Using Abattoir-Based Surveillance to Establish Foot-and-Mouth Disease Non-Structural Protein Seropositivity in Cattle and Pigs in Cambodia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
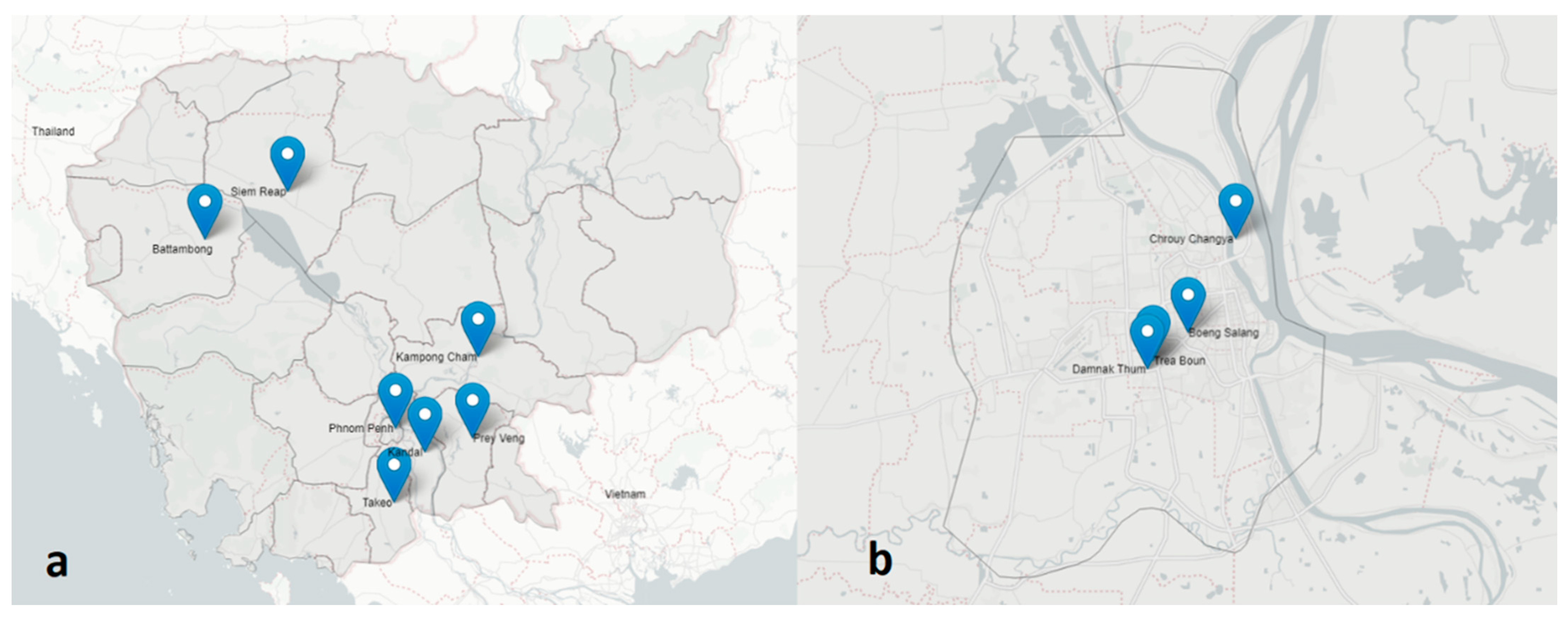
2.1. Study Design
2.2. Sampling and Sample Collection
2.3. Sample Handling and Processing
2.4. FMD NSP Serological Analysis
2.5. Data Analysis
3. Results
3.1. Seroprevalence of FMDV NSP Antibodies in Cattle
3.2. Seroprevalence of FMD NSP in Pigs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandersen, S.; Mowat, N. Foot-and-mouth disease: Host range and pathogenesis. Curr. Top. Microbiol. Immunol. 2005, 288, 9–42. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.J.; Gubbins, S.; King, D.P. Understanding the transmission of foot-and-mouth disease virus at different scales. Curr. Opin. Virol. 2018, 28, 85–91. [Google Scholar] [CrossRef]
- Rweyemamu, M.; Roeder, P.; Mackay, D.; Sumption, K.; Brownlie, J.; Leforban, Y.; Valarcher, J.F.; Knowles, N.J.; Saraiva, V. Epidemiological patterns of foot-and-mouth disease worldwide. Transbound. Emerg. Dis. 2008, 55, 57–72. [Google Scholar] [CrossRef]
- Young, J.R.; Suon, S.; Andrews, C.J.; Henry, L.A.; Windsor, P.A. Assessment of financial impact of foot-and-mouth disease on smallholder cattle farmers in Southern Cambodia. Transbound. Emerg. Dis. 2013, 60, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Kitching, R.P. Clinical variation in foot-and-mouth disease: Cattle. Rev. Sci. Tech. Off. Int. Epiz. 2002, 21, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, L. A review of the status of foot-and-mouth disease in South-East Asia and approaches to control and eradication. Rev. Sci. Tech. Off. Int. Epiz. 2002, 21, 465–472. [Google Scholar] [CrossRef]
- Poolkhet, C.; Kasemsuwan, S.; Seng, S.; Keartha, C.; Sokmao, C.; Shin, M.; Kalpravidh, W.; Hinrichs, J. Social network analysis of cattle movement in Kampong Cham, Kampong Speu, and Takeo, Cambodia. Acta Trop. 2016, 159, 44–49. [Google Scholar] [CrossRef]
- Blacksell, S.D.; Siengsanan-Lamont, J.; Kamolsiripichaiporn, S.; Gleeson, L.J.; Windsor, P.A. A history of FMD research and control programmes in Southeast Asia: Lessons from the past informing the future. Epidemiol. Infect. 2019, 147, e171. [Google Scholar] [CrossRef]
- Tum, S.; Robertson, I.D.; Edwards, J.; Abila, R.; Morzaria, S. Seroprevalence of foot-and-mouth disease in the southern provinces of Cambodia. Trop. Anim. Health Prod. 2015, 47, 541–547. [Google Scholar] [CrossRef]
- Sothoeun, S.; Young, J.; Windsor, P. Livestock infectious disease status in Cambodia. In Cattle Health, Production and Trade in Cambodia, Proceedings of the Three ACIAR-Funded Projects Presented at an International Workshop, Phnom Penh, Cambodia, 7–8 June 2011; Young, J., Rast, L., Sothoeun, S., Windsor, P., Eds.; ACIAR: Canberra, Australia, 2013; p. 44. [Google Scholar]
- MAFF. Five-Year Strategic Plan 2019–2023 for Agriculture Sector; Ministry of Agriculture Forestry and Fisheries: Phnom Penh, Cambodia, 2019. [Google Scholar]
- Royal Government of Cambodia. National Strategic Development Plan (2019–2023); Open Development Cambodia: Phnom Penh, Cambodia, 2019. [Google Scholar]
- General Directorate of Animal Health and Production (GDAHP). Annual Report; GDAHP: Phnom Penh, Cambodia, 2020. [Google Scholar]
- Parida, S. Vaccination against foot-and-mouth disease virus: Strategies and effectiveness. Expert Rev. Vaccines 2009, 8, 347–365. [Google Scholar] [CrossRef]
- Sieng, S.; Walkden-Brown, S.W.; Kerr, J. Effect of vaccine storage temperatures and dose rate on antibody responses to foot-and-mouth disease vaccination in Cambodia. Vet. Med. Sci. 2018, 4, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Young, J.R.; Suon, S.; Olmo, L.; Bun, C.; Hok, C.; Ashley, K.; Bush, R.D.; Windsor, P.A. Investigation of smallholder farmer biosecurity and implications for sustainable foot-and-mouth disease control in Cambodia. Transbound. Emerg. Dis. 2017, 64, 2000–2012. [Google Scholar] [CrossRef] [PubMed]
- Young, J.R.; Suon, S.; Rast, L.; Nampanya, S.; Windsor, P.A.; Bush, R.D. Benefit-Cost Analysis of Foot-and-Mouth Disease Control in Large Ruminants in Cambodia. Transbound. Emerg. Dis. 2016, 63, 508–522. [Google Scholar] [CrossRef]
- Cannon, R.M.; Roe, R.T. Livestock Disease Surveys: A Field Manual for Veterinarians; Australian Bureau of Animal Health: Canberra, Australia, 1982. [Google Scholar]
- ID.vet. ID Soft™ Software, France. 2014. Available online: https://www.id-vet.com/products/ (accessed on 14 May 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 14 May 2025).
- Kim, J.H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 2019, 72, 558. [Google Scholar] [CrossRef]
- Graul, C. LeafletR: Interactive Web-Maps Based on the Leaflet JavaScript Library, Version 0.4-0. Available online: https://rdrr.io/cran/leafletR/ (accessed on 14 May 2025).
- Netrabukkana, P.; Cappelle, J.; Trevennec, C.; Roger, F.; Goutard, F.; Buchy, P.; Robertson, I.D.; Fenwick, S. Epidemiological analysis of Influenza A infection in Cambodian pigs and recommendations for surveillance strategies. Transbound. Emerg. Dis. 2015, 62, e37–e44. [Google Scholar] [CrossRef]
- Horm, S.V.; Tarantola, A.; Rith, S.; Ly, S.; Gambaretti, J.; Duong, V.; Sorn, S.; Holl, D.; Allal, L.; Kalpravidh, W.; et al. Intense circulation of A/H5N1 and other avian influenza viruses in Cambodian live-bird markets with serological evidence of sub-clinical human infections. Emerg. Microbes Infect. 2016, 5, e70. [Google Scholar] [CrossRef]
- Cappelle, J.; Duong, V.; Pring, L.; Kong, L.; Yakovleff, M.; Prasetyo, D.B.; Peng, B.; Choeung, R.; Duboz, R.; Ong, S. Intensive circulation of Japanese encephalitis virus in peri-urban sentinel pigs near Phnom Penh, Cambodia. PLoS Negl. Trop. Dis. 2016, 10, e0005149. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, E.; Söderberg, R.; Ström Hallenberg, G.; Kroesna, K.; Ly, S.; Sear, B.; Unger, F.; Tum, S.; Nguyen-Viet, H.; Lindahl, J.F. Japanese encephalitis in small-scale pig farming in rural Cambodia: Pig seroprevalence and farmer awareness. Pathogens 2021, 10, 578. [Google Scholar] [CrossRef]
- Cappelle, J.; Hoem, T.; Hul, V.; Furey, N.; Nguon, K.; Prigent, S.; Dupon, L.; Ken, S.; Neung, C.; Hok, V.; et al. Nipah virus circulation at human-bat interfaces, Cambodia. Bull. World Health Organ. 2020, 98, 539–547. [Google Scholar] [CrossRef]
- Suy, P. Foot-and-Mouth Disease Hits Cattle. Khmer Times. 15 August 2017. Available online: https://www.khmertimeskh.com/78153/foot-mouth-disease-hits-cattle/ (accessed on 14 May 2025).
- Pech, S. Foot-and-Mouth Affecting Cattle. Khmer Times. 8 February 2017. Available online: https://www.khmertimeskh.com/12183/foot-and-mouth-affecting-cattle/ (accessed on 14 May 2025).
- Siengsanan-Lamont, J.; Douangngeun, B.; Theppangna, W.; Khounsy, S.; Phommachanh, P.; Kamolsiripichaiporn, S.; Udon, R.; Seeyo, K.B.; Selleck, P.W.; Matsumoto, N. Seroepidemiology of foot-and-mouth disease using passive surveillance techniques in selected provinces of Lao PDR. Trop. Anim. Health Prod. 2021, 53, 303. [Google Scholar] [CrossRef]
- Blacksell, S.D.; Khounsy, S.; Conlan, J.V.; Gleeson, L.J.; Colling, A.; Westbury, H.A. Foot and mouth disease in the Lao People’s Democratic Republic: II. Seroprevalence estimates, using structured surveillance and surveys of abattoirs. Rev. Sci. Tech. Off. Int. Epiz. 2008, 27, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Chamnanpood, P.; Cleland, P.C.; Baldock, F.C.; Gleeson, L.J. The minor role of pigs in outbreaks of foot-and-mouth disease of northern Thailand. Aust. Vet. J. 1995, 72, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.; Sieng, S.; Scoizec, A. Working with traders to understand livestock movements and spread of animal disease in Cambodia and Lao PDR. In Animal Biosecurity in the Mekong: Future Directions for Research and Development, Proceedings of the International Workshop, Siem Reap, Cambodia, 10–13 August 2010; Adams, L.B., Gray, G.D., Murray, G., Eds.; ACIAR: Canberra, Australia, 2012; Volume 137, pp. 59–64. [Google Scholar]
- Tum, S.; Bourn, D. Foot and Mouth Disease in Cambodia. Available online: https://www.researchgate.net/publication/315786318_Foot_and_mouth_disease_in_Cambodia (accessed on 14 May 2025).
- Sieng, S.; Hawkins, C.; Madin, B.; Kerr, J. Characteristics of livestock traders and trading in Cambodia. In Animal Biosecurity in the Mekong: Future Directions for Research and Development, Proceedings of the International Workshop, Siem Reap, Cambodia, 10–13 August 2010; Adams, L.B., Gray, G.D., Murray, G., Eds.; ACIAR: Canberra, Australia, 2012; Volume 137, p. 45. [Google Scholar]
- World Organisation for Animal Health. Lumpy Skin Disease (LSD) Coordination Meeting for South East Asia. 11 June 2021. Available online: https://rr-asia.oie.int/en/events/lumpy-skin-disease-lsd-coordination-meeting-for-south-east-asia/ (accessed on 14 May 2025).
- Muñoz-Pérez, C.; Bosch, J.; Ito, S.; Martínez-Avilés, M.; Sánchez-Vizcaíno, J.M. Quantitative risk assessment of African swine fever introduction into Spain by legal import of live pigs. Pathogens 2022, 11, 76. [Google Scholar] [CrossRef]
- Xu, X. Cambodia Imports 1,500 Pigs from Thailand per Day Amid Ban of Import from Vietnam. Xinhua. 2019. Available online: http://www.xinhuanet.com/english/2019-05/21/c_138077390.htm (accessed on 14 May 2025).
- Lee, F.; Jong, M.-H.; Yang, D.-W. Presence of antibodies to non-structural proteins of foot-and-mouth disease virus in repeatedly vaccinated cattle. Vet. Microbiol. 2006, 115, 14–20. [Google Scholar] [CrossRef]
- Ter Beek, V. ASF Cambodia: 1st Outbreak as Virus Travels South. 4 April 2019. Available online: https://www.pigprogress.net/health-nutrition/asf-cambodia-1st-outbreak-as-virus-travels-south/ (accessed on 14 May 2025).
- Chea, V. Cambodia Reduces Live Pig Imports to Stop Disease. Khmer Times. 1 October 2021. Available online: https://www.khmertimeskh.com/50945014/cambodia-reduces-live-pig-imports-to-stop-disease/ (accessed on 14 May 2025).
- Cleland, P.C.; Baldock, F.C.; Chamnanpood, P.; Gleeson, L.J. Village level risk factors for foot-and-mouth disease in northern Thailand. Prev. Vet. Med. 1996, 26, 253–261. [Google Scholar] [CrossRef]
- Sieng, S.; Patrick, I.W.; Windsor, P.A.; Walkden-Brown, S.W.; Sar, C.; Smith, R.G.B.; Kong, R. Knowledge, attitudes, and practices of smallholder farmers on foot-and-mouth disease control in two Cambodian provinces. Transbound. Emerg. Dis. 2022, 69, 1983–1998. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.; Sieng, S.; Scoizec, A. Working with traders to understand livestock movements and spread of animal diseases in Cambodia and Lao PDR. In Cattle Health, Production and Trade in Cambodia, Proceedings of the Three ACIAR-Funded Projects Presented at an International Workshop, Phnom Penh, Cambodia, 7–8 June 2011; Young, J., Rast, L., Sothoeun, S., Windsor, P., Eds.; ACIAR: Canberra, Australia, 2013; p. 101. [Google Scholar]
- Robiolo, B.; Seki, C.; Fondevilla, N.; Grigera, P.; Scodeller, E.; Periolo, O.; La Torre, J.; Mattion, N. Analysis of the immune response to FMDV structural and non-structural proteins in cattle in Argentina by the combined use of liquid phase and 3ABC-ELISA tests. Vaccine 2006, 24, 997–1008. [Google Scholar] [CrossRef]
- Mohapatra, J.K.; Pandey, L.K.; Sanyal, A.; Pattnaik, B. Recombinant non-structural polyprotein 3AB-based serodiagnostic strategy for FMD surveillance in bovines irrespective of vaccination. J. Virol. Methods 2011, 177, 184–192. [Google Scholar] [CrossRef]
- Remond, M.; Kaiser, C.; Lebreton, F.O.; Moutou, F.; Crucière, C. Residual foot-and-mouth disease virus antibodies in French cattle and sheep six years after the vaccination ban. Vet. Res. 2001, 32, 81–86. [Google Scholar] [CrossRef][Green Version]
- Fakhrul-Islam, K.; Jalal, M.S.; Poddr, S.; Quader, N. Clinical investigation of foot and mouth disease of cattle in Batiaghata Upazilla veterinary hospital, Bangladesh. Vet. Sci. Res. Rev. 2016, 2, 76–81. [Google Scholar] [CrossRef]
- Edwards, J.R. Strategy for the control of foot-and-mouth disease in Southeast Asia (SEAFMD). Dev. Biol. 2004, 119, 423–431. [Google Scholar]
- Doel, T.R. Optimisation of the immune response to foot-and-mouth disease vaccines. Vaccine 1999, 17, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Garland, A. Vital elements for the successful control of foot-and-mouth disease by vaccination. Vaccine 1999, 17, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Pannu, A.; Khurana, S.K.; Rajak, K.K.; Mahajan, S.; Dahiya, S.S.; Mittal, D.; Sharma, R.; Yadav, S.; Saini, M.; Singh, N.; et al. Systematic Follow-Up Investigation of NSP Seroreactors and In-Contact Cattle and Buffaloes for Foot-and-Mouth Disease Virus Using Probang Sampling. BMC Vet. Res. 2025, 21, 86. [Google Scholar] [CrossRef]


| Province/Abattoir Name | Cattle | Pigs | ||||
|---|---|---|---|---|---|---|
| Total | Positive | NSP Prevalence % (95% CI) | Total | Positive | NSP Prevalence % (95% CI) | |
| Phnom Penh/Boeng Salang | 207 | 83 | 40.1 (33.3–47.1) | 245 | 1 | 0.4 (0.0–2.2) |
| Chrouy Changya | 340 | 137 | 40.2 (35.0–45.7) | - | - | |
| Damnak Thum * | - | - | 185 | 3 | 1.6 (0.3–4.6) | |
| Trea Boun * | - | - | 295 | 3 | 1.0 (0.2–2.9) | |
| Battambang/Krong Battambang | 110 | 60 | 54.5 (44.7–64.0) | 125 | 0 | 0.0 (0.0–2.9) |
| Takeo/Krong Doun Kaev | 32 | 12 | 37.5 (21.1–56.3) | 191 | 2 | 1.0 (0.1–3.7) |
| Kampong Cham/Krong Kampong Cham | 3 | 0 | 0.0 (0.0–70.7) | 27 | 0 | 0.0 (0.0–12.7) |
| Siem Reap/Krong Siem Reap | 109 | 57 | 52.2 (42.5–62.0) | 127 | 0 | 0.0 (0.0–2.8) |
| Kandal/Krong Ta Khmau | 7 | 0 | 0.0 (0.0–41.0) | 21 | 0 | 0.0 (0.0–16.1) |
| Prey Veng/Neak Loeung | 31 | 14 | 45.1 (27.3–64.0) | 183 | 0 | 0.0 (0.0–2.0) |
| Overall | 839 | 363 | 43.2 (39.8–46.7) | 1399 | 9 | 0.6 (0.2–1.2) |
| Province of Animal Origin | Cattle | Pigs | ||
|---|---|---|---|---|
| Total | NSP Prevalence % | Total | NSP Prevalence % | |
| Battambang | 122 | 50.8 (62/122) | 68 | 0 |
| Bonteay Meanchey | 1 | 100 (1/1) | - | - |
| Kampong Cham | 49 | 47.0 (23/49) | 14 | 0 |
| Kampong Chhnang | 3 | 100 (3/3) | - | - |
| Kampong Speu | 39 | 43.6 (17/39) | 603 | 0.8 (5/603) |
| Kampong Thom | 10 | 80.0 (8/10) | - | - |
| Kampot | - | - | 12 | 0 |
| Kap | 3 | 33.3 (1/3) | - | - |
| Kandal | - | - | 6 | 0 |
| Oddor Meanchey | 1 | 0 | - | - |
| Phnom Penh | 16 | 31.2 (5/16) | - | - |
| Preash Vihear | 12 | 33.3 (4/12) | - | - |
| Pailin | - | - | 33 | 0 |
| Prey Veng | 34 | 41.1 (14/34) | 20 | 0 |
| Pursat | 42 | 47.6 (20/42) | - | - |
| Siem Reap | 105 | 53.3 (56/105) | 191 | 0 |
| Sihanoukville | - | - | 31 | 0 |
| Svay Reing | 4 | 50 (2/4) | 9 | 0 |
| Takeo | 162 | 43.2 (70/162) | 83 | 0 |
| Tboung Khmom | - | - | 68 | 0 |
| Thailand | 236 | 32.6 (77/236) | 250 | 1.6 (4/250) |
| Unknown | - | - | 11 | 0 |
| Total | 839 | 43.2 (363/839) | 1399 | 0.6 (9/1399) |
| Categories | Total | Positive | Seroprevalence (95% CI) | p-Value (Chi-Square) |
|---|---|---|---|---|
| Cattle | ||||
| Import | 236 | 77 | 32.6 (26.6–39.0) | |
| Local | 603 | 286 | 47.4 (43.3–51.5) | 0.0001 |
| Male | 458 | 174 | 38.0 (33.5–42.6) | |
| Female | 381 | 189 | 49.6 (44.4–54.7) | 0.0009 |
| Less than or equal to 3 years | 392 | 165 | 42.0 (37.1–47.1) | |
| More than 3 years | 447 | 198 | 44.2 (39.6–49.0) | 0.566 |
| Pigs | ||||
| Import | 250 | 4 | 1.6 (0.4–4.0) | |
| Unknown | 10 | 0 | 0 (0–30.0) | |
| Commercial | 833 | 5 | 0.6 (0.1–1.3) | |
| Smallholder | 306 | 0 | 0 (0–1.1) | |
| Female | 799 | 6 | 0.7 (0.2–1.6) | |
| Male | 600 | 3 | 0.5 (0.1–1.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Siengsanan-Lamont, J.; Tum, S.; Selleck, P.W.; Areerob, J.; Young, J.R.; Gleeson, L.J.; Blacksell, S.D. Using Abattoir-Based Surveillance to Establish Foot-and-Mouth Disease Non-Structural Protein Seropositivity in Cattle and Pigs in Cambodia. Animals 2025, 15, 1624. https://doi.org/10.3390/ani15111624
Kong L, Siengsanan-Lamont J, Tum S, Selleck PW, Areerob J, Young JR, Gleeson LJ, Blacksell SD. Using Abattoir-Based Surveillance to Establish Foot-and-Mouth Disease Non-Structural Protein Seropositivity in Cattle and Pigs in Cambodia. Animals. 2025; 15(11):1624. https://doi.org/10.3390/ani15111624
Chicago/Turabian StyleKong, Lida, Jarunee Siengsanan-Lamont, Sothyra Tum, Paul W. Selleck, Jeeranan Areerob, James R. Young, Laurence J. Gleeson, and Stuart D. Blacksell. 2025. "Using Abattoir-Based Surveillance to Establish Foot-and-Mouth Disease Non-Structural Protein Seropositivity in Cattle and Pigs in Cambodia" Animals 15, no. 11: 1624. https://doi.org/10.3390/ani15111624
APA StyleKong, L., Siengsanan-Lamont, J., Tum, S., Selleck, P. W., Areerob, J., Young, J. R., Gleeson, L. J., & Blacksell, S. D. (2025). Using Abattoir-Based Surveillance to Establish Foot-and-Mouth Disease Non-Structural Protein Seropositivity in Cattle and Pigs in Cambodia. Animals, 15(11), 1624. https://doi.org/10.3390/ani15111624






