolskee, R.F.; Mosinger, B. Release of endogenous opioids from duodenal enteroendocrine cells requires Trpm5. Gastroenterology 2009, 137, 598–606. [Google Scholar] [CrossRef]
- Nihei, K.; Iwanaga, T. Localization of Met-enkephalin-Arg6-Gly7-Leu8-like immunoreactivity in the gastrointestinal tract of rat and pig. J. Histochem. Cytochem. 1985, 33, 1001–1006. [Google Scholar] [CrossRef]
- Epstein, M.L.; Hudis, J.; Dahl, J.L. The development of peptidergic neurons in the foregut of the chick. J. Neurosci. 1983, 3, 2431–2447. [Google Scholar] [CrossRef]
- Proszkowiec-Weglarz, M. Gastrointestinal anatomy and physiology. In Sturkie’s Avian Physiology, 7th ed.; Scanes, C.G., Dridi, S., Eds.; Academic Press: New York, NY, USA, 2022; pp. 485–498. [Google Scholar]
- Clark, S.J.; Smith, T.W. Peristalsis abolishes the release of methionine-enkephalin from guinea-pig ileum in vitro. Eur. J. Pharmacol. 1981, 70, 421–424. [Google Scholar] [CrossRef]
- Clark, S.J.; Smith, J.W. The release of met-enkephalin from the guinea pig ileum at rest and during peristalitic activity. Life Sci. 1983, 33, 465–468. [Google Scholar] [CrossRef]
- Money, S.R.; Petroianu, A.; Gintzler, A.R.; Jaffe, B.M. Meal-stimulated release of Methionine-enkephalin into the canine jejunal lumen. J. Clin. Investig. 1988, 81, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Hook, V.Y.; Eiden, L.E. (Met)enkephalin and carboxypeptidase processing enzyme are co-released from chromaffin cells by cholinergic stimulation. Biochem. Biophys. Res. Commun. 1985, 128, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Pierzchała-Koziec, K.; Van Loon, G.R. Effects of nicotine on the concentration of native and cryptic Met- and Leu-enkephalin in peripheral tissues. J. Physiol. Pharmacol. 1994, 45, 319–330. [Google Scholar]
- Jaszcza, K.; Scanes, C.G.; Capcarova, M.; Pierzchała-Koziec, K. Effects of opioid and cholinergic receptors inhibition on the intestine hormones concentration and release in rats. J. Anim. Feed Sci. 2020, 29, 266–275. [Google Scholar] [CrossRef]
- Georgiev, I.P.; Georgieva, T.M.; Pfaffl, M.; Hammon, H.M.; Blum, J.W. Insulin-like growth factor and insulin receptors in intestinal mucosa of neonatal calves. J. Endocrinol. 2003, 176, 121–132. [Google Scholar] [CrossRef]
- Al Haj Ali, M.; Mensah-Brown, E.; Chandranath, S.I.; Adeghate, E.; Adem, A. Distribution of insulin like growth factor-1 (IGF-1) and its receptor in the intestines of the one-humped camel (Camelus dromedarius). Growth Factors 2003, 21, 131–137. [Google Scholar] [CrossRef]
- Reinecke, M.; Betzler, D.; Drakenberg, K.; Falkmer, S.; Sara, V.R. Occurrence of members of the insulin superfamily in central nervous system and digestive tract of protochordates. Histochemistry 1993, 99, 277–285. [Google Scholar] [CrossRef]
- Thompson, J.M.; Di Gregorio, A. Insulin-like genes in ascidians: Findings in Ciona and hypotheses on the evolutionary origins of the pancreas. Genesis 2015, 53, 82–104. [Google Scholar] [CrossRef]
- Steeb, C.B.; Trahair, J.F.; Read, L.C. Administration of insulin-like growth factor-I (IGF-I) peptides for three days stimulates proliferation of the small intestinal epithelium in rats. Gut 1995, 37, 630–638. [Google Scholar] [CrossRef][Green Version]
- Steeb, C.B.; Shoubridge, C.A.; Tivey, D.R.; Read, L.C. Systemic infusion of IGF-I or LR(3)IGF-I stimulates visceral organ growth and proliferation of gut tissues in suckling rats. Am. J. Physiol. 1997, 272, G522–G533. [Google Scholar] [CrossRef]
- Wheeler, E.E.; Challacombe, D.N. The trophic action of growth hormone, insulin-like growth factor-I, and insulin on human duodenal mucosa cultured in vitro. Gut 1997, 40, 57–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez, V. Somatostatin. In Handbook of Biologically Active Peptides, 2nd ed.; Kastin, A.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1320–1329. [Google Scholar]
- Alison, B.C. The distribution and ontogeny of gastrin/CCK-, somatostatin- and neurotensin-immunoreactive cells in the gastrointestinal tract of the chicken. Histol. Histopath. 1989, 4, 55–62. [Google Scholar] [PubMed]
- Schubert, M.L.; Makhlouf, G.M. Gastrin secretion induced by distention is mediated by gastric cholinergic and vasoactive intestinal peptide neurons in rats. Gastroenterology 1993, 104, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Saffouri, B.; DuVal, J.W.; Makhlouf, G.M. Stimulation of gastrin secretion in vitro by intraluminal chemicals: Regulation by intramural cholinergic and noncholinergic neurons. Gastroenterology 1984, 87, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y. Mechanisms for regulation of gastrin and somatostatin release from isolated rat stomach during gastric distention. World J. Gastroenterol. 2003, 9, 129–133. [Google Scholar] [CrossRef]
- Bulc, M.; Palus, K.; Całka, J. The influence of a hyperglycemic condition on the population of somatostatin enteric neurons in the porcine gastrointestinal tract. Animals 2020, 10, 142. [Google Scholar] [CrossRef]
- Gonkowski, S.; Rytel, L. Somatostatin as an active substance in the mammalian enteric nervous system. Int. J. Mol. Sci. 2019, 20, 4461. [Google Scholar] [CrossRef]
- Shamsi, B.H.; Chatoo, M.; Xu, X.K.; Xu, X.; Chen, X.Q. Versatile functions of somatostatin and somatostatin receptors in the gastrointestinal system. Front. Endocrinol. 2021, 12, 652363. [Google Scholar] [CrossRef]
- The, F.O.; Boeckxstaens, G.E.; Snoek, S.A.; Cash, J.L.; Bennink, R.; Larosa, G.J.; van den Wijngaard, R.M.; Greaves, D.R.; de Jonge, W.J. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology 2007, 133, 1219–1228. [Google Scholar] [CrossRef]
- Campbell, A.M.; Zagon, I.S.; McLaughlin, P.J. Astrocyte proliferation is regulated by the OGF-OGFr axis in vitro and in experimental autoimmune encephalomyelitis. Brain Res. Bull. 2013, 90, 43–51. [Google Scholar] [CrossRef]
- Zagon, I.S.; Wu, Y.; McLaughlin, P.J. Opioid growth factor-dependent DNA synthesis in the neonatal rat aorta. Am. J. Physiol. 1996, 270, R22–R32. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Wu, Y.; McLaughlin, P.J. Met-enkephalin decreased mouse esophageal cell proliferation, naltrexone hydrochloride increased mouse esophageal cell proliferation. Am. J. Physiol. 1997, 272, R1094–R1104. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Wu, Y.; McLaughlin, P.J. The opioid growth factor, [Met5]-enkephalin, and the zeta opioid receptor are present in human and mouse skin and tonically act to inhibit DNA synthesis in the epidermis. J. Investig. Dermatol. 1996, 106, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Donahue, R.N.; Bonneau, R.H.; McLaughlin, P.J. T lymphocyte proliferation is suppressed by the opioid growth factor ([Met(5)]-enkephalin)-opioid growth factor receptor axis: Implication for the treatment of autoimmune diseases. Immunobiology 2011, 216, 579–590. [Google Scholar] [CrossRef]
- Zagon, I.S.; Donahue, R.N.; Bonneau, R.H.; McLaughlin, P.J. B lymphocyte proliferation is suppressed by the opioid growth factor-opioid growth factor receptor axis: Implication for the treatment of autoimmune diseases. Immunobiology 2011, 216, 173–183. [Google Scholar] [CrossRef]
- Zagon, I.S.; Sassani, J.W.; McLaughlin, P.J. Opioid growth factor modulates corneal epithelial outgrowth in tissue culture. Am. J. Physiol. 1995, 268, R946–R950. [Google Scholar] [CrossRef]
- Donahue, R.N.; McLaughlin, P.J.; Zagon, I.S. Cell proliferation of human ovarian cancer is regulated by the opioid growth factor-opioid growth factor receptor axis. Am. J. Physiol. 2009, 296, R1716–R1725. [Google Scholar] [CrossRef]
- Huang, H.; Liu, B.; Qu, N.; Zhang, S.; Bai, X.; Handley, M.; Shan, F. Research progress of opioid growth factor in immune-related diseases and cancer diseases. Int. Immunopharmacol. 2021, 99, 107713. [Google Scholar] [CrossRef]
- Qu, N.; Wang, X.; Meng, Y.; Fengping Shan, F. Prospective oncotarget for gynecological cancer: Opioid growth factor (OGF)—Opioid growth factor receptor (OGFr) axis. Int. Immunopharmacol. 2019, 75, 105723. [Google Scholar] [CrossRef]
- Steine-Martin, A.; Hauser, K.F. Opioid-dependent growth of glial cultures: Suppression of astrocyte DNA synthesis by Met-enkephalin. Life Sci. 1990, 46, 91–96. [Google Scholar] [CrossRef]
- Kishi, H.; Mishima, H.K.; Sakamoto, I.; Yamashita, U. Stimulation of retinal pigment epithelial cell growth by neuropeptides in vitro. Curr. Eye Res. 1996, 15, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Pierzchala, K.; Van Loon, G.R. Plasma native and peptidase-derivable Met-enkephalin responses to restraint stress in rats. Adaptation to repeated restraint. J. Clin. Investig. 1990, 85, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Lee, K.Y.; Chang, T.M.; Chey, W.Y. MEK inhibits secretin release and pancreatic secretion: Roles of secretin-releasing peptide and somatostatin. Am. J. Physiol. 2001, 280, G890–G896. [Google Scholar] [CrossRef]
- Schubert, M.L.; Bitar, K.N.; Makhlouf, G.M. Regulation of gastrin and somatostatin secretion by cholinergic and noncholinergic intramural neurons. Am. J. Physiol. 1982, 243, G442–G447. [Google Scholar] [CrossRef]
- Konturek, S.J. Cholinergic control of gastric acid secretion in man. Scand. J. Gastroenterol. Suppl. 1982, 72, 1–5. [Google Scholar] [PubMed]
- Bohin, N.; McGowan, K.P.; Keeley, T.M.; Carlson, E.A.; Yan, K.S.; Samuelson, L.C. Insulin-like growth factor-1 and mTORC1 signaling promote the intestinal regenerative response after irradiation injury. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 797–810. [Google Scholar] [CrossRef]
- Bortvedt, S.F.; Lund, P.K. Insulin-like growth factor 1: Common mediator of multiple enterotrophic hormones and growth factors. Curr. Opin. Gastroenterol. 2012, 28, 89–98. [Google Scholar] [CrossRef]
- Nelson, D.W.; Murali, S.G.; Liu, X.; Koopmann, M.C.; Holst, J.J.; Ney, D.M. Insulin-like growth factor I and glucagon-like peptide-2 responses to fasting followed by controlled or ad libitum refeeding in rats. Am. J. Physiol. 2008, 294, R1175–R1184. [Google Scholar] [CrossRef][Green Version]
- Wilson, S.P. Insulin-like growth factor I enhances proenkephalin synthesis and dopamine β-hydroxylase activity in adrenal chromaffin cells. Life Sci. 1991, 49, 269–272. [Google Scholar] [CrossRef]
- de Figueiredo, C.S.; Raony, I.; Medina, S.V.; de Mell Silva, E.; dos Santos, A.A.; de Araujo, E.G. Insulin-like growth factor-1 stimulates retinal cell proliferation via activation of multiple signaling pathways. Curr. Res. Neurobiol. 2022, 4, 100068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Torregrossa, M.M.; Jutkiewicz, E.M.; Shi, Y.-G.; Rice, K.C.; Woods, J.H.; Watson, S.J.; Ko, M.-C. Endogenous opioids upregulate brain-derived neurotrophic factor mRNA through δ- and μ-opioid receptors independent of antidepressant-like effects. Eur. J. Neurosci. 2006, 23, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Murali, S.G.; Holst, J.J.; Ney, D.M. Enteral nutrients potentiate the intestinotrophic action of glucagon-like peptide-2 in association with increased insulin-like growth factor-I responses in rats. Am. J. Physiol. 2008, 295, R1794–R1802. [Google Scholar] [CrossRef] [PubMed]


| Primer | F/R | Gene Sequence | Fragment Size (bp) |
|---|---|---|---|
| pPENK | F | 5′-CAGCTCTTTGGCTTCATCT-3′ | 102 |
| R | 5′-AGAGGCCAATGGAAGTGAGA-3′ | ||
| 18S rRNA | F | 5′-CTTTGGTCGCTCGCTCCTC-3′ | 115 |
| R | 5′-CTGACCGGGTTGGTTTTGAT-3′ |
| Adjusted R2 | p-Value | |
|---|---|---|
| Proventriculus | ||
| Day 0 | ||
| Met-enkephalin vs. IGF-1 | 0.138 | 0.0595 |
| Met-enkephalin vs. SRIF | 0.158 | 0.0465 |
| SRIF vs. IGF-1 | 0.897 | 1.52 × 10−10 |
| Day 1 | ||
| Met-enkephalin vs. IGF-1 | −0.055 | 0.948 |
| Met-enkephalin vs. SRIF | −0.044 | 0.659 |
| SRIF vs. IGF-1 | 0.488 | 0.00037 |
| Duodenum | ||
| Day 0 | ||
| Met-enkephalin vs. IGF-1 | 0.899 | 1.34 × 10−10 |
| Met-enkephalin vs. SRIF | −0.0012 | 0.390 |
| SRIF vs. IGF-1 | −0.051 | 0.783 |
| Day 1 | ||
| Met-enkephalin vs. IGF-1 | 0.388 | 0.00199 |
| Met-enkephalin vs. SRIF | 0.166 | 0.0424 |
| SRIF vs. IGF-1 | −0.049 | 0.734 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scanes, C.G.; Jaszcza, K.; Gajewska, A.; Pierzchala-Koziec, K. Effects of Cholinergic and Opioid Antagonists on In Vitro Release of Met-Enkephalin, Somatostatin and Insulin-like Growth Factor-1 by and PENK Expression in Crop, Proventriculus and Duodenum of Newly Hatched Chickens. Animals 2025, 15, 1702. https://doi.org/10.3390/ani15121702
Scanes CG, Jaszcza K, Gajewska A, Pierzchala-Koziec K. Effects of Cholinergic and Opioid Antagonists on In Vitro Release of Met-Enkephalin, Somatostatin and Insulin-like Growth Factor-1 by and PENK Expression in Crop, Proventriculus and Duodenum of Newly Hatched Chickens. Animals. 2025; 15(12):1702. https://doi.org/10.3390/ani15121702
Chicago/Turabian StyleScanes, Colin G., Klaudia Jaszcza, Alina Gajewska, and Krystyna Pierzchala-Koziec. 2025. "Effects of Cholinergic and Opioid Antagonists on In Vitro Release of Met-Enkephalin, Somatostatin and Insulin-like Growth Factor-1 by and PENK Expression in Crop, Proventriculus and Duodenum of Newly Hatched Chickens" Animals 15, no. 12: 1702. https://doi.org/10.3390/ani15121702
APA StyleScanes, C. G., Jaszcza, K., Gajewska, A., & Pierzchala-Koziec, K. (2025). Effects of Cholinergic and Opioid Antagonists on In Vitro Release of Met-Enkephalin, Somatostatin and Insulin-like Growth Factor-1 by and PENK Expression in Crop, Proventriculus and Duodenum of Newly Hatched Chickens. Animals, 15(12), 1702. https://doi.org/10.3390/ani15121702







