Effects of Heat Stress on Estrus Expression and Pregnancy in Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Farm Conditions
2.2. Microclimate of the Barn
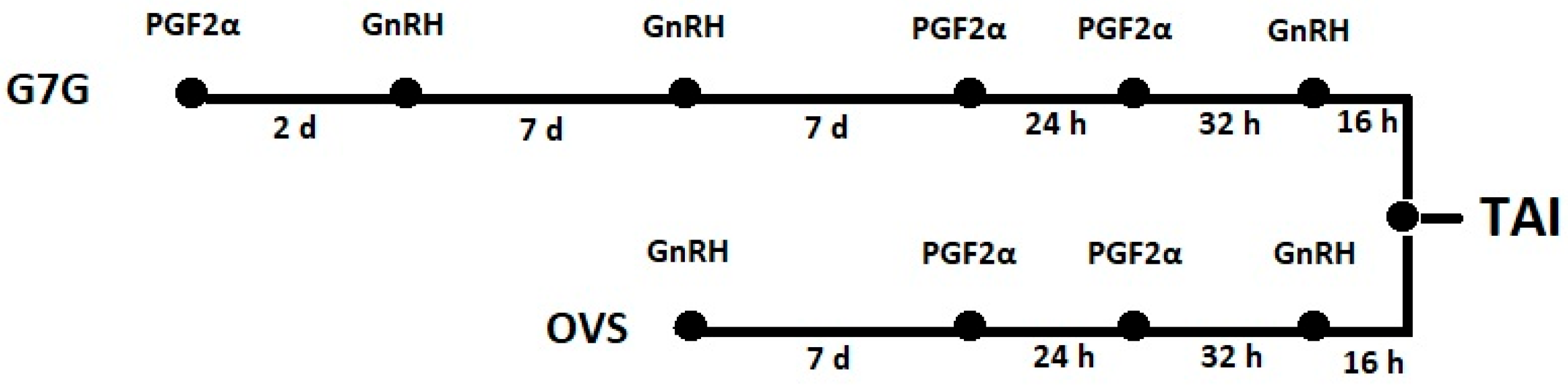
2.3. Study Design
- (1)
- Following the voluntary waiting period after calving, cows at 48 ± 3 DIM were enrolled in the G7G protocol, leading to their first timed artificial insemination (TAI). The G7G program was scheduled as follows (Figure 1): injection of PGF2α (cloprostenol; 0.250 mg, 2 mL, Syncoprost; Vetem S.p.A., Porto Empedocle, Italy) and a GnRH (gonadorelin 50 μg, 2 mL, Ovarelin; Ceva Sante Animale, Libourne, France) 2 d later, followed by a 7-day Ovsynch (OVS) protocol with double PGF, (GnRH, 7 d, PGF2α, 24 h, PGF2α, 32 h, GnRH, 16 h TAI) initiated 7 d later (Figure 1).
- (2)
- After a negative pregnancy diagnosis, cows were enrolled in an OVS program (Figure 1) with double PGF (GnRH, 7 d, PGF2α, 24 h, PGF2α, 32 h, GnRH, 16 h TAI).
2.4. Samplings and Blood Analysis
2.5. Estrus Behavior Measurements
2.6. Statistical Analysis
3. Results
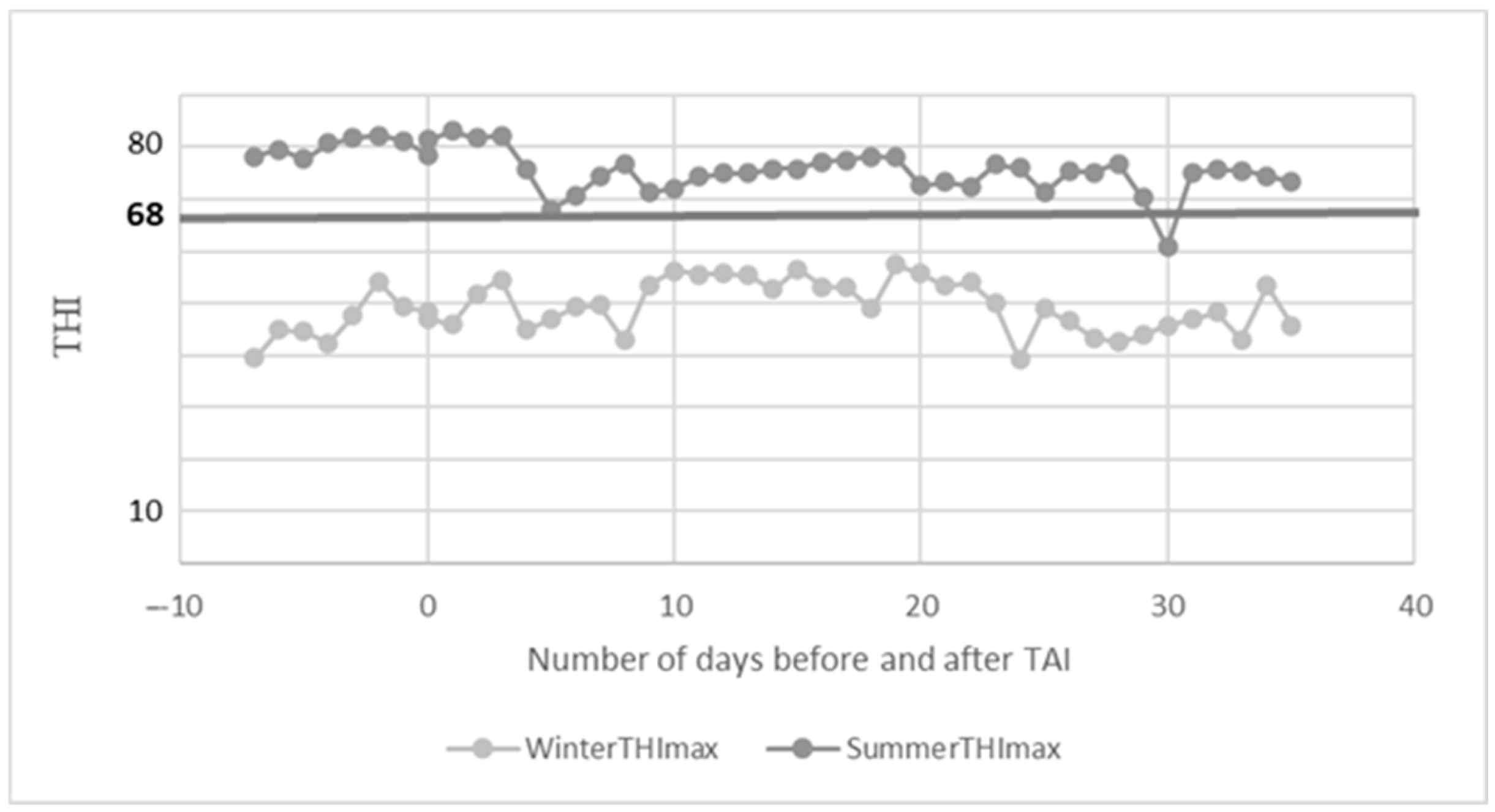
3.1. Barn Microclimate
3.2. Hormone Concentrations
3.3. Estrus Behavior
3.4. Estrus Intensity Changes in Heat Stress and Thermoneutral Period
3.5. Pregnancy Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roelofs, J.; López-Gatius, F.; Hunter, R.H.F.; van Eerdenburg, F.J.C.M.; Hanzen, C. When is a cow in estrus? Clinical and practical aspects. Theriogenology 2010, 74, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.A.; Collier, R.J.; Stone, A.E. Invited review: Physiological and behavioral effects of heat stress in dairy Cows. J. Dairy Sci. 2020, 103, 6751–6770. [Google Scholar] [CrossRef] [PubMed]
- Kadzere, C.T.; Murphy, M.R.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- De Rensis, F.; Scaramuzzi, R.J. Heat stress and seasonal effects on reproduction in the dairy cow—A review. Theriogenology 2003, 60, 1139–1151. [Google Scholar] [CrossRef]
- Schütz, K.E.; Cox, N.R.; Matthews, L.R. How important is shade to dairy cattle? Choice between shade or lying following different levels of lying deprivation. Appl. Anim. Behav. Sci. 2008, 114, 307–318. [Google Scholar] [CrossRef]
- Pereira, M.H.C.; Wiltbank, M.C.; Barbosa, L.F.S.P.; Costa, W.M.; Carvalho, M.A.P.; Vasconcelos, J.L.M. Effect of adding a gonadotropin-releasing-hormone treatment at the beginning and a second prostaglandin F2α treatment at the end of an estradiol-based protocol for timed artificial insemination in lactating dairy cows during cool or hot seasons of the year. J. Dairy Sci. 2015, 98, 947–959. [Google Scholar] [CrossRef]
- Roth, Z. Reproductive physiology and endocrinology responses of cows exposed to environmental heat stress—Experiences from the past and lessons for the present. Theriogenology 2020, 155, 150–156. [Google Scholar] [CrossRef]
- Wolfenson, D.; Roth, Z.; Meidan, R. Impaired reproduction in heat-stressed cattle: Basic and applied aspects. Anim. Reprod. Sci. 2000, 60–61, 535–547. [Google Scholar] [CrossRef]
- Wolfenson, D.; Roth, Z. Impact of heat stress on cow reproduction and fertility. Anim. Front. 2019, 9, 32–38. [Google Scholar] [CrossRef]
- Mietkiewska, K.; Kordowitzki, P.; Pareek, C.S. Effects of heat stress on bovine oocytes and early embryonic development—An update. Cells 2022, 11, 4073. [Google Scholar] [CrossRef]
- Roth, Z.; Meidan, R.; Shaham-Albalancy, A.; Braw-Tal, R.; Wolfenson, D. Delayed effect of heat stress on steroid production in medium-sized and preovulatory bovine follicles. Reproduction 2001, 121, 745–751. [Google Scholar] [CrossRef] [PubMed]
- García-Ispierto, I.; López-Gatius, F.; Bech-Sabat, G.; Santolaria, P.; Yániz, J.L.; Nogareda, C.; De Rensis, F.; López-Béjar, M. Climate factors affecting conception rate of high producing dairy cows in northeastern Spain. Theriogenology 2007, 67, 1379–1385. [Google Scholar] [CrossRef]
- Al-Katanani, Y.M.; Webb, D.W.; Hansen, P.J. Factors affecting seasonal variation in 90-day nonreturn rate to first service in lactating Holstein cows in a hot climate. J. Dairy Sci. 1999, 82, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.J.; Aréchiga, C.F. Strategies for managing reproduction in the heat-stressed dairy cow. J. Anim. Sci. 1999, 77 (Suppl. 2), 36–50. [Google Scholar] [CrossRef]
- Gwazdauskas, F.C.; Thatcher, W.W.; Kiddy, C.A.; Pape, M.J.; Wilcox, C.J. Hormonal pattern during heat stress following PGF2alpha-tham salt induced luteal regression in heifers. Theriogenology 1981, 16, 271–285. [Google Scholar] [CrossRef]
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy cow reproduction under the influence of heat stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 978–986. [Google Scholar] [CrossRef]
- Reith, S.; Hoy, S. Behavioural signs of estrus and the potential of fully automated systems for detection of estrus in dairy cattle. Animal 2018, 12, 398–407. [Google Scholar] [CrossRef]
- López-Gatius, F.; Santolaria, P.; Mundet, I.; Yàniz, J.L. Walking activity at estrus and subsequent fertility in dairy cows. Theriogenology 2005, 63, 1419–1429. [Google Scholar] [CrossRef]
- Gwazdauskas, F.C.; Lineweaver, J.A.; McGilliard, M.L. Environmental and management factors affecting estrous activity in dairy cattle. J. Dairy Sci. 1983, 66, 1510–1514. [Google Scholar] [CrossRef]
- Butler, W.R. Nutritional effects on resumption of ovarian cyclicity and conception rate in postpartum dairy cows. In Fertility in the High-Producing Dairy Cow; Diskin, M.G., Ed.; BSAS: Edinburgh, UK, 2001; pp. 133–145. [Google Scholar]
- Ronchi, B.; Stradaioli, G.; Supplizi, A.V.; Bernabucci, U.; Lacetera, N.; Accorsi, P.A.; Nardone, A.; Seren, E. Influence of heat stress or feed restriction on plasma progesterone, oestradiol-17beta, LH, FSH, prolactin and cortisol in Holstein heifers. Livest. Prod. Sci. 2001, 68, 231–241. [Google Scholar] [CrossRef]
- Mader, T.L.; Davis, M.S.; Brown-Brandl, T. Environmental factors influencing heat stress in feedlot cattle. J. Anim. Sci. 2006, 84, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Reiczigel, J.; Solymosi, N.; Könyves, L.; Maróti-Agóts, A.; Kern, A.; Bartyik, J. Examination of heat stress caused milk production loss by the use of temperature-humidity indices. Magy. Állatorv. Lapja 2009, 131, 137–144. [Google Scholar]
- Collier, R.J.; Zimbelman, R.B.; Rhoads, R.P.; Rhoads, M.L.; Baumgard, L.H. A re-evaluation of the impact of temperature humidity index (THI) and black globe humidity index (BGHI) on milk production in high producing dairy cows. In Proceedings of the Western Dairy Management Conference, Reno, NV, USA, 9–11 March 2011; pp. 113–126. [Google Scholar]
- Zemjanis, R. Diagnostic and Therapeutic Techniques in Animal Reproduction, 2nd ed.; Williams and Wilkins: Baltimore, MD, USA, 1970; pp. 29–45. [Google Scholar]
- Romano, J.E.; Thompson, J.A.; Kramer, D.C.; Westhusin, M.E.; Forrest, D.W.; Tomaszweski, M.A. Early pregnancy diagnosis by palpation per rectum: Influence on embryo/fetal viability in dairy cattle. Theriogenology 2007, 67, 486–493. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2024. Available online: https://www.r-project.org/ (accessed on 15 August 2024).
- Gaude, I.; Kempf, A.; Strüve, K.D.; Hoedemaker, M. Estrus signs in Holstein Friesian dairy cows and their reliability for ovulation detection in the context of visual estrus detection. Livest. Sci. 2021, 245, 104449. [Google Scholar] [CrossRef]
- Senger, P.L. Pathways to Pregnancy and Parturition, 3rd ed.; Current Conceptions Inc.: Pullman, WA, USA, 2012. [Google Scholar]
- Schüller, L.K.; Michaelis, I.; Heuieser, W. Impact of heat stress on estrus expression and follicle size in estrus under field condition in dairy cows. Theriogenology 2017, 102, 48–53. [Google Scholar] [CrossRef]
- Yaniz, J.L.; Santolaria, P.; Giribet, A.; Lopez-Gatius, F. Factors affecting walking activity at estrus during postpartum period and subsequent fertility in dairy cows. Theriogenology 2006, 66, 1943–1950. [Google Scholar] [CrossRef]
- Orihuela, A. Some factors affecting the behavioural manifestation of oestrus in cattle: A review. Appl. Anim. Behav. Sci. 2000, 70, 1–16. [Google Scholar] [CrossRef]
- Sakatani, M.; Balboula, A.Z.; Yamanaka, K.; Takahashi, M. Effect of summer heat environment on body temperature, estrous cycles and blood antioxidant levels in Japanese Black cow. Anim. Sci. 2012, 83, 394–402. [Google Scholar] [CrossRef]
- López-Gatius, F.; Mirzaei, A.; Santolaria, P.; Bech-Sábat, G.; Nogareda, C.; García-Ispierto, I.; Hanzen, C.; Yániz, J.L. Factors affecting the response to the specific treatment of several forms of clinical anestrus in high producing dairy cows. Theriogenology 2008, 69, 1095–1103. [Google Scholar] [CrossRef]
- Morton, J.M.; Tranter, W.P.; Mayer, D.G.; Jonsson, N.N. Effects of environmental heat on conception rates in lactating dairy cows: Critical periods of exposure. J. Dairy Sci. 2007, 90, 2271–2278. [Google Scholar] [CrossRef]
- Schüller, L.K.; Burfeind, O.; Heuwieser, W. Impact of heat stress on conception rate of dairy cows in the moderate climate considering different temperature humidity index thresholds, periods relative to breeding, and heat load indices. Theriogenology 2014, 81, 1050e7. [Google Scholar] [CrossRef]
- Gilad, E.; Median, R.; Berman, A.; Graber, Y.; Wolfenson, D. Effect of heat stress on tonic and GnRH-induced gonadotrophin secretion in relation to concentration of oestradiol in plasma of cyclic cow. J. Reprod. Fertil. 1993, 99, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Reames, P.S.; Hatler, T.B.; Hayes, S.H.; Ray, D.L.; Silvia, W.J. Differential regulation of estrous behavior and luteinizing hormone secretion by estradiol-17β in ovariectomized dairy cows. Theriogenology 2011, 75, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Khodaei-Motlagh, M.; Shahneh, A.Z.; Masoumi, R.; De Renis, F. Alterations in reproductive hormones during heat stress in dairy cattle. Afr. J. Biotechnol. 2010, 10, 5552–5558. [Google Scholar]
- Roth, Z.; Median, R.; Braw-Tal, R.; Wolfenson, D. Immediate and delayed effect of heat stress on follicular developments and its association with plasma FSH and inhibin concentration in cow. J. Reprod. Fertil. 2000, 120, 80–83. [Google Scholar]
- De Rensis, F.; Marconi, P.; Capelli, T.; Gatti, F.; Facciolongo, F.; Franzini, S.; Scaramuzzi, R.J. Fertility in postpartum dairy cows in winter or summer following estrous synchronization and fixed time A.I. after the induction of an LH surge with Gonadotropin-releasing hormone (GnRH) or human chorionic gonadotropin (hCG). Theriogenology 2002, 58, 1675–1687. [Google Scholar] [CrossRef]
- O’Calagan, D.; Boland, M.P. Nutritional effects on ovulation, embryo development and the establishment of pregnancy in ruminants. Anim. Sci. 1999, 68, 299–314. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Baez, G.M.; Cochrane, F.; Barletta, R.V.; Trayford, C.R.; Joseph, R.T. Effect of a second treatment with prostaglandin F2α during the Ovsynch protocol on luteolysis and pregnancy in dairy cows. J. Dairy Sci. 2015, 98, 8644–8654. [Google Scholar] [CrossRef]
- Yotov, S.; Fasulkov, I.; Atanasov, A.; Kistanova, E.; Sinapov, B.; Ivanova, B.; Yarkov, D.; Zaimova, D. Influence of ovarian status and steroid hormone concentration on day of timed artificial insemination (TAI) on the reproductive performance of dairy cows inseminated with sexed semen. Animals 2023, 13, 896. [Google Scholar] [CrossRef]
- Alnimer, M.; De Rosa, G.; Grasso, F.; Napolitana, F.; Bordi, A. Effect of climate on the response of three oestrus synchronization techniques in lactating dairy cows. Anim. Reprod. Sci. 2002, 71, 157–168. [Google Scholar] [CrossRef]

| Summer | Winter | |
|---|---|---|
| Ambient temperature (°C; average; min.; max.) | 23; 14; 35 | 5; −4; 14 |
| Relative humidity (%; average; min.; max.) | 67; 32; 95 | 77; 39; 95 |
| THI 1 (average; min.; max.) | 70; 57; 83 | 44; 28; 57 |
| Summer Mean (95% CI 1) | Winter Mean (95%CI) | p Value | |
|---|---|---|---|
| Estradiol (pg/mL) | |||
| G7G 2 | 34.6 (24.8; 44.4) | 38.2 (29.0;47.3) | 0.525 |
| OVS | 18.9 (9.26; 28.6) | 40.6 (31.3; 50.0) | <0.001 |
| Luteinizing hormone (mIU/mL) | |||
| G7G | 0.54 (0.22; 0.86) | 0.34 (0.08; 0.61) | 0.322 |
| OVS | 0.25 (0.01; 0.62) | 0.28 (0.01; 0.64) | 0.928 |
| Insulin (µIU/mL) | |||
| G7G | 20.8 (13.9; 27.8) | 15.8 (8.74; 22.8) | 0.2581 |
| OVS | 21.2 (14.0; 28.3) | 21.2 (13.8; 28.7) | 0.9914 |
| Prolactin (ng/mL) | |||
| G7G | 32.4 (16.2; 48.5) | 35.0 (19.7; 50.2) | 0.7780 |
| OVS | 30.4 (13.6; 47.3) | 37.1 (19.4; 54.8) | 0.5023 |
| Insulin-like growth factor 1 (ng/mL) | |||
| G7G | 121.0 (80.7; 161.0) | 144.0 (106.8; 181) | 0.3105 |
| OVS | 149.0 (110; 189) | 183.0 (145.0; 221.0) | 0.1800 |
| Summer | Winter | p-Value 1 | |
|---|---|---|---|
| G7G 2 | |||
| intensive estrus | 3 | 9 | 0.058 |
| silent estrus | 5 | 4 | |
| no estrus | 7 | 2 | |
| OVS | |||
| intensive estrus | 9 | 10 | 0.154 |
| silent estrus | 4 | 0 | |
| no estrus | 2 | 3 | |
| G7G | |||
| pregnant | 3 | 7 | 0.245 |
| non-pregnant | 12 | 8 | |
| OVS | |||
| pregnant | 0 | 4 | 0.035 |
| non-pregnant | 15 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szalai, S.; Bodnár, Á.; Fébel, H.; Bakony, M.; Jurkovich, V. Effects of Heat Stress on Estrus Expression and Pregnancy in Dairy Cows. Animals 2025, 15, 1688. https://doi.org/10.3390/ani15121688
Szalai S, Bodnár Á, Fébel H, Bakony M, Jurkovich V. Effects of Heat Stress on Estrus Expression and Pregnancy in Dairy Cows. Animals. 2025; 15(12):1688. https://doi.org/10.3390/ani15121688
Chicago/Turabian StyleSzalai, Szilvia, Ákos Bodnár, Hedvig Fébel, Mikolt Bakony, and Viktor Jurkovich. 2025. "Effects of Heat Stress on Estrus Expression and Pregnancy in Dairy Cows" Animals 15, no. 12: 1688. https://doi.org/10.3390/ani15121688
APA StyleSzalai, S., Bodnár, Á., Fébel, H., Bakony, M., & Jurkovich, V. (2025). Effects of Heat Stress on Estrus Expression and Pregnancy in Dairy Cows. Animals, 15(12), 1688. https://doi.org/10.3390/ani15121688







