Toll-like Receptor Expression Patterns in the Female Reproductive Tract of Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Animals, Experimental Design and Sample Collection
2.3. RNA Extracted and qRT-PCR
2.4. Immunohistochemistry
2.5. Statistical Analysis
3. Results
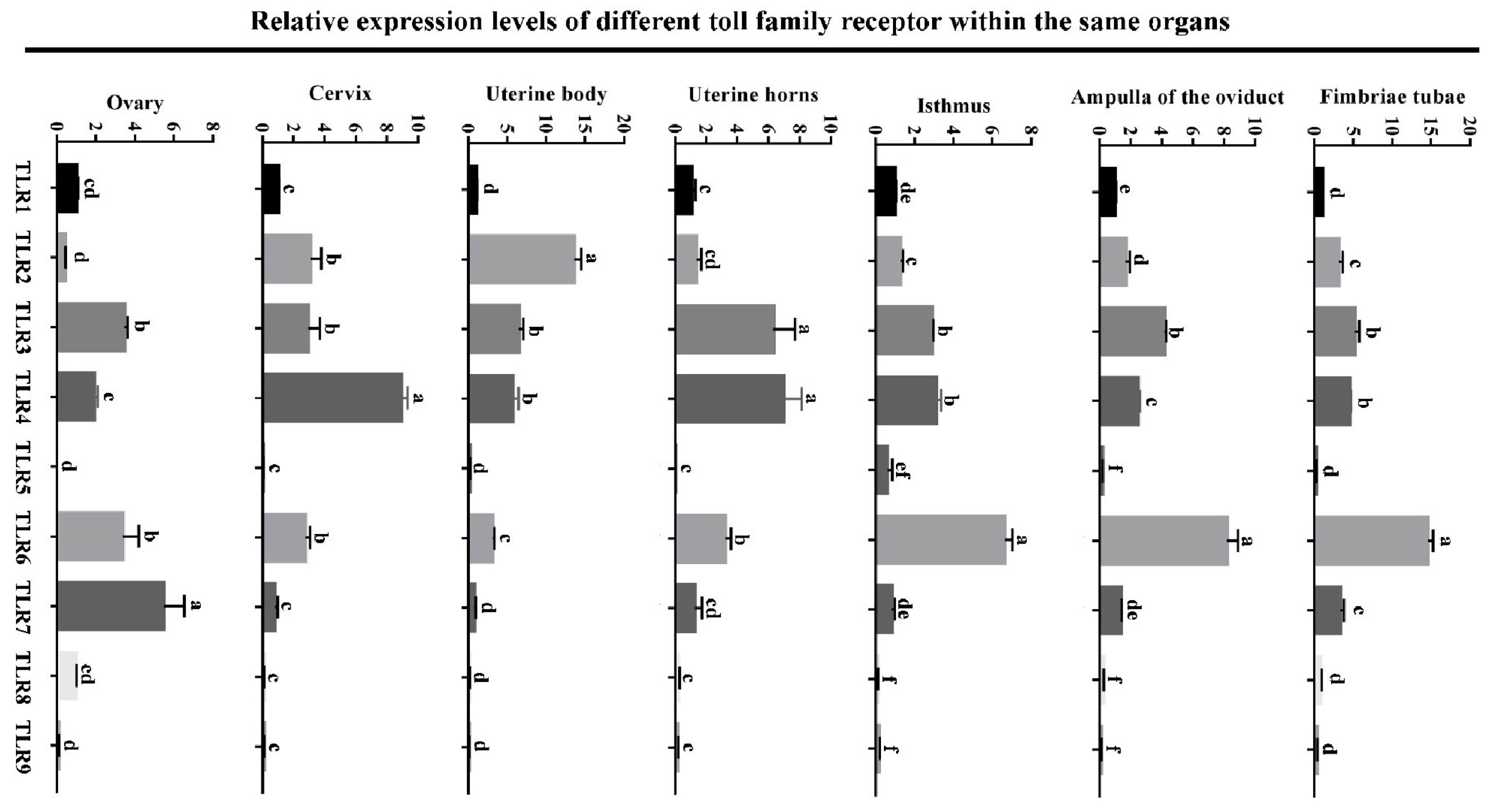
3.1. Expression Levels of Toll-like Receptor Family in Different Reproductive Organs
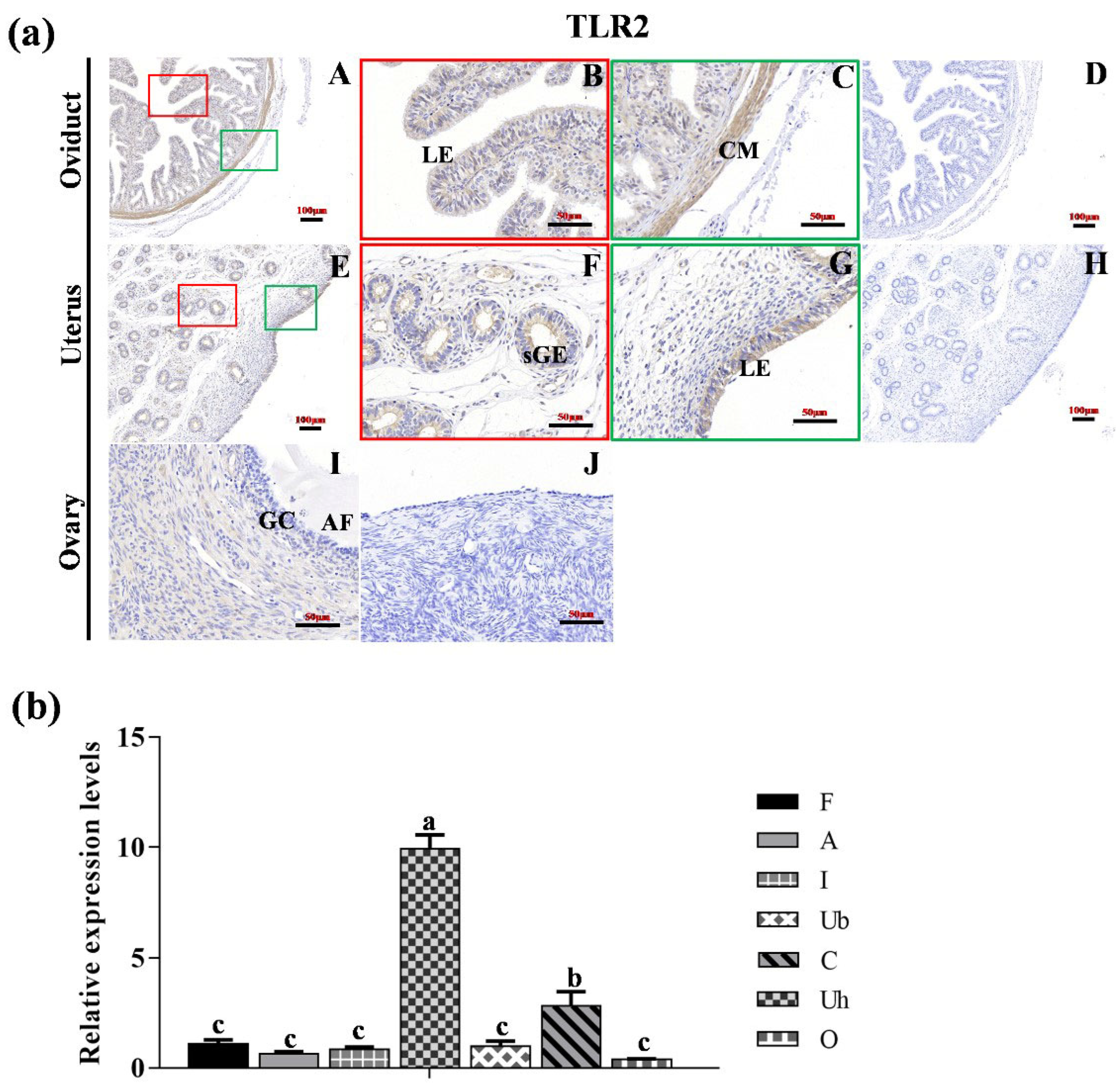
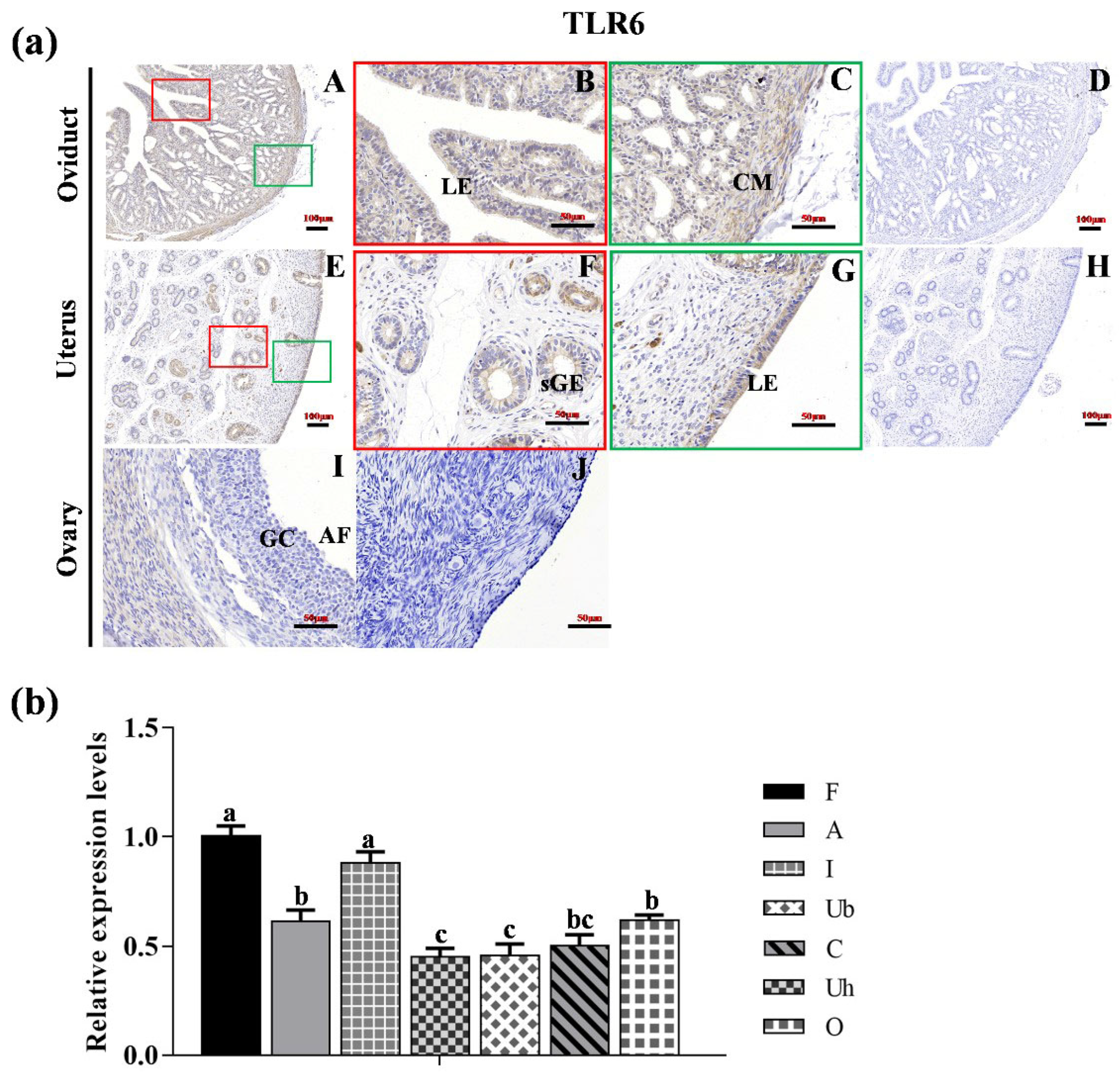
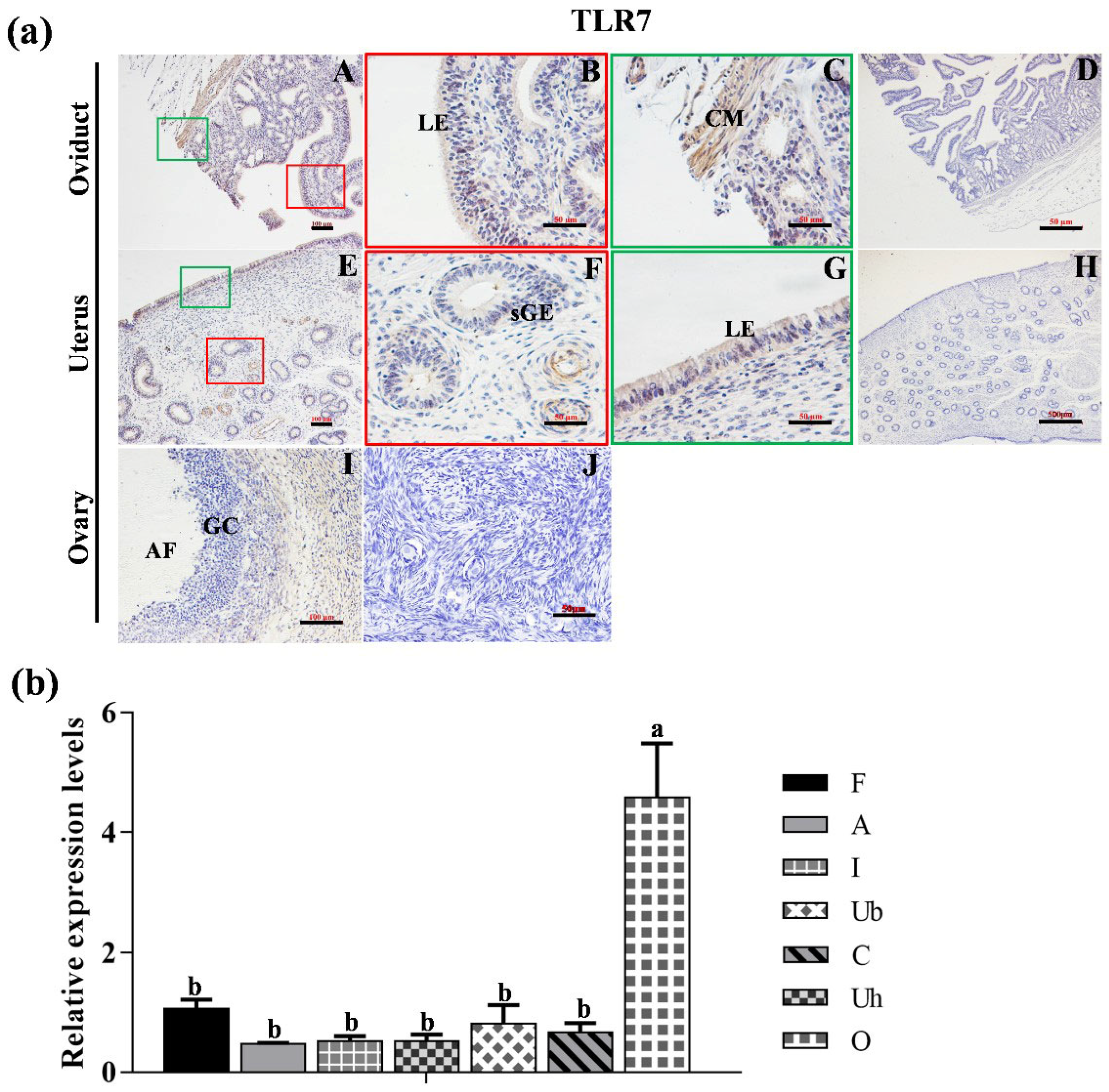
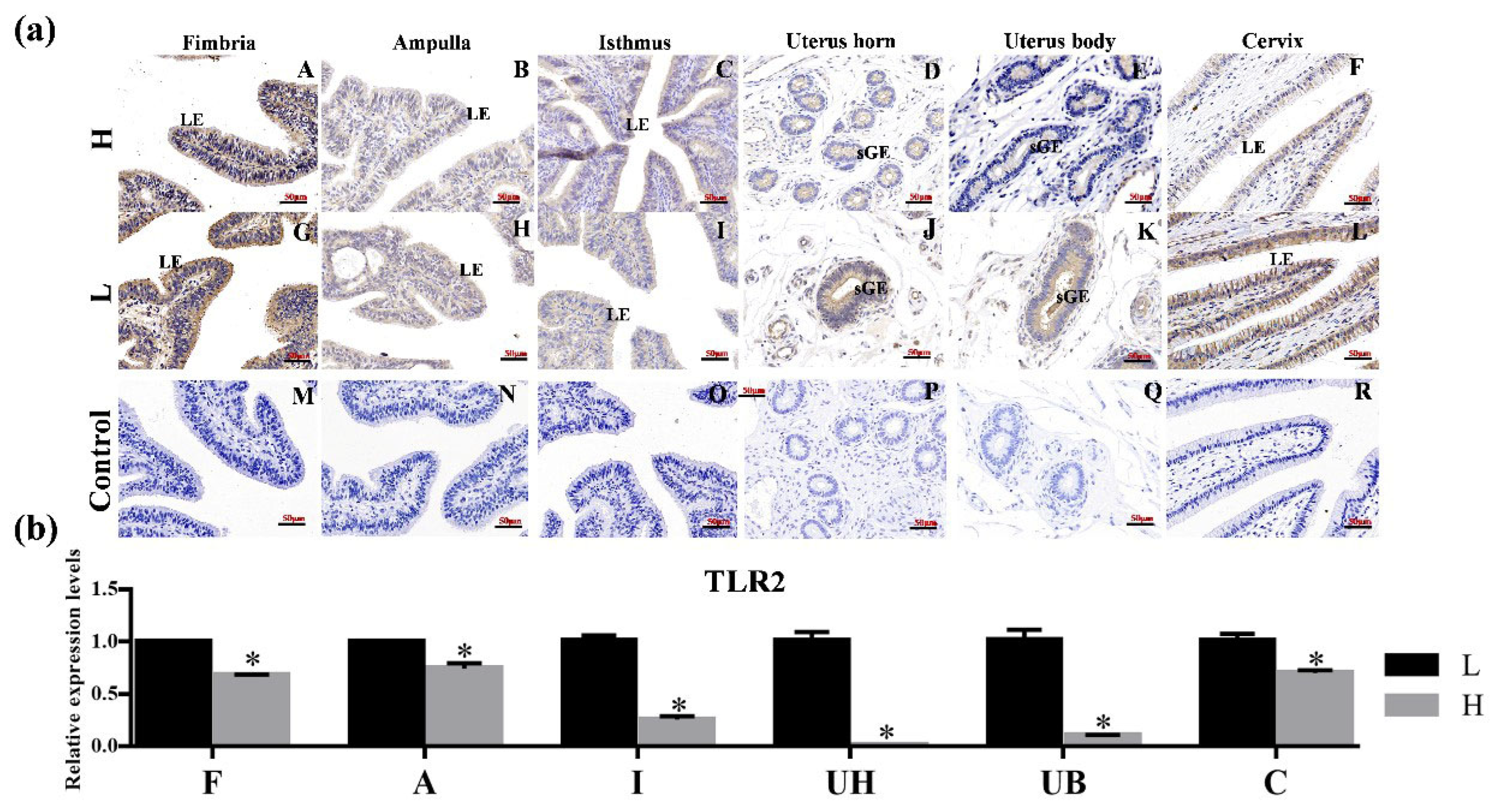
3.2. Localization of TLR2, TLR6 and TLR7 in Various Reproductive Organs and mRNA Expression in Hu Sheep Tissues
3.3. Localization of TLR2, TLR6 and TLR7 in Follicles at Different Developmental Stages in Hu Sheep
3.4. Protein and mRNA Levels of TLR2, TLR6 and TLR7 in Different Tissues of HP and LP Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, M.; Zhang, Y.; Lu, Y.; Han, J.; Guo, T.; Cui, P.; Brannstrom, M.; Shao, L.R.; Billig, H. Regulatory mechanisms of HMGB1 and its receptors in polycystic ovary syndrome-driven gravid uterine inflammation. FEBS J. 2023, 290, 1874–1906. [Google Scholar] [CrossRef] [PubMed]
- Babaei, K.; Azimi Nezhad, M.; Sedigh Ziabari, S.N.; Mirzajani, E.; Mozdarani, H.; Sharami, S.H.; Farzadi, S.; Mirhafez, S.R.; Naghdipour Mirsadeghi, M.; Norollahi, S.E.; et al. TLR signaling pathway and the effects of main immune cells and epigenetics factors on the diagnosis and treatment of infertility and sterility. Heliyon 2024, 10, e35345. [Google Scholar] [CrossRef] [PubMed]
- Tirumurugaan, K.G.; Dhanasekaran, S.; Raj, G.D.; Raja, A.; Kumanan, K.; Ramaswamy, V. Differential expression of toll-like receptor mRNA in selected tissues of goat (Capra hircus). Vet. Immunol. Immunopathol. 2010, 133, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Vahanan, B.M.; Raj, G.D.; Pawar, R.M.; Gopinath, V.P.; Raja, A.; Thangavelu, A. Expression profile of toll like receptors in a range of water buffalo tissues (Bubalus bubalis). Vet. Immunol. Immunopathol. 2008, 126, 149–155. [Google Scholar] [CrossRef]
- Pruett, S.B.; Zheng, Q.; Fan, R.; Matthews, K.; Schwab, C. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol 2004, 33, 147–155. [Google Scholar] [CrossRef]
- Kokkinopoulos, I.; Jordan, W.J.; Ritter, M.A. Toll-like receptor mRNA expression patterns in human dendritic cells and monocytes. Mol. Immunol. 2005, 42, 957–968. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Sandor, F.; Ortiz, Y.; Bowen, G.N.; Counter, S.L.; Wang, T.C.; Finberg, R.W. Use of murine embryonic fibroblasts to define Toll-like receptor activation and specificity. J. Endotoxin Res. 2004, 10, 419–424. [Google Scholar] [CrossRef]
- Zarember, K.A.; Godowski, P.J. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J. Immunol. 2002, 168, 554–561. [Google Scholar] [CrossRef]
- Hornung, V.; Rothenfusser, S.; Britsch, S.; Krug, A.; Jahrsdorfer, B.; Giese, T.; Endres, S.; Hartmann, G. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002, 168, 4531–4537. [Google Scholar] [CrossRef]
- Menzies, M.; Ingham, A. Identification and expression of Toll-like receptors 1-10 in selected bovine and ovine tissues. Vet. Immunol. Immunopathol. 2006, 109, 23–30. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, R.; Ma, L.; Wang, Y.; Pan, B.; Cai, J.; Wang, M. Eimeria tenella: Expression profiling of toll-like receptors and associated cytokines in the cecum of infected day-old and three-week old SPF chickens. Exp. Parasitol. 2012, 130, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Huggett, J.F.; Dheda, K.; Kim, L.U.; Zumla, A.; Rook, G.A. Myobacterium tuberculosis induces selective up-regulation of TLRs in the mononuclear leukocytes of patients with active pulmonary tuberculosis. J. Immunol. 2006, 176, 3010–3018. [Google Scholar] [CrossRef]
- Chuang, T.; Ulevitch, R.J. Identification of hTLR10: A novel human Toll-like receptor preferentially expressed in immune cells. Biochim. Biophys. Acta 2001, 1518, 157–161. [Google Scholar] [CrossRef] [PubMed]
- McGuire, K.; Jones, M.; Werling, D.; Williams, J.L.; Glass, E.J.; Jann, O. Radiation hybrid mapping of all 10 characterized bovine Toll-like receptors. Anim. Genet. 2006, 37, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Werling, D.; Coffey, T.J. Pattern recognition receptors in companion and farm animals—The key to unlocking the door to animal disease? Vet. J. 2007, 174, 240–251. [Google Scholar] [CrossRef]
- Chang, J.S.; Russell, G.C.; Jann, O.; Glass, E.J.; Werling, D.; Haig, D.M. Molecular cloning and characterization of Toll-like receptors 1-10 in sheep. Vet. Immunol. Immunopathol. 2009, 127, 94–105. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Xu, H.; Ofori, E.; Elovitz, M.A. Toll-like receptors in the uterus, cervix, and placenta: Is pregnancy an immunosuppressed state? Am. J. Obstet. Gynecol. 2007, 197, 296.e1–296.e6. [Google Scholar] [CrossRef]
- Chen, C.; Zibiao, H.; Ming, Z.; Shiyi, C.; Ruixia, L.; Jie, W.; SongJia, L. Expression pattern of Toll-like receptors (TLRs) in different organs and effects of lipopolysaccharide on the expression of TLR 2 and 4 in reproductive organs of female rabbit. Dev. Comp. Immunol. 2014, 46, 341–348. [Google Scholar] [CrossRef]
- Liu, Z.; Shimada, M.; Richards, J.S. The involvement of the Toll-like receptor family in ovulation. J. Assist. Reprod. Genet. 2008, 25, 223–228. [Google Scholar] [CrossRef]
- Richards, J.S.; Liu, Z.; Shimada, M. Immune-like mechanisms in ovulation. Trends Endocrinol. Metab. 2008, 19, 191–196. [Google Scholar] [CrossRef]
- Richards, J.S. Delivery of the oocyte from the follicle to the oviduct: A time of vulnerability. In The Future of the Oocyte: Basic and Clinical Aspects; Springer: Berlin/Heidelberg, Germany, 2002; Volume 41, pp. 43–62. [Google Scholar]
- Fang, Y.; Hu, J. Toll-like receptor and its roles in myocardial ischemic/reperfusion injury. Med. Sci. Monit. 2011, 17, RA100–RA109. [Google Scholar] [CrossRef] [PubMed]
- Holst, B.; Raby, A.C.; Hall, J.E.; Labéta, M.O. Complement takes its Toll: An inflammatory crosstalk between Toll-like receptors and the receptors for the complement anaphylatoxin C5a. Anaesthesia 2012, 67, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Barbato, O.; Filipescu, I.E.; Traina, G.; Leonardi, L.; Polisca, A.; Troisi, A.; Guelfi, G.; Piro, F.; Brecchia, G. Effects of local lipopolysaccharide administration on the expression of Toll-like receptor 4 and pro-inflammatory cytokines in uterus and oviduct of rabbit does. Theriogenology 2018, 107, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Cai, S.Y.; Shao, J.Z.; Chen, J. Toll-Like Receptors, Associated Biological Roles, and Signaling Networks in Non-Mammals. Front. Immunol. 2018, 9, 1523. [Google Scholar] [CrossRef]
- Huang, Y.; Yao, X.L.; Meng, J.Z.; Liu, Y.; Jiang, X.L.; Chen, J.W.; Li, P.F.; Ren, Y.S.; Liu, W.Z.; Yao, J.B.; et al. Intrafollicular expression and potential regulatory role of cocaine- and amphetamine-regulated transcript in the ovine ovary. Domest. Anim. Endocrinol. 2016, 54, 30–36. [Google Scholar] [CrossRef]
- Yao, X.D.; Fernandez, S.; Kelly, M.M.; Kaushic, C.; Rosenthal, K.L. Expression of Toll-like receptors in murine vaginal epithelium is affected by the estrous cycle and stromal cells. J. Reprod. Immunol. 2007, 75, 106–119. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Barua, A. Female Reproductive System and Immunology. Adv. Exp. Med. Biol. 2017, 1001, 33–57. [Google Scholar] [CrossRef]
- Kowalewska, M.; Herman, A.P.; Szczepkowska, A.; Skipor, J. The effect of melatonin from slow-release implants on basic and TLR-4-mediated gene expression of inflammatory cytokines and their receptors in the choroid plexus in ewes. Res. Vet. Sci. 2017, 113, 50–55. [Google Scholar] [CrossRef]
- Ateya, A.I.; Hussein, M.S.; Ghanem, H.M.; Saleh, R.M.; El-Domany, W.B.; Elseady, Y.Y. Expression profiles of immunity and reproductive genes during transition period in Holstein cattle. Reprod. Domest. Anim. 2018, 53, 352–358. [Google Scholar] [CrossRef]
- Amjadi, F.; Zandieh, Z.; Salehi, E.; Jafari, R.; Ghasemi, N.; Aflatoonian, A.; Fazeli, A.; Aflatoonian, R. Variable localization of Toll-like receptors in human fallopian tube epithelial cells. Clin. Exp. Reprod. Med. 2018, 45, 1–9. [Google Scholar] [CrossRef]
- Feng, X.; Li, F.; Wang, F.; Zhang, G.; Pang, J.; Ren, C.; Zhang, T.; Yang, H.; Wang, Z.; Zhang, Y. Genome-wide differential expression profiling of mRNAs and lncRNAs associated with prolificacy in Hu sheep. Biosci. Rep. 2018, 38, BSR20171350. [Google Scholar] [CrossRef] [PubMed]
- Darville, T.; O’Neill, J.M.; Andrews, C.W.; Nagarajan, U.M.; Stahl, L.; Ojcius, D.M. Toll-Like Receptor-2, but Not Toll-Like Receptor-4, Is Essential for Development of Oviduct Pathology in Chlamydial Genital Tract Infection. J. Immunol. 2003, 171, 6187–6197. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Nasu, K.; Nishida, M.; Matsumoto, H.; Yuge, A.; Narahara, H. Human oviductal stromal fibroblasts, but not oviductal epithelial cells, express Toll-like receptor 4: The site-specific mucosal immunity of the human fallopian tube against bacterial infection. Am. J. Reprod. Immunol. 2006, 56, 91–101. [Google Scholar] [CrossRef]
- Derbigny, W.A.; Shobe, L.R.; Kamran, J.C.; Toomey, K.S.; Ofner, S. Identifying a role for Toll-like receptor 3 in the innate immune response to Chlamydia muridarum infection in murine oviduct epithelial cells. Infect. Immun. 2012, 80, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Schaefer, T.M.; Fahey, J.V.; Wright, J.A.; Wira, C.R. Antiviral responses of human Fallopian tube epithelial cells to toll-like receptor 3 agonist poly(I:C). Fertil. Steril. 2008, 89, 1497–1506. [Google Scholar] [CrossRef]
- Nasu, K.; Itoh, H.; Yuge, A.; Nishida, M.; Narahara, H. Human oviductal epithelial cells express Toll-like receptor 3 and respond to double-stranded RNA: Fallopian tube-specific mucosal immunity against viral infection. Hum. Reprod. 2007, 22, 356–361. [Google Scholar] [CrossRef]
- Atli, M.O.; Kose, M.; Hitit, M.; Kaya, M.S.; Bozkaya, F. Expression patterns of Toll-like receptors in the ovine corpus luteum during the early pregnancy and prostaglandin F2alpha-induced luteolysis. Theriogenology 2018, 111, 25–33. [Google Scholar] [CrossRef]
- Aboussahoud, W.S.; Smith, H.; Stevens, A.; Wangsaputra, I.; Hunter, H.R.; Kimber, S.J.; Seif, M.W.; Brison, D.R. The expression and activity of Toll-like receptors in the preimplantation human embryo suggest a new role for innate immunity. Hum. Reprod. 2021, 36, 2661–2675. [Google Scholar] [CrossRef]
- Silva, E.; Henriques, S.; Brito, S.; Ferreira-Dias, G.; Lopes-da-Costa, L.; Mateus, L. Oestrous cycle-related changes in production of Toll-like receptors and prostaglandins in the canine endometrium. J. Reprod. Immunol. 2012, 96, 45–57. [Google Scholar] [CrossRef]
- Gerard, N.; Caillaud, M.; Martoriati, A.; Goudet, G.; Lalmanach, A.C. The interleukin-1 system and female reproduction. J. Endocrinol. 2004, 180, 203–212. [Google Scholar] [CrossRef]
- Shimada, M.; Hernandez-Gonzalez, I.; Gonzalez-Robanya, I.; Richards, J.S. Induced expression of pattern recognition receptors in cumulus oocyte complexes: Novel evidence for innate immune-like functions during ovulation. Mol. Endocrinol. 2006, 20, 3228–3239. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.F.; Guo, S.; Yang, J.; Liu, J.F.; Shaukat, A.; Zhao, G.; Wu, H.C.; Deng, G.Z. Matrine alleviates Staphylococcus aureus lipoteichoic acid-induced endometritis via suppression of TLR2-mediated NF-kappa B activation. Int. Immunopharmacol. 2019, 70, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Gu, T.; Xu, Q.; Zhu, G.; Chen, G. Effects of Salmonella enterica serovar Enteritidis infection on egg production and the immune response of the laying duck Anas platyrhynchos. PeerJ 2019, 7, e6359. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, L.; Yang, P.; Zhang, Q.; Waqas, Y.; Liu, T.; Zhang, L.; Wang, S.; Chen, W.; Le, Y.; et al. Expression of TLR2/4 in the sperm-storing oviduct of the Chinese soft-shelled turtle Pelodiscus sinensis during hibernation season. Ecol. Evol. 2015, 5, 4466–4479. [Google Scholar] [CrossRef]
- Chotimanukul, S.; Sirivaidyapong, S. The localization of Toll-like receptor 2 (TLR2) in the endometrium and the cervix of dogs at different stages of the oestrous cycle and with pyometra. Reprod. Domest. Anim. 2012, 47 (Suppl. S6), 351–355. [Google Scholar] [CrossRef]
- Turner, M.L.; Cronin, J.G.; Healey, G.D.; Sheldon, I.M. Epithelial and Stromal Cells of Bovine Endometrium Have Roles in Innate Immunity and Initiate Inflammatory Responses to Bacterial Lipopeptides In Vitro via Toll-Like Receptors TLR2, TLR1, and TLR6. Endocrinology 2014, 155, 1453–1465. [Google Scholar] [CrossRef]
- Ghasemi, N.; Amjadi, F.; Salehi, E.; Shakeri, M.; Aflatoonian, A.; Aflatoonian, R. Expression of Toll-like receptors 7-10 in human fallopian tubes. Iran. J. Reprod. Med. 2014, 12, 389–394. [Google Scholar]
- Ruiz-Gonzalez, I.; Minten, M.; Wang, X.; Dunlap, K.A.; Bazer, F.W. Involvement of TLR7 and TLR8 in conceptus development and establishment of pregnancy in sheep. Reproduction 2015, 149, 305–316. [Google Scholar] [CrossRef]
- Aflatoonian, R.; Tuckerman, E.; Elliott, S.L.; Bruce, C.; Aflatoonian, A.; Li, T.C.; Fazeli, A. Menstrual cycle-dependent changes of Toll-like receptors in endometrium. Human. Reprod. 2007, 22, 586–593. [Google Scholar] [CrossRef]
- Herath, S.; Fischer, D.P.; Werling, D.; Williams, E.J.; Lilly, S.T.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Expression and function of Toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology 2006, 147, 562–570. [Google Scholar] [CrossRef]
- Laffont, S.; Rouquie, N.; Azar, P.; Seillet, C.; Plumas, J.; Aspord, C.; Guery, J.C. X-Chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-alpha production of plasmacytoid dendritic cells from women. J. Immunol. 2014, 193, 5444–5452. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Ojeda, M.; Martinez-Garcia, M.A.; Alpanes, M.; Luque-Ramirez, M.; Escobar-Morreale, H.F. Association of TLR2 S450S and ICAM1 K469E polymorphisms with polycystic ovary syndrome (PCOS) and obesity. J. Reprod. Immunol. 2016, 113, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Luttgenau, J.; Herzog, K.; Struve, K.; Latter, S.; Boos, A.; Bruckmaier, R.M.; Bollwein, H.; Kowalewski, M.P. LPS-mediated effects and spatio-temporal expression of TLR2 and TLR4 in the bovine corpus luteum. Reproduction 2016, 151, 391–399. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Sequence 5′-3′ | PCR Product Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| TLR1 | F-CCCAACTTTGTCCAGAGCGA | 262 | 60 |
| R-CTGCTGCTTTTCCCATCAGTT | |||
| TLR2 | F-ACGGGCTGTGGTACATGAAG | 212 | 60 |
| R-TTTGCCAGGGACAAGGTCTC | |||
| TLR3 | F-TTTTCTTGGTTGGGGCACCT | 193 | 60 |
| R-CCACCCTTCGAAGCATCAGT | |||
| TLR4 | F-TCCACCTGATGCTTCTTGCT | 203 | 60 |
| R-GATGATATTGGCGGCGATGG | |||
| TLR5 | F-GGGAGACTGCCTTGACCTTC | 289 | 60 |
| R-GAGATTGGGCAGGTTTCGGA | |||
| TLR6 | F-TCCAATCACCACGAGTCTCA | 158 | 60 |
| R-GCAAGTGAGCAACAGGTAGT | |||
| TLR7 | F-AAACTCTGCCCTGTGATGTC | 148 | 60 |
| R-GAGATGCCTGCTATGTGGTT | |||
| TLR8 | F-CCCGAAGCTATCCTTGCGAT | 294 | 60 |
| R-GCAGCAACTCCCTTAGGTGT | |||
| TLR9 | F-CTGCTGCTGTCCTACAACCA | 244 | 60 |
| R-CGCGGAACCAGTCTTTCTCT | |||
| GAPDH | F-GTCAAGGCAGAGAACGGGAA | 232 | 60 |
| R-GGTTCACGCCCATCACAAAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zheng, J.; Yang, H.; Feng, X.; Li, F.; Pang, J.; Yao, X.; Wang, F.; Zhang, Y. Toll-like Receptor Expression Patterns in the Female Reproductive Tract of Sheep. Animals 2025, 15, 1704. https://doi.org/10.3390/ani15121704
Wang Z, Zheng J, Yang H, Feng X, Li F, Pang J, Yao X, Wang F, Zhang Y. Toll-like Receptor Expression Patterns in the Female Reproductive Tract of Sheep. Animals. 2025; 15(12):1704. https://doi.org/10.3390/ani15121704
Chicago/Turabian StyleWang, Zhibo, Jian Zheng, Hua Yang, Xu Feng, Fengzhe Li, Jing Pang, Xiaolei Yao, Feng Wang, and Yanli Zhang. 2025. "Toll-like Receptor Expression Patterns in the Female Reproductive Tract of Sheep" Animals 15, no. 12: 1704. https://doi.org/10.3390/ani15121704
APA StyleWang, Z., Zheng, J., Yang, H., Feng, X., Li, F., Pang, J., Yao, X., Wang, F., & Zhang, Y. (2025). Toll-like Receptor Expression Patterns in the Female Reproductive Tract of Sheep. Animals, 15(12), 1704. https://doi.org/10.3390/ani15121704





