A Study on the Differences in Rumen Microbiota–Liver Gluconeogenesis–Mitochondrial Interaction Between Tibetan Sheep and Hu Sheep in the Qinghai–Tibet Plateau
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Experimental Animals and Sample Collection
2.3. Liver Tissue Gluconeogenesis and Mitochondrial Function Measurements
2.4. Total RNA Extraction and Gene Expression Measurement in Liver Tissue
2.5. Total DNA Extraction of Rumen Microorganisms and Determination of Bacterial Population Density
2.6. Statistical Analysis of the Data
3. Results
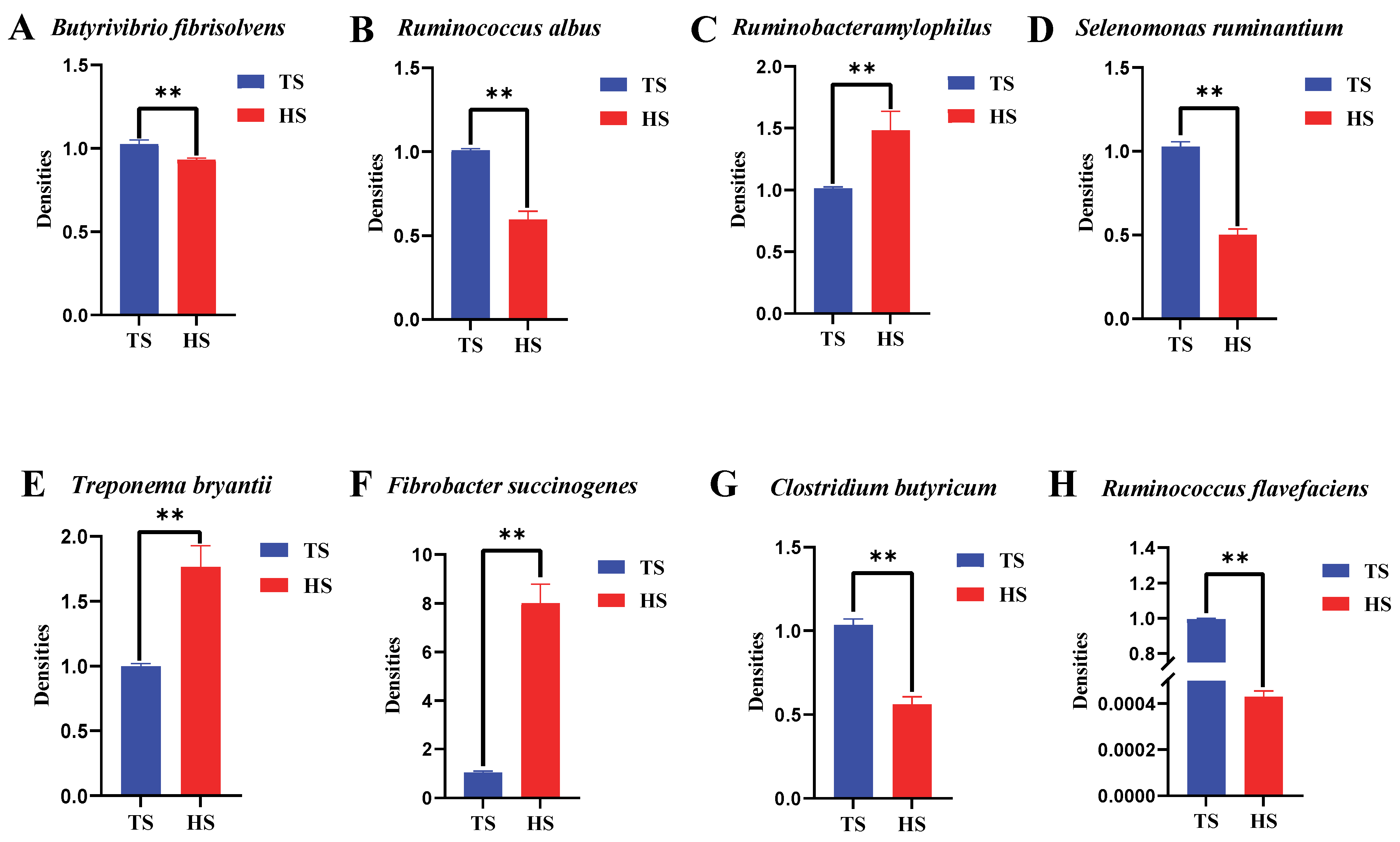
3.1. Differences in Rumen Flora Density Between Tibetan Sheep and Hu Sheep
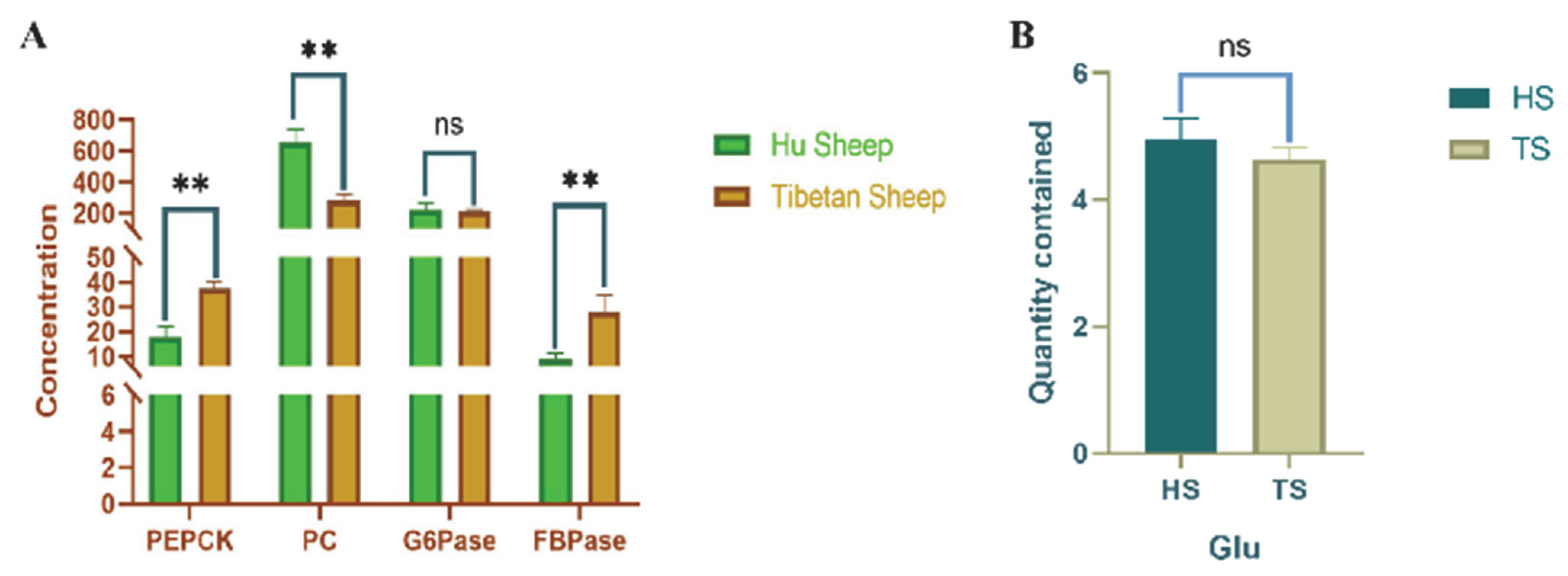
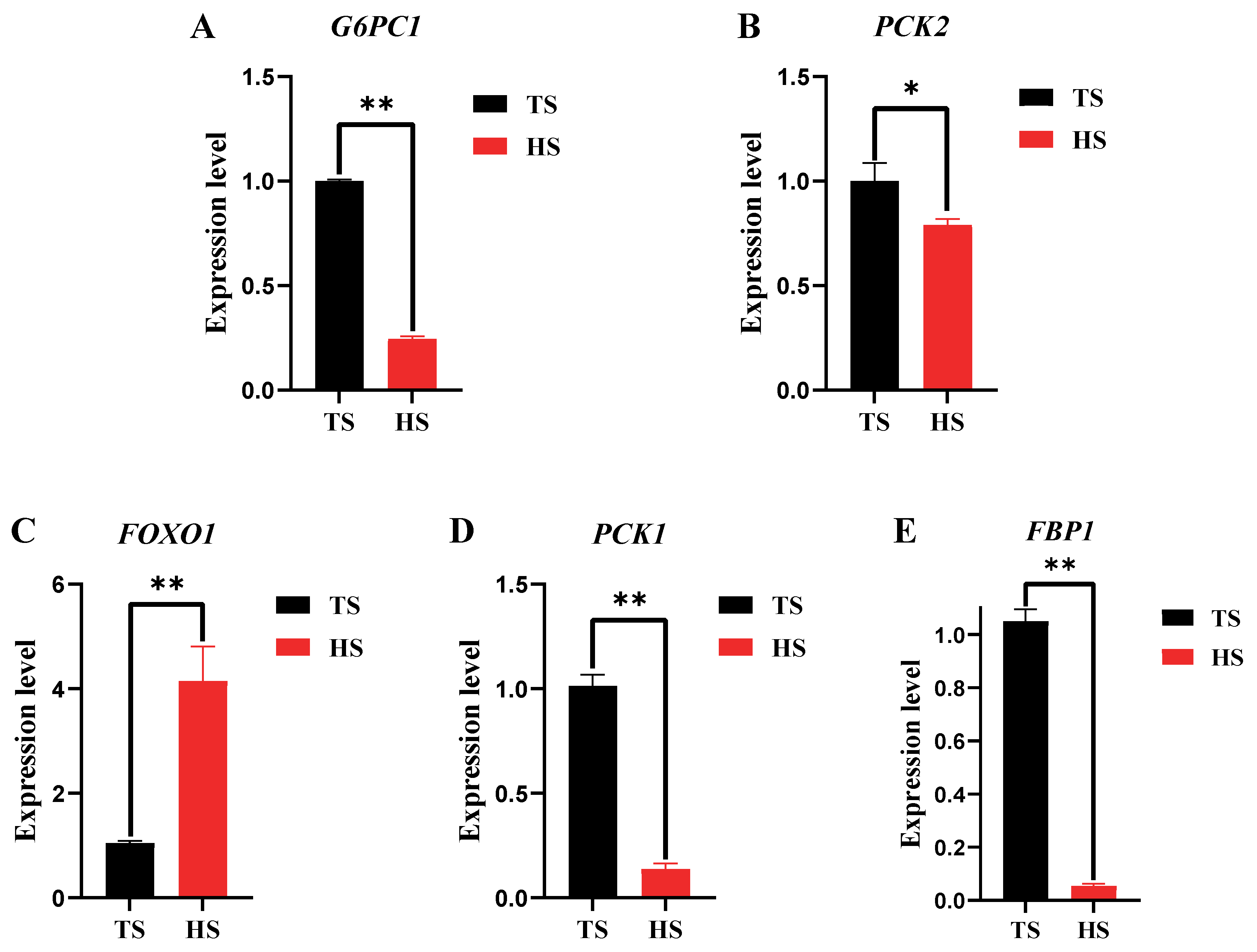
3.2. Determination of Hepatic Glyoxylase Activity and Related Gene Expression in Tibetan Sheep and Hu Sheep
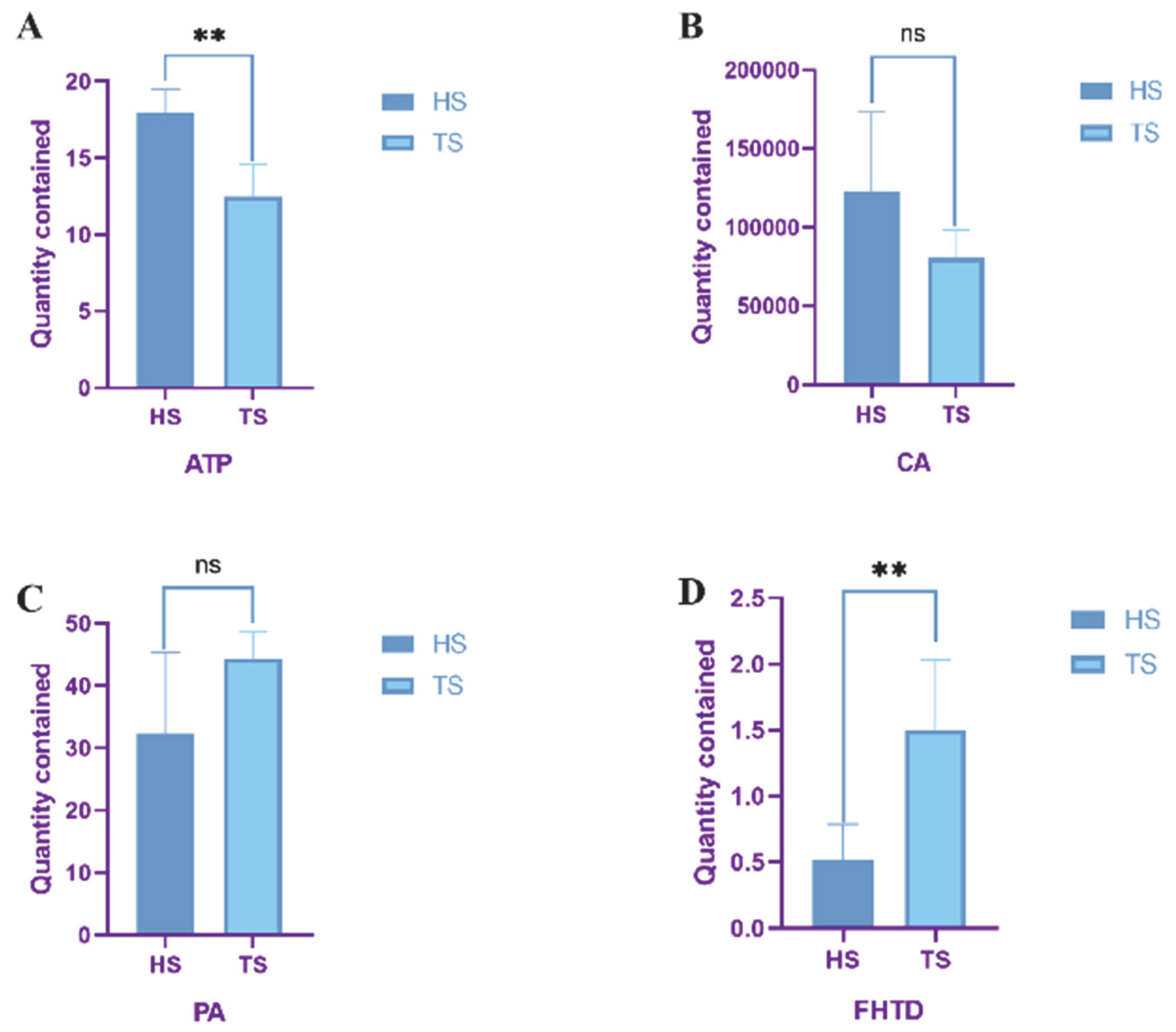
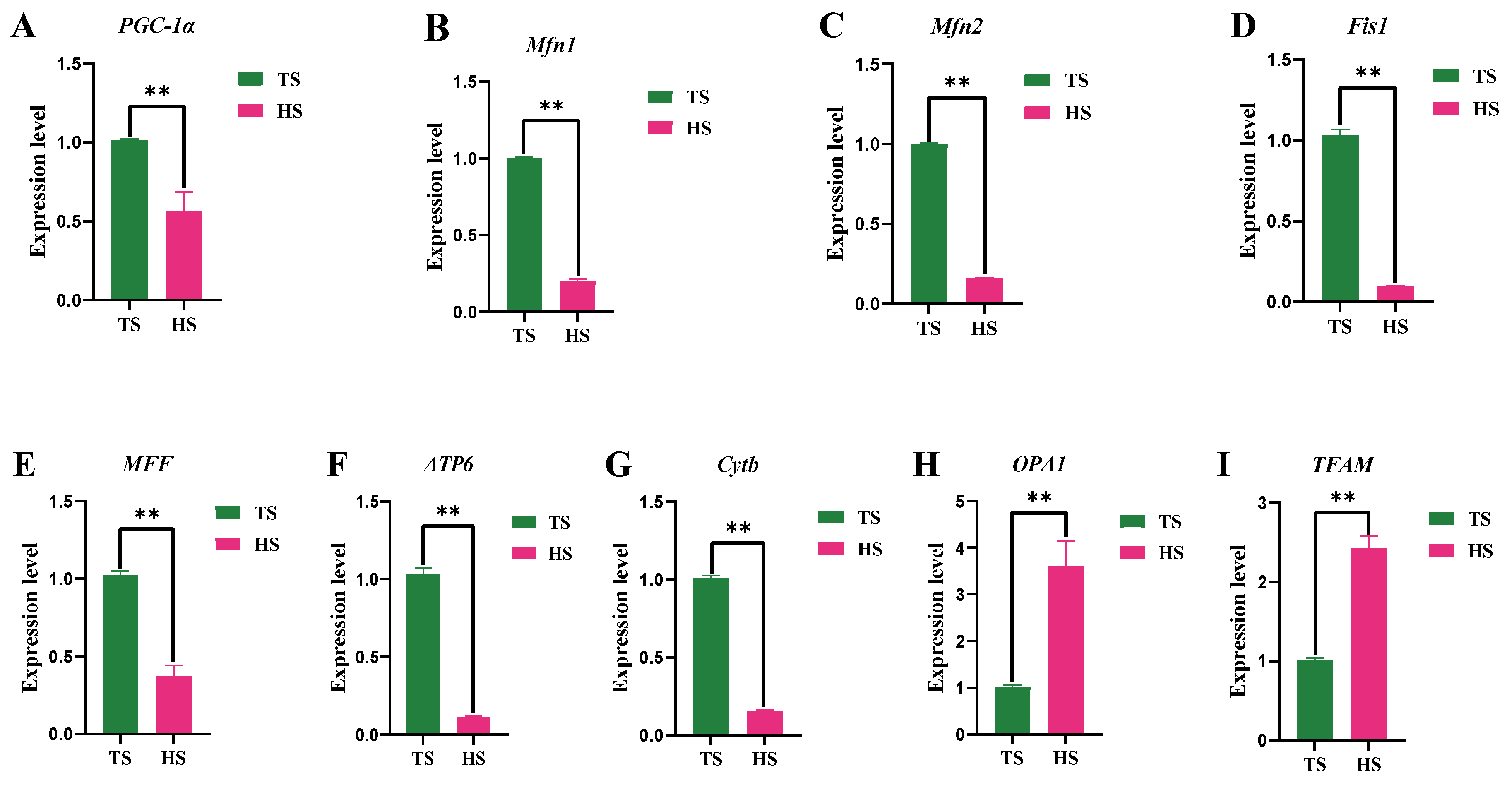
3.3. Determination of Mitochondrial Function in Tibetan Sheep and Hu Sheep
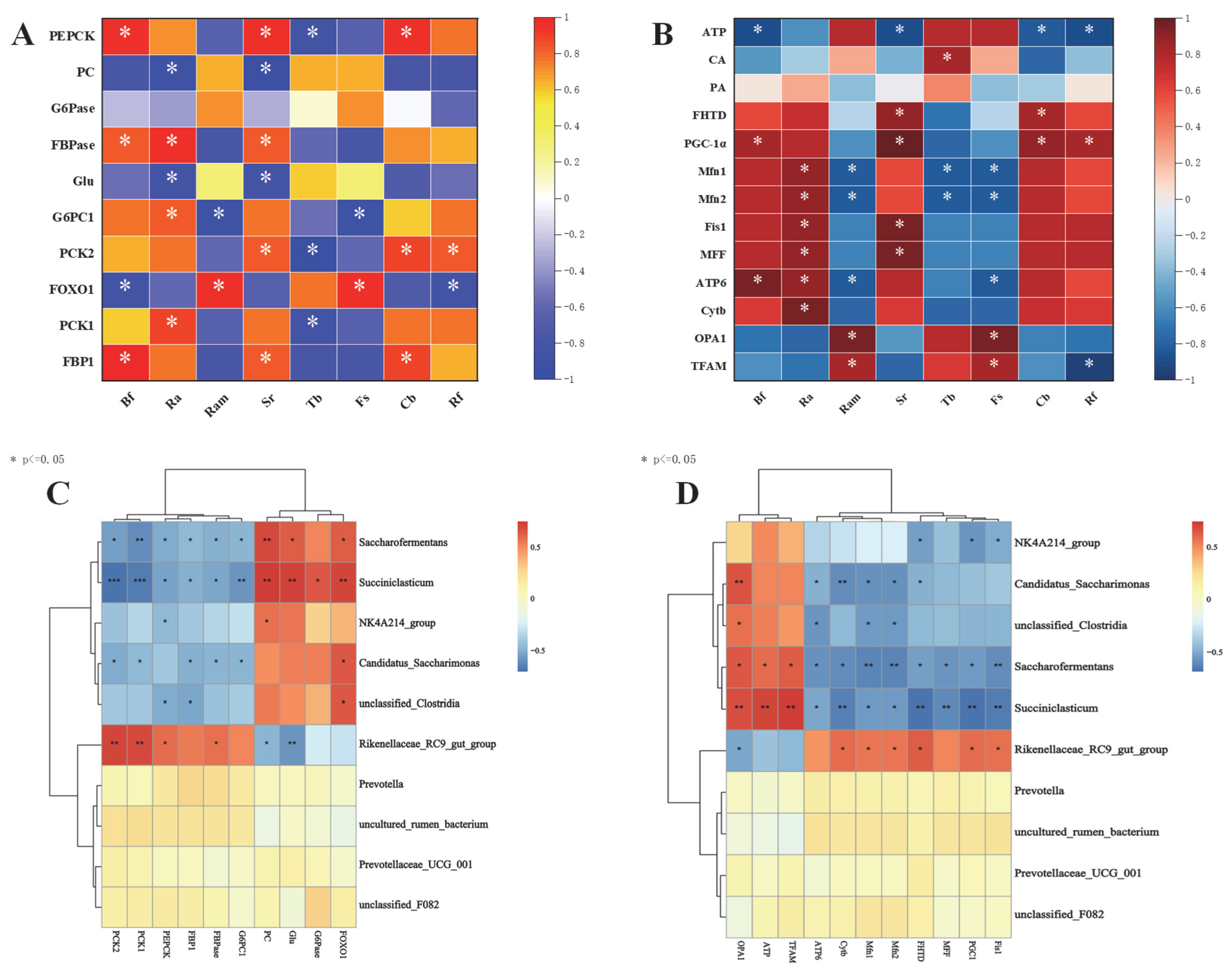
3.4. Correlation Analysis of Rumen Microbial–Hepatic Gluconeogenesis–Mitochondrial Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witt, K.E.; Huerta-Sanchez, E. Convergent evolution in human and domesticate adaptation to high-altitude environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180235. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Fang, C.; Liu, L.; Li, M.; Liu, W. Environmental driving of adaptation mechanism on rumen microorganisms of sheep based on metagenomics and metabolomics data analysis. Int. J. Mol. Sci. 2024, 25, 10957. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J. Degradation of cellulose and hemicellulose by ruminal microorganisms. Microorganisms 2022, 10, 2345. [Google Scholar] [CrossRef] [PubMed]
- Newbold, C.J.; Ramos-Morales, E. Review: Ruminal microbiome and microbial metabolome: Effects of diet and ruminant host. Animal 2020, 14, s78–s86. [Google Scholar] [CrossRef]
- Russell, J.B.; Muck, R.E.; Weimer, P.J. Quantitative analysis of cellulose degradation and growth of cellulolytic bacteria in the rumen. FEMS Microbiol. Ecol. 2009, 67, 183–197. [Google Scholar] [CrossRef]
- Tajima, K.; Nonaka, I.; Higuchi, K.; Takusari, N.; Kurihara, M.; Takenaka, A.; Mitsumori, M.; Kajikawa, H.; Aminov, R.I. Influence of high temperature and humidity on rumen bacterial diversity in holstein heifers. Anaerobe 2007, 13, 57–64. [Google Scholar] [CrossRef]
- Liu, E.; Sun, M.; He, C.; Mao, K.; Li, Q.; Zhang, J.; Wu, D.; Wang, S.; Zheng, C.; Li, W.; et al. Rumen microbial metabolic responses of dairy cows to the honeycomb flavonoids supplement under heat-stress conditions. Front. Vet. Sci. 2022, 9, 845911. [Google Scholar] [CrossRef]
- Cankaya, M.; Hernandez, A.M.; Ciftci, M.; Beydemir, S.; Ozdemir, H.; Budak, H.; Gulcin, I.; Comakli, V.; Emircupani, T.; Ekinci, D.; et al. An analysis of expression patterns of genes encoding proteins with catalytic activities. BMC Genom. 2007, 8, 232. [Google Scholar] [CrossRef]
- Mithieux, G.; Andreelli, F.; Magnan, C. Intestinal gluconeogenesis: Key signal of central control of energy and glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 419–423. [Google Scholar] [CrossRef]
- Aschenbach, J.R.; Kristensen, N.B.; Donkin, S.S.; Hammon, H.M.; Penner, G.B. Gluconeogenesis in dairy cows: The secret of making sweet milk from sour dough. IUBMB Life 2010, 62, 869–877. [Google Scholar] [CrossRef]
- Bergman, E.N. Glucose metabolism in ruminants as related to hypoglycemia and ketosis. Cornell Vet. 1973, 63, 341–382. [Google Scholar]
- Agca, C.; Greenfield, R.B.; Hartwell, J.R.; Donkin, S.S. Cloning and characterization of bovine cytosolic and mitochondrial PEPCK during transition to lactation. Physiol. Genom. 2002, 11, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, H.; Daitoku, H.; Fukamizu, A. Nutrient control of phosphorylation and translocation of foxo1 in c57BL/6 and db/db mice. Int. J. Mol. Med. 2006, 18, 433–439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Claxton, D.P.; Overway, E.M.; Oeser, J.K.; O’Brien, R.M.; Mchaourab, H.S. Biophysical and functional properties of purified glucose-6-phosphatase catalytic subunit 1. J. Biol. Chem. 2022, 298, 101520. [Google Scholar] [CrossRef]
- Davis, R.E.; Williams, M. Mitochondrial function and dysfunction: An update. J. Pharmacol. Exp. Ther. 2012, 342, 598–607. [Google Scholar] [CrossRef]
- Niles, N.R.; Bitensky, L.; Chayen, J.; Cunningham, G.J.; Braimbridge, M.V. The value of histochemistry in the analysis of myocardial dysfunction. Lancet 1964, 1, 963–965. [Google Scholar] [CrossRef]
- Scarpulla, R.C.; Vega, R.B.; Kelly, D.P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 2012, 23, 459–466. [Google Scholar] [CrossRef]
- Svensson, K.; Schnyder, S.; Cardel, B.; Handschin, C. Loss of renal tubular PGC-1alpha exacerbates diet-induced renal steatosis and age-related urinary sodium excretion in mice. PLoS ONE 2016, 11, e0158716. [Google Scholar] [CrossRef]
- Zhan, M.; Usman, I.; Yu, J.; Ruan, L.; Bian, X.; Yang, J.; Yang, S.; Sun, L.; Kanwar, Y.S. Perturbations in mitochondrial dynamics by p66shc lead to renal tubular oxidative injury in human diabetic nephropathy. Clin. Sci. 2018, 132, 1297–1314. [Google Scholar] [CrossRef]
- Franco-Obregon, A.; Gilbert, J.A. The microbiome-mitochondrion connection: Common ancestries, common mechanisms, common goals. mSystems 2017, 2, e00018-17. [Google Scholar] [CrossRef]
- Bajpai, P.; Darra, A.; Agrawal, A. Microbe-mitochondrion crosstalk and health: An emerging paradigm. Mitochondrion 2018, 39, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Yardeni, T.; Tanes, C.E.; Bittinger, K.; Mattei, L.M.; Schaefer, P.M.; Singh, L.N.; Wu, G.D.; Murdock, D.G.; Wallace, D.C. Host mitochondria influence gut microbiome diversity: A role for ROS. Sci. Signal. 2019, 12, eaaw3159. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Chen, Q.; Sha, Y.; Liu, X.; He, Y.; Chen, X.; Yang, W.; Gao, M.; Huang, W.; Wang, J.; He, J.; et al. Unique rumen micromorphology and microbiota-metabolite interactions: Features and strategies for tibetan sheep adaptation to the plateau. Front. Microbiol. 2024, 15, 1471732. [Google Scholar] [CrossRef]
- Rozman, G.I.; Yin, G.; Borovok, I.; Berg, M.M.; Yeoman, C.J.; Dassa, B.; Yu, Z.; Mizrahi, I.; Flint, H.J.; Bayer, E.A.; et al. Functional phylotyping approach for assessing intraspecific diversity of Ruminococcus albus within the rumen microbiome. FEMS Microbiol. Lett. 2015, 362, 1–10. [Google Scholar]
- Bolte, L.A.; Vich, V.A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- Yeoman, C.J.; Fields, C.J.; Lepercq, P.; Ruiz, P.; Forano, E.; White, B.A.; Mosoni, P. In vivo competitions between Fibrobacter succinogenes, Ruminococcus flavefaciens, and Ruminoccus albus in a gnotobiotic sheep model revealed by multi-omic analyses. mBio 2021, 12, e03533-20. [Google Scholar] [CrossRef]
- Anderson, K.L. Biochemical analysis of starch degradation by Ruminobacter amylophilus 70. Appl. Environ. Microbiol. 1995, 61, 1488–1491. [Google Scholar] [CrossRef]
- Fall, F.; Mamede, L.; Schioppa, L.; Ledoux, A.; De Tullio, P.; Michels, P.; Frederich, M.; Quetin-Leclercq, J. Trypanosoma brucei: Metabolomics for analysis of cellular metabolism and drug discovery. Metabolomics 2022, 18, 20. [Google Scholar] [CrossRef]
- Kingsley, V.V.; Hoeniger, J.F. Growth, structure, and classification of selenomonas. Bacteriol. Rev. 1973, 37, 479–521. [Google Scholar] [CrossRef]
- Jeyanathan, J.; Martin, C.; Morgavi, D.P. The use of direct-fed microbials for mitigation of ruminant methane emissions: A review. Animal 2014, 8, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Palevich, N.; Kelly, W.J.; Leahy, S.C.; Denman, S.; Altermann, E.; Rakonjac, J.; Attwood, G.T. Comparative genomics of rumen butyrivibrio sUncovers a continuum of polysaccharide-degrading capabilities. Appl. Environ. Microbiol. 2019, 86, e01993-19. [Google Scholar] [CrossRef] [PubMed]
- Felig, P. Interaction of insulin and amino acid metabolism in the regulation of gluconeogenesis. Isr. J. Med. Sci. 1972, 8, 262–270. [Google Scholar] [PubMed]
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef]
- Valle, M. Pyruvate carboxylase, structure and function. Subcell Biochem. 2017, 83, 291–322. [Google Scholar]
- Wang, X.; Li, X.; Zhao, C.; Hu, P.; Chen, H.; Liu, Z.; Liu, G.; Wang, Z. Correlation between composition of the bacterial community and concentration of volatile fatty acids in the rumen during the transition period and ketosis in dairy cows. Appl. Environ. Microbiol. 2012, 78, 2386–2392. [Google Scholar] [CrossRef]
- van Gylswyk, N.O. Succiniclasticum ruminis gen. Nov., Sp. Nov., A ruminal bacterium converting succinate to propionate as the sole energy-yielding mechanism. Int. J. Syst. Bacteriol. 1995, 45, 297–300. [Google Scholar] [CrossRef]
- Brearley, M.C.; Daniel, Z.; Loughna, P.T.; Parr, T.; Brameld, J.M. The phosphoenolpyruvate carboxykinase (PEPCK) inhibitor, 3-mercaptopicolinic acid (3-MPA), induces myogenic differentiation in c2c12 cells. Sci. Rep. 2020, 10, 22177. [Google Scholar] [CrossRef]
- Gizak, A.; Duda, P.; Wisniewski, J.; Rakus, D. Fructose-1,6-bisphosphatase: From a glucose metabolism enzyme to multifaceted regulator of a cell fate. Adv. Biol. Regul. 2019, 72, 41–50. [Google Scholar] [CrossRef]
- Lin, X.; Pan, X.M.; Peng, Z.K.; Wang, K.; Tang, N. Glucose-6 phosphatase catalytic subunit inhibits the proliferation of liver cancer cells by inducing cell cycle arrest. Zhonghua Gan Zang Bing Za Zhi 2022, 30, 213–219. [Google Scholar]
- Oh, K.; Han, H.; Kim, M.; Koo, S. CREB and FoxO1: Two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep. 2013, 46, 567–574. [Google Scholar] [CrossRef]
- Luo, L.; Luo, J.; Cai, Y.; Fu, M.; Li, W.; Shi, L.; Liu, J.; Dong, R.; Xu, X.; Tu, L.; et al. Inulin-type fructans change the gut microbiota and prevent the development of diabetic nephropathy. Pharmacol. Res. 2022, 183, 106367. [Google Scholar] [CrossRef]
- Frazier, K.; Manzoor, S.; Carroll, K.; DeLeon, O.; Miyoshi, S.; Miyoshi, J.; St, G.M.; Tan, A.; Chrisler, E.A.; Izumo, M.; et al. Gut microbes and the liver circadian clock partition glucose and lipid metabolism. J. Clin. Investig. 2023, 133, e162515. [Google Scholar] [CrossRef]
- Flythe, M.D.; Aiken, G.E. Effects of hops (Humulus lupulus L.) Extract on volatile fatty acid production by rumen bacteria. J. Appl. Microbiol. 2010, 109, 1169–1176. [Google Scholar] [CrossRef]
- Hasegawa, K.; Sakamaki, Y.; Tamaki, M.; Wakino, S. PCK1 protects against mitoribosomal defects in diabetic nephropathy in mouse models. J. Am. Soc. Nephrol. 2023, 34, 1343–1365. [Google Scholar] [CrossRef]
- Suzuki, T.A.; Martins, F.M.; Phifer-Rixey, M.; Nachman, M.W. The gut microbiota and bergmann’s rule in wild house mice. Mol. Ecol. 2020, 29, 2300–2311. [Google Scholar] [CrossRef]
- Grattagliano, I.; Russmann, S.; Diogo, C.; Bonfrate, L.; Oliveira, P.J.; Wang, D.Q.; Portincasa, P. Mitochondria in chronic liver disease. Curr. Drug Targets 2011, 12, 879–893. [Google Scholar] [CrossRef]
- MacDonald, M.J.; Fahien, L.A.; Buss, J.D.; Hasan, N.M.; Fallon, M.J.; Kendrick, M.A. Citrate oscillates in liver and pancreatic beta cell mitochondria and in INS-1 insulinoma cells. J. Biol. Chem. 2003, 278, 51894–51900. [Google Scholar] [CrossRef]
- Eyenga, P.; Rey, B.; Eyenga, L.; Sheu, S.S. Regulation of oxidative phosphorylation of liver mitochondria in sepsis. Cells 2022, 11, 1598. [Google Scholar] [CrossRef]
- Kadenbach, B. Complex IV—The regulatory center of mitochondrial oxidative phosphorylation. Mitochondrion 2021, 58, 296–302. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Banuls, C.; Diaz-Morales, N.; Hernandez-Mijares, A.; Rocha, M.; Victor, V.M. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017, 11, 637–645. [Google Scholar] [CrossRef]
- Al, O.M.; Salah, A.; El-Hattab, A.W. Mitochondrial fission and fusion: Molecular mechanisms, biological functions, and related disorders. Membranes 2022, 12, 893. [Google Scholar] [CrossRef]
- Halling, J.F.; Pilegaard, H. PGC-1alpha-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Elzo, M.A.; Dang, S.; Jia, X.; Lai, S. Genetic diversity of ATP8 and ATP6 genes is associated with high-altitude adaptation in yak. Mitochondrial DNA A DNA Mapp. Seq. Anal. 2018, 29, 385–393. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, L.; Zhang, M.; Tang, H.; Huang, Y.; Su, Y.; Ding, Y.; Li, C.; Wang, M.; Zhou, Y.; et al. A novel protein CYTB-187AA encoded by the mitochondrial gene CYTB modulates mammalian early development. Cell Metab. 2024, 36, 1586–1597. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1–28. [Google Scholar] [CrossRef]

| Gene | Primers (5′–3′) | Length | Annealing Temperature | ID |
|---|---|---|---|---|
| FBP1 | F: CCAGCTGCTCAACTCGCTTT R: CCAGCTATTCCATAGAGGTGCG | 90 bp | 60 °C | XM_004004092.5 |
| G6PC1 | F: GCGGCTGAACACAAAGGGAA R: AAGGTAGCGCCCAAAGTTGT | 135 bp | 60 °C | XM_012186137.4 |
| PCK2 | F: GCGGCTGAACACAAAGGGAA R: AAGGTAGCGCCCAAAGTTGT | 84 bp | 60 °C | XM_015096868.3 |
| FOXO1 | F: TCAGTCAACATCCGCAGTCA R: CAGGCGGTTCATACCCGAG | 82 bp | 60 °C | XM_027973596.2 |
| PCK1 | F: GGGAGTTCGTGGAGAGTAGC R: GCCTCTTGATCACACCCTCC | 123 bp | 60 °C | XM_004014441.5 |
| PGC-1α | F: AGCTCCACGACTCCAGACA R: GTCGGAATCTGTGGAAGAGTGT | 86 bp | 60 °C | JF449960.1 |
| β-acting | F: AGCCTTCCTTCCTGGGCATGGA R: GGACAGCACCGTGTTGGCGTAGA | 113 bp | 60 °C | NM_001009784 |
| Gene | Primers (5′–3′) | Length | Annealing Temperature | ID |
|---|---|---|---|---|
| Mfn1 | F: TGGGCATCATCGTTGTTGGA R: AAAGGCTCTCTCCTTGGCAC | 137 bp | 60 °C | XM_004003134.5 |
| Mfn2 | F: ATGAACTGCACCGCCACATA R: TTGAGGTCGTAGCTGAGGGA | 196 bp | 60 °C | XM_004013714.5 |
| Fis1 | F: TGAAGTATGTGCGAGGGCTG R: CCATGCCCACTAGTCCATCTTT | 108 bp | 60 °C | XM_027961118.1 |
| MFF | F: TCCAGCACGTGCATACTGAG R: CCGCCCCACTCACTAAATGT | 107 bp | 60 °C | XM_027965256.1 |
| ATP6 | F: GCCTCCTACCCCACTCATTTAC R: GGTGTCCCTTGTGGTAGGAAA | 145 bp | 60 °C | KT750051.1 |
| Cytb | F: ACCCACTTAACACTCCCCCT R: GAGAGGATTAGGGCGAGGAC | 112 bp | 60 °C | FR873152.1 |
| OPA1 | F: CAGTTAAGGACGTCATTGCAGC R: CTGGCCAAAAATTCCTGTGGG | 112 bp | 60 °C | XM_060404590.1 |
| TFAM | F: ATGGAAGTTGGACGAGAAGACC R: AAAGCTGCTCAGGCTCACTT | 73 bp | 60 °C | XM_027962472.2 |
| β-actin | F: AGCCTTCCTTCCTGGGCATGGA R: GGACAGCACCGTGTTGGCGTAGA | 113 bp | 60 °C | NM_001009784 |
| Gene | Primers (5′–3′) | Length | Annealing Temperature | ID |
|---|---|---|---|---|
| Bf | F: CCTGACTAAGAAGCACCGGC R: GTAAAACCGCCTACGCTCCC | 107 bp | 60 °C | U41167.1 |
| Ra | F: GGGCTTAACCCCTGAACTGC R: TCGCCACTGATGTTCCTCCT | 114 bp | 60 °C | X85098.1 |
| Ram | F: GGGGACAACACCTGGAAACG R: CTTGGTAGGCCGTTACCCCA | 124 bp | 60 °C | Y15992.1 |
| Sr | F: AGAAAGCCACGGCTAACTAC R: TCTCCTGCACTCAAGAAGAC | 169 bp | 60 °C | AB198442.1 |
| Tb | F: ATGGCAGGTACAGAGTGAAG R: TTCAAGGAGTCGGGTTTCAG | 95 bp | 60 °C | NR-118718.2 |
| Rf | F: CTAATCAGACGCGAGCCCAT R: ACATGCAAGTCGAACGGAGT | 196 bp | 60 °C | LT976286.1 |
| Fs | F: GATGAGCTTGCGTCCGATT R: ATTCCCTACTGCTGCCTCC | 110 bp | 60 °C | EU606019.1 |
| Cb | F: CATTGGGACTGAGACACGGC R: AAGACCGTCATCACTCACGC | 108 bp | 60 °C | NR_042144.1 |
| Bacterium | F: CCTACGGGAGGCAGCAG R: TTACCGCGGCTGCTGG | 181 bp | 60 °C | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Sha, Y.; Liu, X.; Gao, M.; Chen, X.; Yang, W.; Huang, W.; Wang, J.; He, Y.; Gao, X.; et al. A Study on the Differences in Rumen Microbiota–Liver Gluconeogenesis–Mitochondrial Interaction Between Tibetan Sheep and Hu Sheep in the Qinghai–Tibet Plateau. Animals 2025, 15, 1603. https://doi.org/10.3390/ani15111603
Chen Q, Sha Y, Liu X, Gao M, Chen X, Yang W, Huang W, Wang J, He Y, Gao X, et al. A Study on the Differences in Rumen Microbiota–Liver Gluconeogenesis–Mitochondrial Interaction Between Tibetan Sheep and Hu Sheep in the Qinghai–Tibet Plateau. Animals. 2025; 15(11):1603. https://doi.org/10.3390/ani15111603
Chicago/Turabian StyleChen, Qianling, Yuzhu Sha, Xiu Liu, Min Gao, Xiaowei Chen, Wenxin Yang, Wei Huang, Jiqing Wang, Yapeng He, Xu Gao, and et al. 2025. "A Study on the Differences in Rumen Microbiota–Liver Gluconeogenesis–Mitochondrial Interaction Between Tibetan Sheep and Hu Sheep in the Qinghai–Tibet Plateau" Animals 15, no. 11: 1603. https://doi.org/10.3390/ani15111603
APA StyleChen, Q., Sha, Y., Liu, X., Gao, M., Chen, X., Yang, W., Huang, W., Wang, J., He, Y., Gao, X., & He, Y. (2025). A Study on the Differences in Rumen Microbiota–Liver Gluconeogenesis–Mitochondrial Interaction Between Tibetan Sheep and Hu Sheep in the Qinghai–Tibet Plateau. Animals, 15(11), 1603. https://doi.org/10.3390/ani15111603





