Growth Performance and Realized Heritability in a Mass-Selected Strain of Silver Pomfret (Pampus argenteus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Parental Origin
2.2. Hatching and Rearing
2.3. Sampling and Growth Measurement
2.4. Statistics on the Rate of Hatching and Fertilization
2.5. Growth Performance Parameters
2.6. Estimation of Genetic Parameters
2.7. Statistical Analyses
3. Results
3.1. The Fertilization Rate and Hatching Rate
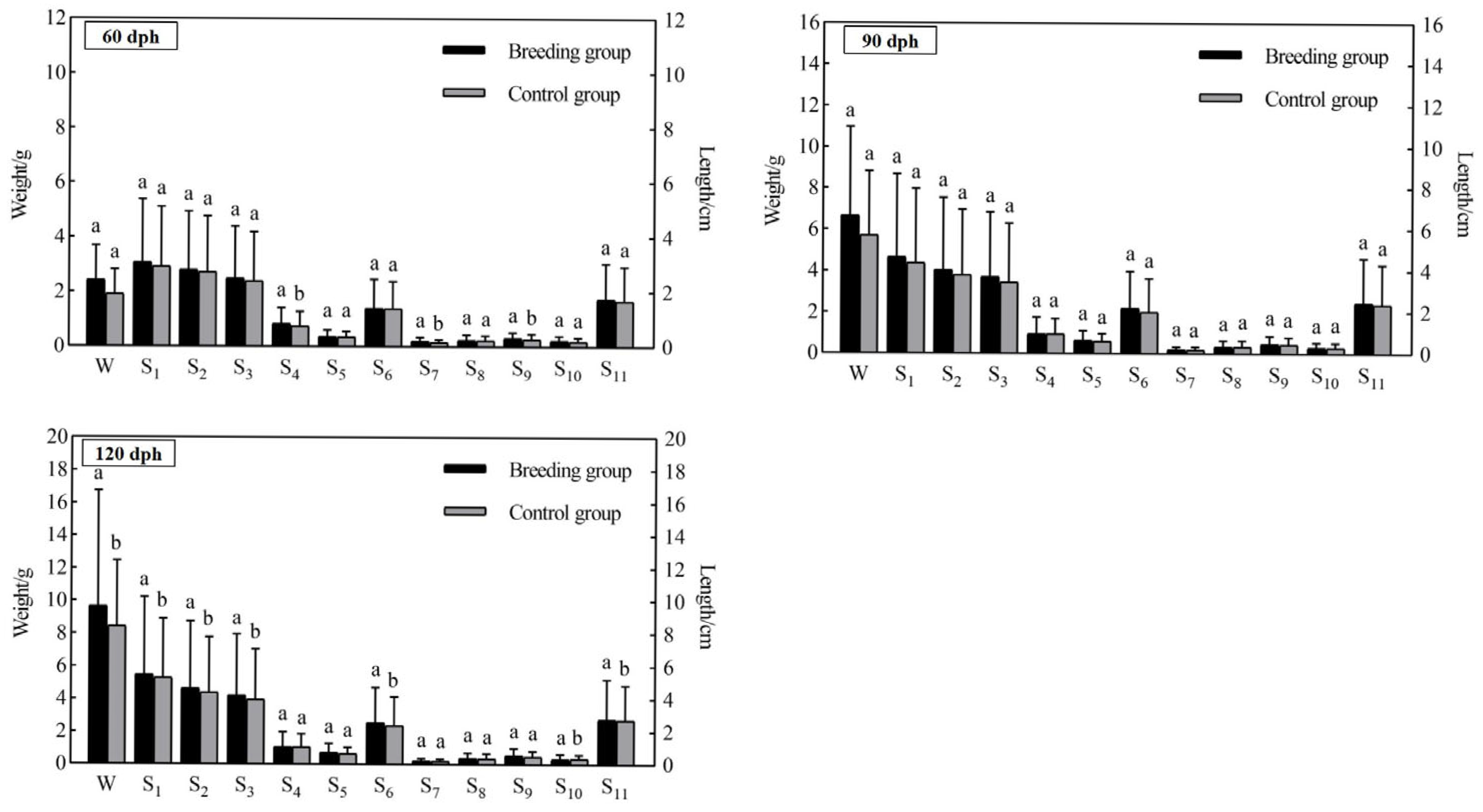
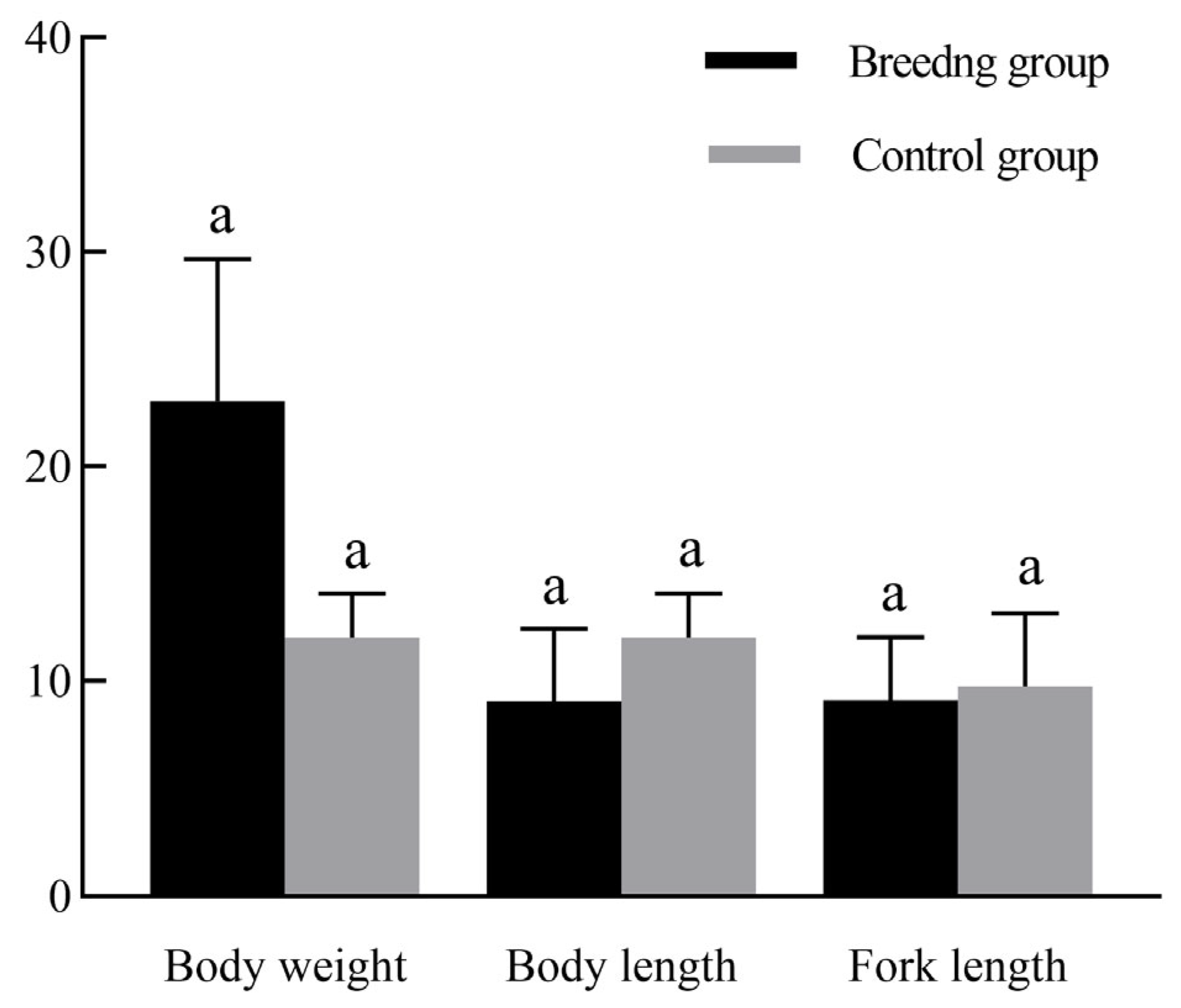
3.2. Growth Performance Comparison
3.3. Genetic Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newkirk, G.F. Applied breeding of commercially important molluscs: A summary of discussion. Aquaculture 1983, 33, 415–422. [Google Scholar] [CrossRef]
- Langdon, C.; Evans, F.; Jacobson, D.; Blouin, M. Yields of cultured Pacific oysters Crassostrea gigas Thunberg improved after one generation of selection. Aquaculture 2003, 220, 227–244. [Google Scholar] [CrossRef]
- Sheridan, A.K. Genetic improvement of oyster production—A critique. Aquaculture 1997, 153, 165–179. [Google Scholar] [CrossRef]
- Hulata, G. Genetic manipulations in aquaculture: A review of stock improvement by classical and modern technologies. Genetica 2001, 111, 155–173. [Google Scholar] [CrossRef]
- Khasani, I. and Sopian, A. Selection response and reproduction performance of selected giant freshwater prawn (Macrobrachium rosenbergii). AACL Bioflux 2021, 14, 1068–1077. [Google Scholar]
- Li, Q.; Wang, Q.Z.; Liu, S.K.; Kong, L.F. Selection response and realized heritability for growth in three stocks of the Pacific oyster Crassostrea gigas. Fish. Sci. 2011, 77, 643–648. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Li, Q.; Kong, L.F.; Yu, R.H. Response to selection for fast growth in the second generation of pacific oyster (Crassostrea gigas). J. Ocean. Univ. China 2012, 11, 413–418. [Google Scholar] [CrossRef]
- Sun, C.F.; Dong, J.J.; Li, W.H.; Tian, Y.Y.; Hu, J.; Ye, X. Response to four generations of selection for growth performance traits in mandarin fish (Siniperca chuatsi). Aquaculture 2022, 548, 737590. [Google Scholar] [CrossRef]
- Vandeputte, M.; Corraze, G.; Doerflinger, J.; Enez, F.; Clota, F.; Terrier, F.; Horat, M.; Larroquet, L.; Petit, V.; Haffray, P.; et al. Realised genetic gains on growth, survival, feed conversion ratio and quality traits after ten generations of multi-trait selection in rainbow trout Oncorhynchus mykiss, fed a standard diet or a “future” fish-free and soy-free diet. Aquacult. Rep. 2022, 27, 101363. [Google Scholar] [CrossRef]
- Wang, X.B.; Li, Q.; Yu, H.; Kong, L.F. Genetic variation assessed with microsatellites in mass selection lines of the pacific oyster (Crassostrea gigas) in China. J. Ocean. Univ. China 2016, 15, 1039–1045. [Google Scholar] [CrossRef]
- Hu, X.S.; Li, C.T.; Shang, M.; Ge, Y.L.; Jia, Z.Y.; Wang, S.H.; Zhang, Q.J.; Shi, L.Y. Inheritance of growth traits in songpu mirror carp (Cyprinus carpio L.) cultured in northeast china. Aquaculture 2017, 477, 1–5. [Google Scholar]
- Imron, I.; Iswanto, B.; Suparapto, R.; Marnis, H. Development of genetically improved farmed African Catfish, Clarias gariepinus; a review and lessons learned from indonesian fish breeding program. IOP Conf. Ser. Earth Environ. Sci. 2020, 593, 012032. [Google Scholar] [CrossRef]
- Janhunen, M.; Kause, A.; Vehviläinen, H.; Nousiainen, A.; Koskinen, H. Accounting for early rearing density effects on growth in the genetic evaluation of rainbow trout, Oncorhynchus mykiss. J. Anim. Sci. 2013, 91, 5144–5152. [Google Scholar]
- Bentsen, H.B.; Gjerde, B.; Eknath, A.E.; de Vera, M.S.P.; Velasco, R.R.; Danting, J.C.; Dionisio, E.E.; Longalong, F.M.; Reyes, R.A.; Abella, T.A.; et al. Genetic improvement of farmed tilapias: Response to five generations of selection for increased body weight at harvest in oreochromis niloticus and the further impact of the project. Aquaculture 2017, 468, 206–217. [Google Scholar]
- Liu, F.; Li, Y.Z.; Du, M.; Shao, C.W.; Chen, S.L. Analysis of phenotypic and genetic parameters for growth-related traits in the half smooth tongue sole, Cynoglossus semilaevis. Chin. J. Oceanol. Limn. 2016, 34, 163–169. [Google Scholar] [CrossRef]
- Cui, Z.X.; Liu, Y.; Liu, J.; Luan, W.S. Molecular identification of pampus fishes (Perciformes, Stromateidae). Ichthyol Res. 2010, 57, 32–39. [Google Scholar]
- Davis, P.; Wheeler, A. The occurrence of Pampus argenteus (Euphrasen, 1788), (Osteichthyes, Perciformes, Stromateoidei, Stromateidae) in the North Sea. J. Fish Biol. 1985, 26, 105–109. [Google Scholar] [CrossRef]
- Sun, P.; Yin, F.; Shi, Z.; Peng, S. Genetic structure of silver pomfret (Pampus argenteus (Euphrasen, 1788)) in the Arabian Sea, Bay of Bengal, and South China Sea as indicated by mitochondrial COI gene sequences. J. Appl. Ichthyol. 2013, 29, 733–737. [Google Scholar] [CrossRef]
- Zhao, F.; Zhuang, P.; Song, C.; Shi, Z.H.; Zhang, L.Z. Amino acid and fatty acid compositions and nutritional quality of muscle in the pomfret, Pampus punctatissimus. Food Chem. 2010, 118, 224–227. [Google Scholar] [CrossRef]
- Campos-Montes, G.R.; Montaldo, H.H.; Martínez-Ortega, A.; Jiménez, A.M.; Castillo-Juárez, H. Genetic parameters for growth and survival traits in pacific white shrimp penaeus (Litopenaeus) vannamei from a nucleus population undergoing a two-stage selection program. Aquacult Int. 2013, 21, 299–310. [Google Scholar] [CrossRef]
- Sheng, Z.L.; Chen, Y.S. Quantitative Genetics; Science Press: Beijing, China, 1999; pp. 164–173. [Google Scholar]
- Huang, X.; Zhang, C.; Hu, J.B.; Tao, S.S.; Tang, J.; Huang, L.; Zheng, C.J.; Xu, S.L.; Wang, Y.J. Analyses of growth performance and realized heritability of the second generation of Indo-Pacific Pampus argenteus. J. Fish Biol. 2023, 102, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Akvaforsk. Selection and Breeding Programs in Aquaculture; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Lallias, D.; Quillet, E.; Bégout, M.L.; Aupérin, B.; Khaw, H.L.; Millot, S.; Valotaire, C.; Kernéais, T.; Labbé, L.; Prunet, P.; et al. Genetic variability of environmental sensitivity revealed by phenotypic variation in body weight and (its) correlations to physiological and behavioral traits. PLoS ONE 2017, 12, e0189943. [Google Scholar] [CrossRef]
- Li, J.; Ou, Y.; Wen, J.; Hu, R.; Zhu, C.; Lan, J.; Li, J.; Zhou, H. Correlation among growth performance, morphological traits and body mass of Eleutheronema tetradactyuulum cultured in indoor circulating aquaculture system and aquaculture pond. South China Fish. Sci. 2020, 16, 27–35. [Google Scholar]
- Zhang, C.; Jacques, K.J.; Zhang, S.; Xu, S.L.; Wang, Y.J.; Wang, D.L. Analyses of growth performance and realized heritability of Pampus argenteus in a breeding program in China. Front. Mar. Sci. 2022, 9, 935924. [Google Scholar] [CrossRef]
- Tong, X.H.; Ge, B.M.; Wang, H.L.; Yang, X.L.; Zhang, Z.M.; Ju, Y.; Lu, Y. Correlation between morphological traits and body weight of Japanese Flounder during different periods. Agric. Sci. Hubei 2014, 53, 863–865. [Google Scholar]
- Chen, X.; Qin, S.; Ye, S. Analysis of the fish community structure and its correlation with environmental factors in leqing bay. J. Zhejiang Agric. Sci. 2023, 64, 1806–1812. [Google Scholar]
- Canosa, L.F.; Bertucci, J.I. The effect of environmental stressors on growth in fish and its endocrine control. Front. Endocrinol. 2023, 14, 1109461. [Google Scholar] [CrossRef]
- Zenger, K.R.; Khatkar, M.S.; Jones, D.B.; Khalilisamani, N.; Jerry, D.R.; Raadsma, H.W. Genomic selection in aquaculture: Application, limitations and opportunities with special reference to marine shrimp and pearl oysters. Front. Genet. 2019, 9, 693. [Google Scholar] [CrossRef]
- Wu, X.B.; Li, X.M.; Zhu, Y.J.; Gong, J.L.; Zhu, T.B.; Ni, J.J.; Yang, D.G. Effects of water temperature on the growth, antioxidant capacity, and gut microbiota of Percocypris pingi juveniles. Fishes 2022, 7, 374. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Hagras, A.E.; Elbaghdady, H.A.M.; Monier, M.N. Effects of dissolved oxygen and fish size on nile tilapia, Oreochromis niloticus (L.): Growth performance, whole-body composition, and innate immunity. Aquacult. Int. 2015, 23, 1261–1274. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, S.Q.; Wang, S. Weight–length relationship and condition factor of gibel carp (Carassius auratus gibelio var. CAS V) at different growth stages and feed formulations. Fishes 2023, 8, 439. [Google Scholar] [CrossRef]
- Saura, M.; Villanueva, B.; Fernández, J.; Toro, M.A. Effect of assortative mating on genetic gain and inbreeding in aquaculture selective breeding programs. Aquaculture 2017, 472, 30–37. [Google Scholar] [CrossRef]
- Zhang, J.C.; Cao, F.J.; Liu, J.Y.; Yuan, R.P. Estimation of genetic parameters and genetic gain in growth and hypoxic tolerance traits of Litopenaeus vannamei. Oceanol. Limnol. Sin. 2016, 47, 869–875. [Google Scholar]
- Kong, J.; Luan, S.; Tan, J.; Sui, J.; Luo, K.; Li, X.P.; Dai, P.; Meng, X.H.; Lu, X.; Chen, B.L.; et al. Progress of study on penaeid shrimp selective breeding. Period. Ocean. Univ. China 2020, 50, 81–97. [Google Scholar]
- Ibarra, A.M.; Ramirez, J.L.; Ruiz, C.A.; Cruz, P.; Avila, S. Realized heritabilities and genetic correlation after dual selection for total weight and shell width in catarina scallop (Argopecten ventricosus). Aquaculture 1999, 175, 227–241. [Google Scholar] [CrossRef]
- Zheng, H.P.; Zhang, G.F.; Liu, X.A.; Guo, X.M. Sustained response to selection in an introduced population of the hermaphroditic bay scallop Argopecten irradians irradians Lamarck (1819). Aquaculture 2006, 255, 579–585. [Google Scholar] [CrossRef]
- Gjedrem, T.; Rye, M. Selection response in fish and shellfish: A review. Rev. Aquac. 2018, 32, 168–179. [Google Scholar] [CrossRef]
- Phuc, T.H.; Vu, N.T.; Nga, N.T.K.; Ky, N.T.; Nguyen, N.H. Assessment of a long-term selective breeding program for giant freshwater prawn Macrobrachium rosenbergii since 2007. Aquaculture 2021, 541, 736745. [Google Scholar] [CrossRef]
- Chandravanshi, S.; Jayakumar, N.; Durairaja, R.; Aanand, S.; Suresh, E.; Sudhan, C.; Kumar, M.K.; Sonwal, M.C.; Sahu, A. Length-weight relationships and condition factor of 15 coastal fish species (Family: Lethrinidae) of the gulf of mannar, southeast coast of india. Thalassas 2024, 41, 27. [Google Scholar] [CrossRef]
- Amaral, I.P.G.; Johnston, I.A. Experimental selection for body size at age modifies early life-history traits and muscle gene expression in adult zebrafish. J. Exp. Biol. 2012, 215, 3895–3904. [Google Scholar] [CrossRef]
- Wang, X.B.; Li, Y.Y.; Hu, J.B.; Zhang, Y.Y.; Zhang, M.; Wang, G.L.; Jiang, H.; Sun, J.C.; Guo, C.Y.; Xu, S.L.; et al. Effects of different photoperiods on growth and ovarian development and maturation of silver pomfret (Pampus argenteus). J. Fish Biol. 2023, 103, 59–72. [Google Scholar] [CrossRef]
- Panya, S.L.; Kongphop, A.; Sujitra, P.; Boison, S.A. Increasing accuracy of realized heritability estimate using realized selection differential. In Proceedings of the Annual Conference of Fisheries, Ottawa, ON, Canada, 5–6 October 2022. [Google Scholar]
- Vandeputte, M.; Haffray, P. Parentage assignment with genomic markers: A major advance for understanding and exploiting genetic variation of quantitative traits in farmed aquatic animals. Front. Genet. 2014, 5, 103389. [Google Scholar] [CrossRef] [PubMed]
- Noble, D.W.A.; McFarlane, S.E.; Keogh, J.S.; Whiting, M.J. Maternal and additive genetic effects contribute to variation in offspring traits in a lizard. Behav. Ecol. 2014, 25, 633–640. [Google Scholar] [CrossRef][Green Version]
- Garant, D. and Kruuk, L.E.B. How to use molecular marker data to measure evolutionary parameters in wild populations. Mol. Ecol. 2005, 14, 1843–1859. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.T. Aquatic Animal Breeding; China Agriculture Press: Beijing, China, 2014. [Google Scholar]
- Bentsen, H.B.; Gjerde, B.; Nguyen, N.H.; Rye, M.; Ponzoni, R.W.; de Vera, M.S.P.; Bolivar, H.L.; Velasco, R.R.; Danting, J.C.; Dionisio, E.E.; et al. Genetic improvement of farmed tilapias: Genetic parameters for body weight at harvest in nile tilapia (Oreochromis niloticus) during five generations of testing in multiple environments. Aquaculture 2012, 338, 56–65. [Google Scholar] [CrossRef]
- Thodesen, J.; Rye, M.; Wang, Y.X.; Li, S.J.; Bentsen, H.B.; Yazdi, M.H.; Gjedrem, T. Genetic improvement of tilapias in China: Genetic parameters and selection responses in growth, survival and external color traits of red tilapia (Oreochromis spp.) after four generations of multi-trait selection. Aquaculture 2013, 396, 32–42. [Google Scholar] [CrossRef]
- Sun, S.; Hu, Y.L.; Lv, D.; Wang, W.J. Evaluation of genetic gain for harvest body weight in turbot (Scophthalmus Maximus, Linnaeus) after three generations. Chin. Agric. Sci. Bull. 2021, 37, 118–123. [Google Scholar]
- Xu, Y.L.; Chen, Z.; Tan, J.Q.; Wen, L.T.; Pan, X.H.; Zhou, K.Q.; Huang, Y.; Lin, Y.; Du, X.S. Genetic parameters estimation for main growth traits of (Cyprinus carpio var. Quanzhouensis). Sci. Guangxi 2022, 29, 801–808. [Google Scholar]
- Falconer, D.S.; Trudy, F.C. Introduction to quantitative genetics. Trends Genet. 1996, 12, 280. [Google Scholar]
- Rasmussen, R.S.; Ostenfeld, T. Intraspecific growth variation among rainbow trout and brook trout: Impact of initial body weight and feeding level. Aquacult. Int. 2010, 18, 933–941. [Google Scholar] [CrossRef]
- Silva, G.F.; Shiotsuki, L.; Dias, L.T.; Teixeira, R.A. Estimation of genetic parameters for weight and length gains in tambaqui (Colossoma macropomum). Braz. J. Biol. 2023, 83, e277423. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, L.; Zhang, S.D.; Wang, W.J.; Yang, J.M.; Zhang, G.F. Heritability estimates for nutritional quality-related traits of the Pacific oyster. J. World Aquacult. Soc. 2019, 50, 738–748. [Google Scholar] [CrossRef]
- Pillai, B.R.; Ponzoni, R.W.; Das Mahapatra, K.; Panda, D. Genetic improvement of giant freshwater prawn Macrobrachium rosenbergii: A review of global status. Rev. Aquacult. 2022, 14, 1285–1299. [Google Scholar] [CrossRef]
- Liu, X.D.; Cai, M.Y.; Wang, Z.Y.; You, Z.Y. The correlation and path analysis for growth-related traits of large yellow croaker Pseudosciaena crocea from min-yuedong tribe. Period. Ocean Univ. China 2008, 38, 916–920. [Google Scholar]
- Liu, X.D.; Cai, M.Y.; Wang, Z.Y.; Zhao, G.T.; Wu, X.W.; Yao, C.L. Correlation analysis of morphometric traits and body weight of large yellow croaker Pseudosciaena crocea at different growth stage. J. Trop. Oceanogr. 2010, 29, 159–163. [Google Scholar]
- Li, X.; Liu, Y.; Blancheton, J.P. Effect of stocking density on performances of juvenile turbot (Scophthalmus maximus) in recirculating aquaculture systems. Chin. J. Oceanol. Limnol. 2013, 31, 514–522. [Google Scholar] [CrossRef]
- Qin, C.L.; Li, C.; Zhang, C.; Tang, J.; Huang, X.; Li, Y.B.; Hu, J.B.; Wang, Y.J. Study on growth performance and realized heritability in mass selection strain of silver pomfret (Pampus argenteus). Res. Sq. 2024. preprint. [Google Scholar]
| Group | N (Animals) | Age (Days) | Body Weight (g) (Mean ± SD) | Body Length (mm) (Mean ± SD) | Sires | Dams | Selection Intensity |
|---|---|---|---|---|---|---|---|
| Fundamental | 2200 | 270 | 52.42 ± 11.51 | -- | -- | -- | -- |
| Breeding | 200 | 270 | 54.26 ± 10.45 | 124.73 ± 7.73 | 67 | 133 | 1.755 |
| Control | 200 | 270 | 47.94 ± 9.68 | 122.29 ± 9.68 | 71 | 129 | -- |
| Birth Day | Body Length of the Fish/(mm) | Feed Types | Feeding Amount | Feeding Time |
|---|---|---|---|---|
| Start feeding–5 days | ˂5 mm | - | - | 6:20 a.m. 9:30 a.m. 11:00 a.m. 1:30 p.m. 3:00 p.m. 5:00 p.m. 8:00 p.m. 10:20 p.m. |
| 5–10 | 5–8 mm | Rotifer | 1 individual/mL | |
| 11–15 | 8–12 mm | Rotifer | 2–3 individuals/mL | |
| 16–20 | 12–18 mm | Rotifer | 2–3 individuals/ml | |
| Artemia nauplii | 0.5–1 individuals/mL | |||
| 21–26 | 18–25 mm | Artemia nauplii | 1–2 individuals/mL | |
| 27–34 | 20–30 mm | Artemia nauplii | 1–2 individuals/mL | |
| 2# YuBao compound feed | - | |||
| 35–55 | 25–46 mm | 2# YuBao compound feed | - | |
| 56–67 | 29–50 mm | 2# YuBao compound feed | - | |
| 3# YuBao compound feed | - | |||
| 68–90 | 33–66 mm | 3# YuBao compound feed | - | |
| 91–120 | >33 mm | 3# YuBao compound feed | - | |
| SaiFeng Nian compound feed | - | |||
| >120 | >40 mm | SaiFeng Nian compound feed | - |
| Group | The Number of Eggs Laid (×104) | The Number of Fertilized Eggs (×104) | The Number of Hatched Fishes (×104) | Fertilization Rate | Hatching Rate |
|---|---|---|---|---|---|
| Breeding group | 65.58 | 27.05 | 10.23 | 41.25 | 37.82 |
| Control group | 60.40 | 19.72 | 7.24 | 32.65 | 36.69 |
| WGR (%) | SGR (%) | AGR (g/Feeding Day) | CF (g/cm3) | |||||
|---|---|---|---|---|---|---|---|---|
| Breeding Group | Control Group | Breeding Group | Control Group | Breeding Group | Control Group | Breeding Group | Control Group | |
| 60–90 dph | 196.87 | 213.05 | 3.63 | 3.80 | 24.23 | 20.03 | 4.30 | 3.80 |
| 90–120 dph | 52.70 | 41.38 | 1.41 | 1.15 | 19.26 | 12.18 | 3.48 | 3.53 |
| 60–120 dph | 353.33 | 342.59 | 5.04 | 4.96 | 43.49 | 32.21 | 3.32 | 3.58 |
| Traits | Age | GG | GG% | |
|---|---|---|---|---|
| Body weight | Day 60 | 0.87 g | 30.90 | 0.49 |
| Day 90 | 2.15 g | 24.14 | 0.47 | |
| Day 120 | 4.26 g | 34.08 | 0.55 | |
| Mean | 2.43 ± 1.17 g | 29.70 ± 5.08 | 0.50 ± 0.04 | |
| Body length | Day 60 | 0.21 cm | 5.11 | 0.21 |
| Day 90 | 0.55 cm | 8.68 | 0.53 | |
| Day 120 | 0.91 cm | 12.93 | 0.57 | |
| Mean | 0.56 ± 0.29 cm | 8.90 ± 3.91 | 0.44 ± 0.20 | |
| Fork length | Day 60 | 0.17 cm | 3.52 | 0.14 |
| Day 90 | 0.56 cm | 8.16 | 0.50 | |
| Day 120 | 0.97 cm | 12.56 | 0.56 | |
| Mean | 0.57 ± 0.33 cm | 8.08 ± 3.69 | 0.40 ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, C.; Li, C.; Tang, J.; Huang, X.; Li, Y.; Hu, J.; Wang, Y. Growth Performance and Realized Heritability in a Mass-Selected Strain of Silver Pomfret (Pampus argenteus). Animals 2025, 15, 1625. https://doi.org/10.3390/ani15111625
Qin C, Li C, Tang J, Huang X, Li Y, Hu J, Wang Y. Growth Performance and Realized Heritability in a Mass-Selected Strain of Silver Pomfret (Pampus argenteus). Animals. 2025; 15(11):1625. https://doi.org/10.3390/ani15111625
Chicago/Turabian StyleQin, Chunlai, Chang Li, Jie Tang, Xiang Huang, Yuanbo Li, Jiabao Hu, and Yajun Wang. 2025. "Growth Performance and Realized Heritability in a Mass-Selected Strain of Silver Pomfret (Pampus argenteus)" Animals 15, no. 11: 1625. https://doi.org/10.3390/ani15111625
APA StyleQin, C., Li, C., Tang, J., Huang, X., Li, Y., Hu, J., & Wang, Y. (2025). Growth Performance and Realized Heritability in a Mass-Selected Strain of Silver Pomfret (Pampus argenteus). Animals, 15(11), 1625. https://doi.org/10.3390/ani15111625





