Simple Summary
This study demonstrates that dietary supplementation with Lactobacillus reuteri LR1 alleviates weaning stress in piglets by enhancing growth and gut–liver axis metabolism. In a 21-day trial with 48 weaned piglets, LR1 significantly improved growth performance, and LR1 enhanced intestinal nutrient absorption via increased ileum villus height and upregulated amino acid transporters. Metabolically, LR1 elevated serum glycine, hydroxyproline, and hepatic taurine levels, with untargeted metabolomics identifying 11 amino-acid-related metabolites upregulated in portal plasma. Histological analysis revealed reduced ileum collagen deposition, and correlation analysis linked collagen dynamics to gut–liver axis amino acid metabolic reprogramming. This study highlights LR1 as a promising probiotic for optimizing nutrient utilization and promoting intestinal development in piglets, and the intestinal extracellular matrix may be a key target for improving amino acid transport in the gut–liver axis of weaned piglets.
Abstract
Weaning stress leads to intestinal dysfunction and impaired growth performance and intestinal development in piglets. This study aims to investigate the effects of Lactobacillus reuteri LR1 on growth performance and amino acid metabolism in the gut–liver axis of weaned piglets. A total of 48 weaned piglets (Duroc × Landrace × Yorkshire, 21 days old) were randomly assigned to the CON group (fed a basal diet) and the LR1 group (fed the basal diet supplemented with 5 × 1010 CFU/kg of Lactobacillus reuteri LR1) with six pens per group and 4 piglets each pen. The results demonstrated that LR1 significantly increased average daily gain (ADG), average daily feed intake (ADFI), and final body weight (p < 0.05). Additionally, LR1 significantly enhanced the villus height of the ileum (p < 0.05) and upregulated the expression of SLC6A19 in the jejunum, as well as SLC6A19, SLC7A1, and SLC38A9 in the ileum (p < 0.05). Amino acid analysis revealed that LR1 elevated the serum concentrations of glycine and hydroxyproline, along with increased taurine in the liver. Masson staining indicated LR1 reduced ileum fiber deposition, with COL3A1 identified as a key component. Furthermore, untargeted metabolomic analysis identified 27 amino acid-related differential metabolites and 11 significantly up-regulated in the plasma of the hepatic portal vein, including L-asparagine, L-citrulline, His-Cys, N-acetyltryptophan, 4-hydroxy-l-isoleucine, Gly-Arg, creatine, ornithine, ectoine, 3-methyl-l-histidine, and stachydrine. Correlation analysis suggested that COL1A2 and COL3A1 were closely associated with these metabolic changes. Overall, these findings suggest that LR1 supplementation promotes growth, improves intestinal morphology, reduces fiber deposition, and enhances amino acid metabolism in the gut–liver axis of weaned piglets.
1. Introduction
In modern swine production, the healthy development of weaned piglets is fundamental to economic profitability in the pig farming industry. However, piglets encounter numerous challenges during their growth [1]. Weaning stress in piglets leads to growth retardation, digestive disorders, and immune suppression, significantly affecting production efficiency and mortality rate [2,3,4]. Therefore, promoting the growth of piglets and improving production performance are not only conducive to promoting the animal welfare of piglets but also to reducing economic loss on farms.
The gut–liver axis represents a bidirectional communication system between the gastrointestinal tract and the liver, influencing physiological and pathological processes in animals significantly [5]. The foundation of piglet growth and development depends on the organism’s ability to efficiently absorb and metabolize amino acids [6,7]. The liver plays a central role in regulating amino acid distribution, as it receives the majority of amino acids transported via the portal vein [8]. Thus, the efficiency of amino acid transport within the gut–liver axis is a key determinant of overall amino acid absorption. However, intestinal barrier dysfunction, such as increased intestinal permeability, allows microbial endotoxins and metabolites to translocate into the portal circulation, triggering hepatic inflammation and oxidative stress [9,10]. Previous studies have found that weaned piglets show high diarrhea and mortality rates due to the underdeveloped digestive and absorptive functions of their intestines [11,12]. Therefore, proper intestinal development is particularly crucial for improving the growth performance and survival of piglets. Probiotics and phytogenic compounds have demonstrated therapeutic potential by suppressing harmful bacterial populations, promoting SCFA synthesis, and inhibiting hepatic stellate cell activation [13,14]. In addition, a previous study demonstrated that Lactobacillus reuteri LR1, which was renamed Limosilactobacillus reuteri (L. reuteri) in 2020 [15], significantly increases amino acid transport in the ileum of weaned piglets, and this process was highly correlated with changes in the extracellular matrix (ECM) [16]. The ECM provides essential structural support for intestinal cells, regulates cellular behavior, participates in signaling pathways, and contributes to the maintenance of the intestinal barrier [17]. The density and stiffness of the ECM are strongly associated with amino acid uptake and protein synthesis in surrounding cells [18]. ECM stiffening activates glycolysis and glutamine metabolism surrounding fibroblasts mechanically and thus coordinates amino acid flux within the microenvironment [19,20]. Therefore, this experiment was conducted to investigate the effects of LR1 on intestinal amino acid metabolism and the intestinal health of weaned pigs, with a view to providing a theoretical basis for the nutrition of piglets.
2. Materials and Methods
All animal protocols applied in this study complied with the Guidelines for the Care and Use of Animals for Research and Teaching at Guangdong Academy of Agricultural Sciences. They were also approved by the Animal Care and Use Committee of the Guangdong Academy of Agricultural Sciences.
2.1. Animals and Samples
In this experiment, 48 weaned piglets (Duroc × Landrace × Yorkshire), 21 days old with an average body weight of 5.1 kg, were randomly assigned to two groups with balanced sex distribution. Each group had 6 pens, with 4 piglets in each pen. The control group (CON) was fed a basal diet. The Lactobacillus reuteri group (LR1) received the basal diet supplemented with 5 × 1010 CFU/kg Lactobacillus reuteri [21]. The basal diet was formulated as a powdered compound feed based on the nutrient requirements of swine outlined by the NRC (2012), and its composition and nutritional levels are shown in Table 1. The probiotic freeze-dried powder was diluted and thoroughly mixed with feed. The experimental period lasted 21 days. Piglets were fed four times at 08:00, 12:00, 16:00, and 20:00 every day, and the diarrhea situation of piglets was observed and recorded. After the experimental period, 1 piglet was randomly selected from each pen and euthanized. Samples of small intestines, livers, and hepatic portal vein plasma were collected. Samples for section preparation were stored in 4% paraformaldehyde, while the remaining samples were stored in a −80 °C freezer.

Table 1.
Ingredients and composition of experimental diets (air-dry basis).
2.2. Quantitative Real-Time PCR
The total RNA of the jejunum and ileum was extracted using TRIzol reagent, and the total RNA concentration was determined. Then, the cDNA was synthesized following the instructions provided in the reverse transcription kit. Primers specific to the target genes were designed based on gene sequences using the Primer 5.0 software and subsequently synthesized after verification through the NCBI database. The corresponding primer sequences are shown in Table 2. cDNA was prepared in a real-time fluorescence quantitative PCR reaction system, and the relative expression of the mRNA of the target genes was calculated by the 2−ΔΔCt method according to the threshold cycling of the internal reference genes and the target genes’ expression; then, the expressions for each target gene were normalized to the control piglets (1.0) [22].

Table 2.
Primer sequence information.
2.3. H&E Staining & Masson Staining
Small intestinal tissues fixed in 4% paraformaldehyde were paraffin-embedded, followed by 4 μm sections and hematoxylin–eosin (H&E) staining. The stained sections were placed under an Axio Scope A1 microscope (Zeiss, Germany) and photographed; the height of the villi and the depths of crypts were counted by using the Image J software win64; and the ratio of the height of the villi to the depth of the crypts was calculated [23].
After staining the nuclei of the above sections with hematoxylin staining solution, the sections were differentiated with hydrochloric acid alcohol and then rinsed with ultrapure water. The sections were immersed in Masson Ponceau acid fuchsin solution and later rinsed with ultrapure water, washed in aqueous glacial acetic acid, and then dehydrated with an alcohol gradient, followed by immersion in xylene solution for clearing. Finally, the sections were sealed with neutral gum and observed using an Axio Scope A1 microscope, and the collagen volume fraction was calculated using Slide Viewer 2.5 and Image J [24].
2.4. Free Amino Acids Concentration Analysis
Fresh liver tissue was collected from piglets. The tissue was mixed with hydrochloric acid, ground by a grinder, and then centrifuged at 4 °C at 12,000 rpm for 15 min. The supernatant was collected, and 10% sodium sulfosalicylate was added to the supernatant (3:1, v/v). The mixture was thoroughly mixed and subjected to a second round of centrifugation. The supernatant was then collected using a sterile syringe, filtered, and transferred into an injection vial for further analysis. Plasma samples were directly mixed with 10% sodium sulfosalicylate, followed by centrifugation to obtain the supernatant. The supernatant was subsequently filtered and transferred into a sample vial for testing. The amino acid concentration of the samples was measured using a fully automated amino acid analyzer (L-8900, Hitachi, Ltd., Tokyo, Japan), and the results were processed and analyzed using its accompanying the EZChrom Elite software (version 3.1.5.b); then, the final results were expressed as nmol/μL. Buffer preparation referenced Yang [25].
2.5. Untargeted Metabolomics Analysis
Blood samples were collected in Vacutainer tubes containing EDTA and centrifuged at 1500× g for 15 min at 4 °C. The plasma was then aliquoted into 150 μL portions and stored at −80 °C until liquid chromatography–mass spectrometry (LC-MS) analysis. Prior to analysis, plasma samples were thawed at 4 °C. A 100 μL aliquot was mixed with 400 μL of cold methanol–acetonitrile (1:1, v/v) to precipitate proteins. The mixture was centrifuged at 14,000× g for 20 min at 4 °C, and the resulting supernatant was dried using a vacuum centrifuge. The dried extract was then reconstituted in 100 μL of acetonitrile–water (1:1, v/v) and centrifuged at 14,000× g for 15 min at 4 °C, and the supernatant was injected for analysis.
Analysis was performed using a UHPLC (Vanquish UHPLC, Thermo, Waltham, MA, USA) coupled to an Orbitrap (Shanghai Applied Protein Technology Co., Ltd., Shanghai, China). The raw MS data were converted into MzXML files using ProteoWizard MSConvert 3.0.22170 before importing into freely available XCMS software 3.22.0. Compound identification of metabolites was performed by comparing the accuracy (m/z) value (<10 ppm) and MS/MS spectra with an in-house database established with available authentic standards.
2.6. Statistical Analysis
Statistical analysis was performed using the SPSS 26.0 software, and the data were expressed as “mean ± SEM”. Statistical treatment was performed using Student’s t-test, with p < 0.05 considered statistically significant. VIP > 1 and a p-value < 0.05 were used to screen significantly changed metabolites; Pearson’s correlation analysis was performed to determine the correlation between two variables in untargeted metabolomics analysis.
3. Results
3.1. Growth Performance and Intestinal Morphology of Weaned Piglets
As shown in Table 3, LR1 significantly increased the final body weight, average daily gain (ADG), and average daily feed intake (ADFI) of piglets compared to the CON group (p < 0.05). However, no significant differences were observed in the ADG-to-ADFI ratio and the incidence of diarrhea between the LR1 group and the CON group.

Table 3.
Effects of LR1 on the growth performance of weaned piglets.
The H&E-staining sections of the duodenum, jejunum, and ileum of the piglets are shown in Figure 1. Compared with the CON group, in the LR1 group, the height of the intestinal villus was significantly increased in the ileum (p < 0.05) but exhibited a significantly lower villus-to-crypt ratio in the duodenum (p < 0.05), as shown in Table 4.
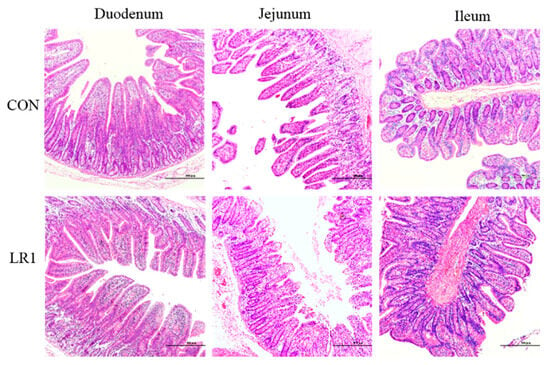
Figure 1.
H&E-stained sections of duodenum, jejunum, and ileum of the piglets. The magnification of the picture is 10 times, and the scale is 500 μm.

Table 4.
Effects of LR1 on intestinal morphology of weaned piglets.
3.2. Amino Acid Transport in the Gut–Liver Axis of Piglets
To evaluate the effect of LR1 on amino acid transport in the gut–liver axis of weaned piglets, the expression of amino acid transport carrier mRNA in the jejunum and ileum of piglets was investigated using q-PCR. The results, presented in Figure 2, indicate that LR1 supplementation significantly increased the mRNA expression of SLC6A19 (Figure 2C) while significantly decreasing the expression of SLC7A1 (Figure 2D) and markedly downregulating SLC7A11, SLC38A2, and SLC38A9 (Figure 2F–H) in the jejunum compared to the CON group. In addition, in the ileum, LR1 supplementation significantly upregulated the expression of SLC6A19, SLC7A1, and SLC38A9 (Figure 2C,D,H) compared to the CON group. However, no significant changes were observed in the expression of SLC3A2 and SLC6A14 in the intestine.
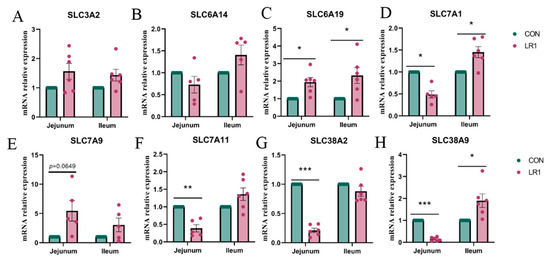
Figure 2.
mRNA expression of amino acid transport carriers in the intestine of piglets. The mRNA expression of SLC3A2 (A), SLC6A14 (B), SLC6A19 (C), SLC7A1 (D), SLC7A9 (E), SLC38A2 (F), SLC38A2 (G), and SLC38A9 (H) in the jejunum and ileum. The values are presented as mean ± SEM, n = 6. * p < 0.05, ** p < 0.01, and *** p < 0.001.
Subsequently, the free amino acid concentrations in the hepatic portal vein sera and livers of weaned piglets were measured by automatic amino acid analyzers. Table 5 shows the free amino acid concentrations in the sera of the experimental piglets. The results show that LR1 significantly increased the concentrations of glycine and hydroxyproline in the portal vein serum and decreased the concentrations of alanine, cysteine, glutamine, histidine, and isoleucine. In contrast to the changes in the portal vein serum, the amino acid concentrations in the liver showed different patterns. In the liver (Table 6), LR1 increased the level of taurine and reduced the concentrations of isoleucine, alanine, leucine, lysine, aspartic acid, ammonia, ornithine, phenylalanine, proline, cysteine, serine, taurine, threonine, glutamic acid, glutamine, histidine, and valine significantly.

Table 5.
Effects of LR1 on free amino acid concentrations in the hepatic portal vein (nmol/μL).

Table 6.
Effects of LR1 on free amino acid concentrations in the liver (nmol/μL).
3.3. Structural Changes in the Extracellular Matrix of the Piglet Intestine
To investigate the effect of LR1 on the extracellular matrix of the intestinal mucosa of weaned pigs, Masson staining was performed to assess the collagen fiber content in the ileum. The sections were then quantified using the Image J software (Figure 3A, left), and the ratio of collagen fibers (blue) to the total tissue surface area was calculated to determine the collagen volume fraction in the ileum. The result showed that CVF was reduced significantly in the LR1 group (Figure 3A, right). Furthermore, the mRNA expression levels of key molecules in the extracellular matrix of the jejunum and ileum were analyzed. The results showed that LR1 significantly decreased the expression of COL1A2 (Figure 3B) and COL1A3 (Figure 3C) in the jejunum, but it had no significant impact on the expression of COL6A1 (Figure 3D) or FN1 (Figure 3E). In the ileum, LR1 significantly reduced the expression of COL1A3 but had no significant impact on the expression of COL1A2, COL6A1, or FN1. The results indicated that LR1 significantly decreased the volume fraction of collagen in the ileum of piglets, suggesting that LR1 can reduce the deposition of collagen fibers in the ileum of weaned piglets.
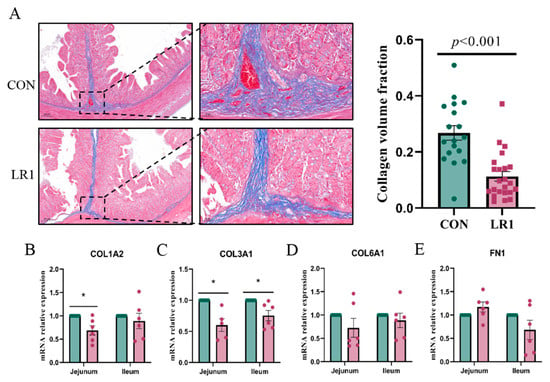
Figure 3.
Effects of LR1 on the structure of the extracellular matrix in the intestine. (A) Masson-stained sections of the ileum of weaned piglets (left) and the collagen volume fraction of the ileum. mRNA expression of COL1A2 (B), COL3A1 (C), COL6A1 (D), and FN1 (E) in the jejunum and ileum. The values are presented as mean ± SEM, n = 6. * p < 0.05.
3.4. Metabolomics Analysis of Hepatic Portal Vein Plasma in Piglets
To investigate the impact of incorporating LR1 into the diet on nutrient metabolism in the digestive tract of underweight pigs, a non-targeted metabolomics analysis was used to assess changes in metabolites within the porcine hepatic portal vein plasma of the piglets. Figure 4A presents a partial least squares discriminant analysis (PLS-DA) plot for samples from both groups. The model evaluation parameters obtained through 7-fold cross-validation yielded a positive Q2 value of 0.617 (left) and a negative Q2 value of 0.543. Q2 > 0.5, indicating that this model is robust and that there are significant differences in metabolite compositions between samples from the two groups. Metabolites exhibiting a fold change (FC) greater than 1.5 or less than 0.67, along with a p-value below 0.05, were classified as differential metabolites and are visually represented using a volcano plot (Figure 4B). In total, 11,523 metabolites were detected in the positive ion mode, with 179 significantly upregulated and 253 significantly downregulated in the LR1. Furthermore, 11,600 metabolites were detected in the negative ion mode, with 244 significantly upregulated and 216 significantly downregulated in the LR1. After combining the amino acid metabolites detected under both modes, significantly differential metabolites were screened based on VIP > 1 and p < 0.05 (Figure 4C). The LR1 group showed a significant increase in the contents of 11 metabolites, including L-asparagine, L-citrulline, His-Cys, N-acetyltryptophan, 4-hydroxy-l-isoleucine, Gly-Arg, creatine, ornithine, ectoine, 3-methyl-l-histidine, and stachydrine.
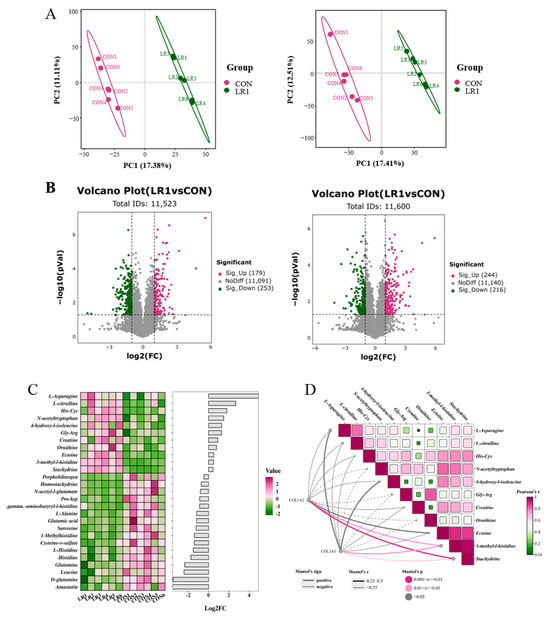
Figure 4.
Effects of LR1 on the metabolites of hepatic portal vein plasma in weaned piglets. (A) Score plot of PLS-DA in positive (left) and negative (right) ion modes. (B) Volcano plot in positive (left) and negative (right) ion modes. (C) Heatmap and histogram of significantly differential metabolites; red color represents high expression, green color represents low expression, and the abscissa is the log2 (FC) of the metabolites in the histogram; VIP > 1, p < 0.05. (D) Correlation network heatmap analysis between the extracellular matrix and amino acid metabolites. Pearson correlation analysis was used for the heatmap, and the network diagram was analyzed using the Mantel test: linear representation of positive relationships between matrices (r sign); p < 0.05 was regarded as a significant correlation between the two matrices, represented by a solid line; p ≥ 0.05 indicated no significant correlation, represented by a dotted line. The larger the absolute value of r (r.abs), the stronger the correlation between the two matrices.
In the above results, LR1 significantly increased the villus height of the ileum in weaned piglets and significantly reduced the CFV in the ileum. To explore the potential link between enhanced amino acid metabolism and these intestinal structural changes, a correlation network heatmap analysis (Figure 4D) was conducted using COL1A2 and COL3A1. The results showed that the decreases in COL1A2 and COL3A1 were closely related to the upregulation of these 11 metabolites. Notably, the downregulation of COL1A2 and COL3A1 exhibited a positive connection with the upregulation of eight metabolites, L-asparagine, L-citrulline, His-Cys, 4-hydroxy-l-isoleucine, creatine, ectoine, 3-methyl-l-histidine, and Stachydrine, and the down-expression of COL1A2 and COL3A1 showed significantly positive correlations with 3-methyl-l-histidine and stachydrine.
4. Discussion
Following the restriction on antibiotic use in animal feed, researchers have proposed various alternatives to fill the gap left by antibiotic growth promoters [26]. Studies have shown that intestinal probiotics are critical to host functions such as nutrient absorption and immunity [27,28]. Gut-derived metabolites, including SCFAs, enhance intestinal barrier function, reducing endotoxin translocation and oxidative stress in the liver [29]. Microbial imbalances elevate trimethylamine N-oxide and lipopolysaccharides, exacerbating hepatic steatosis and fibrosis conversely [30], even impairing neural function and behavioral adaptability [31]. Gut microbiota modulate cardiovascular health through their metabolites, which drive endothelial dysfunction and atherosclerosis [32]. Probiotics restore microbial balance, mitigating myocardial injury via anti-inflammatory pathways [33]. Gut microbes synergistically regulate the gut–liver, gut–brain, and gut–heart axis through metabolites and inflammatory signaling; systemic oxidative stress and probiotic interventions are key mediators of cross-organ health interactions. The above studies highlight that gut-microbiota-derived metabolites critically support organ function and systemic physiological integration.
LR1 has been recognized for its role in maintaining intestinal microbial balance and inhibiting the proliferation of pathogenic bacteria, thereby enhancing the growth performance of piglets, improving intestinal immunity, and promoting gut health [34]. Previous studies have demonstrated that LR1 supplementation produces effects comparable to antibiotic-containing feed in improving the growth performance of 21-day-old weaned piglets [22]. Another study found that LR1 can inhibit the apoptosis of intestinal epithelial cells and maintain the integrity of the intestinal wall in mice by regulating the expression of intestinal tight junction proteins [35]. This indicates that LR1 may potentially serve as one of the feed additives used to replace antibiotics. In addition, LR1 demonstrated the same promoting effect on the growth performance of piglets and had a dominant position within Lactobacillus [36,37]. In our study, the addition of LR1 to the diet significantly increased the final body weight, average daily feed intake, and average daily gain of piglets, remarkably improving the growth performance of piglets, which is also consistent with previous research findings. Although there were no significant effects on the G/F and diarrhea of piglets, there were positive trends in both aspects. Furthermore, LR1 significantly reduced the V/C of the duodenum and significantly increased the villus height of the ileum, which is similar to the research results of Tang [36]. This indicates that LR1 has the potential to be used as an alternative to antibiotic feed additives to promote the growth of piglets and improve intestinal health.
The liver is the governor of the flow of most amino acids in animals, and the total number of amino acids pooled in the hepatic portal vein determines the uptake of amino acids by the animal organism [8]. Yang’s [25] experiment found that LR1 could significantly increase the gene expression of SLC6A19 and SLC38A9 in the ileum, as well as the expression of SLC7A1 in the duodenum. The exogenous addition of supplements to the sow’s diet can likewise boost the expression of SLC7A1 in the piglets’ intestines [38]. In this study, LR1 significantly increased the expression of SLC6A19 in the jejunum and SLC6A19, SLC7A1, and SLC38A9 in the ileum, which is consistent with the abovementioned research results. This may imply that LR1 can regulate the physiological processes that facilitate the flow of amino acids from the intestine to the liver. The different expression trends of SLC7A1 in the jejunum and ileum and the decrease in the concentrations of SLC38A2 and SLC38A9 in the jejunum may be caused by the differences between different intestinal segments [39]. Collectively, these findings imply that LR1 has a positive effect on amino acid transport in the gut–liver axis.
The serum amino acids of piglets play a crucial role in growth performance, as well as in the development of gut health and important biological processes [40]. Consequently, a further assessment of the impacts of LR1 supplementation on the composition of serum amino acids was carried out. The results indicated that LR1 predominantly leads to a reduction in the levels of alanine, cysteine, glutamine, histidine, and isoleucine and increased glycine and hydroxyproline in the piglets’ serum. These findings concurred with a prior study demonstrating that the oral administration of Lactobacillus increases the glycine concentration in the sera of piglets [41]. Another study with similar results reported that the supplementation of nucleotides has a regulatory effect on the relative quantities of glutamic acid, aspartic acid, serine, alanine, glycine, leucine, and valine in the muscle of grass carp [42]. The observed decrease in certain free amino acid concentrations in both the serum and liver suggests that LR1 may contribute to the balance of the intestinal microecology and enhance nutrient absorption efficiency [43]. This improvement enables low-body-weight piglets to absorb amino acids more efficiently and rapidly. After absorption, amino acids are transported more quickly to other tissues and organs for metabolism and synthesis rather than accumulating in the serum of the hepatic portal vein and the liver [44]. Consequently, this process results in reduced amino acid levels in those parts [45]. In our research, the mRNA expression of amino acid transporters was upregulated by LR1, corroborating this hypothesis. On the other hand, it is possible that LR1 enhanced the deamination of amino acids, converting them into keto acids and ammonia [46]. Glutamic acid, serving as a specific precursor for the production of alanine, aspartic acid, proline, ornithine, arginine, and the antioxidant glutathione, can undergo transamination metabolism under the action of corresponding aminotransferases and dehydrogenases [47]. Ammonia is further synthesized into urea in the liver and excreted from the body, while keto acids enter other metabolic pathways to provide energy or synthesize other substances, thereby reducing the amino acid content in the liver [48]. This hypothesis is further supported by our observation of a tendency toward increased urea concentrations in the serum.
ECM is not only the supporting framework for various intestinal cells but also an important medium for the growth, differentiation, migration, and information transmission of these cells in the intestine [49]. When amino acids are restricted, ECM internalization caused by cells or degradation can upregulate the levels of intracellular tyrosine and phenylalanine, and the enhanced metabolism of tyrosine leads to an increase in fumarate, which promotes the TCA cycle and provides an energy source for the metabolic activities of cells [50,51]. LR1 decreases the CFV of the ileum, which may be caused by ECM internalization. Furthermore, collagen is the most abundant structure of the intestinal extracellular matrix, mainly distributed in the basement membrane [52]; the significant decrease in the collagen content demonstrates that LR1 promotes the internalization of ECM deeply in this study. To investigate the connection between changes in the ECM and amino acid metabolism, a correlation analysis was conducted. A positive association was observed between the downregulation of ECM components and the upregulation of amino acid metabolites, which might be attributed to the indirect promotion of amino acid transporter expression caused by the ECM through the activation of the YAP/TAZ pathway, as suggested in previous studies [53]. The results above indicate that the ECM could be an important target for improving intestinal amino acid metabolism in weaned piglets.
5. Conclusions
This study demonstrates that LR1 significantly improves growth performance in weaned piglets by upregulating intestinal amino acid transporter expression to enhance gut–liver axis amino acid transport while suppressing ECM component synthesis to alleviate ileum fibrosis. Correlation analysis identifies ECM remodeling as a pivotal regulatory target for the LR1-mediated modulation of amino acid metabolism. These findings establish a theoretical foundation for optimizing weaned piglet management and provide mechanistic insights for subsequent nutritional interventions.
Author Contributions
Conceptualization, E.X. and H.Y.; methodology, Y.H., Y.W., S.R., Q.W. and Y.X.; software, Y.W., S.R., Q.W. and Y.X.; validation, Y.W., S.R., L.W., Z.J. and E.X.; investigation, Y.H., Y.W., S.R., Q.W. and Y.X.; resources, Q.W., Y.X., L.W. and Z.J.; writing—original draft preparation, Y.H.; writing—review and editing, H.Y.; visualization, H.Y.; supervision, E.X., Z.J. and L.W.; project administration, Z.J. and L.W.; formal analysis, Y.H. and E.X.; data curation and funding acquisition, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32272920), the Guangdong Basic and Applied Basic Research Foundation (2023A1515012491), the earmarked fund for the China Agriculture Research System (CARS-35), and the Building the Main Force of Agricultural Research of Science and Technology Innovation Strategy (R2023PY-JX014), Guangzhou Science and Technology Plan Project (2025A04J5179).
Institutional Review Board Statement
This animal study protocol was approved by the Animal Care and Use Committee of Guangdong Academy of Agricultural Sciences (protocol code 2022m010 and approved on 20220315).
Data Availability Statement
None of the data were deposited in an official repository. The data are available from the authors upon request.
Acknowledgments
We thank all staff at the Pig Nutrition Research Laboratory of the Institute of Animal Science, Guangdong Academy of Agricultural Sciences, for their support and assistance in this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Long, D.W.; Long, B.D.; Nawaratna, G.I.; Wu, G. Oral Administration of L-Arginine Improves the Growth and Survival of Sow-Reared Intrauterine Growth-Restricted Piglets. Animals 2025, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.N.; Spencer, J.D.; Almeida, F.R.C.L.; Patterson, J.L.; Chiarini-Garcia, H.; Dyck, M.K.; Foxcroft, G.R. Consequences of a low litter birth weight phenotype for postnatal lean growth performance and neonatal testicular morphology in the pig. Animal 2013, 7, 1681–1689. [Google Scholar] [CrossRef]
- Kirkden, R.D.; Broom, D.M.; Andersen, I.L. Invited review: Piglet mortality: Management solutions. J. Anim. Sci. 2013, 91, 3361–3389. [Google Scholar] [CrossRef]
- Geiping, L.; Hartmann, M.; Kreienbrock, L.; Grosse, B.E. Killing underweighted low viable newborn piglets: Which health parameters are appropriate to make a decision? Porc. Health Manag. 2022, 8, 25. [Google Scholar] [CrossRef]
- Dumitru, A.; Matei, E.; Cozaru, G.C.; Chisoi, A.; Alexandrescu, L.; Popescu, R.C.; Butcaru, M.P.; Dumitru, E.; Rugina, S.; Tocia, C. Endotoxin Inflammatory Action on Cells by Dysregulated-Immunological-Barrier-Linked ROS-Apoptosis Mechanisms in Gut-Liver Axis. Int. J. Mol. Sci. 2024, 25, 2472. [Google Scholar] [CrossRef]
- Chandel, N.S. Amino Acid Metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040584. [Google Scholar] [CrossRef]
- Azad, H.; Akbar, M.Y.; Sarfraz, J.; Haider, W.; Ghazanfar, S. Simulation studies to identify high-affinity probiotic peptides for inhibiting PAK1 gastric cancer protein: A comparative approach. Comput. Biol. Chem. 2025, 115, 108345. [Google Scholar] [CrossRef]
- Paulusma, C.C.; Lamers, W.H.; Broer, S.; van de Graaf, S. Amino acid metabolism, transport and signalling in the liver revisited. Biochem. Pharmacol. 2022, 201, 115074. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Y.; Zhang, Y.; Dong, J.; Jiang, S.; Tang, Y. DHA-enriched phosphatidylserine ameliorates cyclophosphamide-induced liver injury via regulating the gut-liver axis. Int. Immunopharmacol. 2024, 140, 112895. [Google Scholar] [CrossRef]
- Perumal, S.K.; Arumugam, M.K.; Osna, N.A.; Rasineni, K.; Kharbanda, K.K. Betaine regulates the gut-liver axis: A therapeutic approach for chronic liver diseases. Front. Nutr. 2025, 12, 1478542. [Google Scholar] [CrossRef]
- Mcpeek, A.C.; Patton, B.; Columbus, D.A.; Olver, T.D.; Rodrigues, L.A.; Sands, J.M.; Weber, L.P.; Ferguson, D.P. Low birth weight and reduced postnatal nutrition lead to cardiac dysfunction in piglets. J. Anim. Sci. 2023, 101, skad364. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Wang, H.; Zhang, Q.; Chen, J.; Zhang, X.; Chen, W. Effects of intestinal nutrition and health additives on growth performance, serum biochemical index and digestive enzyme activity in weakly stiff pigs. Chin. J. Vet. Sci. 2019, 39, 2082–2087. [Google Scholar] [CrossRef]
- You, M.; Zhou, L.; Wu, F.; Zhang, L.; Zhu, S.X.; Zhang, H.X. Probiotics for the treatment of hyperlipidemia: Focus on gut-liver axis and lipid metabolism. Pharmacol. Res. 2025, 214, 107694. [Google Scholar] [CrossRef]
- Niu, X.; Chang, G.; Xu, N.; Li, R.; Niu, B.; Mao, R.; Wang, S.; Li, G.; Jiang, J.; Wang, L. Vitamin A-Integrated Cinnamaldehyde Nanoemulsion: A Nanotherapeutic Approach to Counteract Liver Fibrosis via Gut-Liver Axis Modulation. ACS Nano 2025, 19, 10433–10451. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Yi, H.; Yang, G.; Xiong, Y.; Wu, Q.; Xiao, H.; Wen, X.; Yang, X.; Wang, L.; Jiang, Z. Integrated metabolomic and proteomics profiling reveals the promotion of Lactobacillus reuteri LR1 on amino acid metabolism in the gut-liver axis of weaned pigs. Food Funct. 2019, 10, 7387–7396. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, B.; Jin, T.; Ocansey, D.; Jiang, J.; Mao, F. Intestinal Fibrosis in Inflammatory Bowel Disease and the Prospects of Mesenchymal Stem Cell Therapy. Front. Immunol. 2022, 13, 835005. [Google Scholar] [CrossRef]
- Elowsson, R.L.; Lofdahl, A.; Ahrman, E.; Muller, C.; Notermans, T.; Michalikova, B.; Rosmark, O.; Zhou, X.H.; Dellgren, G.; Silverborn, M.; et al. Matrisome Properties of Scaffolds Direct Fibroblasts in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2019, 20, 4013. [Google Scholar] [CrossRef]
- Bertero, T.; Oldham, W.M.; Grasset, E.M.; Bourget, I.; Boulter, E.; Pisano, S.; Hofman, P.; Bellvert, F.; Meneguzzi, G.; Bulavin, D.V.; et al. Tumor-Stroma Mechanics Coordinate Amino Acid Availability to Sustain Tumor Growth and Malignancy. Cell Metab. 2019, 29, 124–140. [Google Scholar] [CrossRef]
- Seo, B.R.; Chen, X.; Ling, L.; Song, Y.H.; Shimpi, A.A.; Choi, S.; Gonzalez, J.; Sapudom, J.; Wang, K.; Andresen, E.R.; et al. Collagen microarchitecture mechanically controls myofibroblast differentiation. Proc. Natl. Acad. Sci. USA 2020, 117, 11387–11398. [Google Scholar] [CrossRef]
- Yang, K.M.; Jiang, Z.Y.; Zheng, C.T.; Wang, L.; Yang, X.F. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 2014, 92, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Wang, L.; Xiong, Y.; Wen, X.; Wang, Z.; Yang, X.; Gao, K.; Jiang, Z. Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. J. Anim. Sci. 2018, 96, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q. Dietary H. illucens Larvae Meal Improves Growth Performance and Intestinal Barrier Function of Weaned Pigs Under the Environment of Enterotoxigenic Escherichia coli K88. Master’s Thesis, Guizhou University, Guiyang, China, 2022. [Google Scholar]
- Liu, C. Effects of Lactobacillus reuteri from Pigs on Intestinal Barrier Function and Extracellular Matrix of Weaned Piglet. Master’s Thesis, Guangxi University, Nanning, China, 2023. [Google Scholar]
- Yang, G. Effects of Lactobacillus reuteri LR1 on the Growth Performance, Intestinal Barrier Function and Amino Acid Transportation in Weaned Piglets. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2018. [Google Scholar]
- de la Fuente-Nunez, C.; Cesaro, A.; Hancock, R. Antibiotic failure: Beyond antimicrobial resistance. Drug Resist. Updates 2023, 71, 101012. [Google Scholar] [CrossRef]
- Mazhar, M.U.; Naz, S.; Zulfiqar, T.; Khan, J.Z.; Hilal, F.; Ghazanfar, S.; Tipu, M.K. Bacillus subtilis (NMCC-path-14) ameliorates acute phase of arthritis via modulating NF-κB and Nrf-2 signaling in mice model. Inflammopharmacology 2025, 33, 1863–1877. [Google Scholar] [CrossRef]
- Ma, L.; Tao, S.; Song, T.; Lyu, W.; Li, Y.; Wang, W.; Shen, Q.; Ni, Y.; Zhu, J.; Zhao, J.; et al. Clostridium butyricum and carbohydrate active enzymes contribute to the reduced fat deposition in pigs. Imeta 2024, 3, e160. [Google Scholar] [CrossRef]
- Yukino-Iwashita, M.; Nagatomo, Y.; Kawai, A.; Taruoka, A.; Yumita, Y.; Kagami, K.; Yasuda, R.; Toya, T.; Ikegami, Y.; Masaki, N.; et al. Short-Chain Fatty Acids in Gut-Heart Axis: Their Role in the Pathology of Heart Failure. J. Pers. Med. 2022, 12, 1805. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, S.; Wu, J.; Ye, T.; Wang, S.; Wang, P.; Xing, D. Butyrate-producing bacteria and the gut-heart axis in atherosclerosis. Clin. Chim. Acta 2020, 507, 236–241. [Google Scholar] [CrossRef]
- He, X.; Hu, M.; Xu, Y.; Xia, F.; Tan, Y.; Wang, Y.; Xiang, H.; Wu, H.; Ji, T.; Xu, Q.; et al. The gut-brain axis underlying hepatic encephalopathy in liver cirrhosis. Nat. Med. 2025, 31, 627–638. [Google Scholar] [CrossRef]
- Abdulrahim, A.O.; Doddapaneni, N.; Salman, N.; Giridharan, A.; Thomas, J.; Sharma, K.; Abboud, E.; Rochill, K.; Shreelakshmi, B.; Gupta, V.; et al. The gut-heart axis: A review of gut microbiota, dysbiosis, and cardiovascular disease development. Ann. Med. Surg. 2025, 87, 177–191. [Google Scholar] [CrossRef]
- He, Z.; Xiong, H.; Cai, Y.; Chen, W.; Shi, M.; Liu, L.; Wu, K.; Deng, X.; Deng, X.; Chen, T. Clostridium butyricum ameliorates post-gastrectomy insulin resistance by regulating the mTORC1 signaling pathway through the gut-liver axis. Microbiol. Res. 2025, 297, 128154. [Google Scholar] [CrossRef]
- Xia, L.; Wu, Z.; Jin, X.; Xu, H.; Zhang, H.; Zhang, T.; Zhu, J.; Zhang, Z. Application of Lactobacillus reuteri in healthy pig breeding. Swine Ind. Sci. 2025, 42, 76–77. [Google Scholar]
- Zhou, Q.; Wu, F.; Chen, S.; Cen, P.; Yang, Q.; Guan, J.; Cen, L.; Zhang, T.; Zhu, H.; Chen, Z. Lactobacillus reuteri improves function of the intestinal barrier in rats with acute liver failure through Nrf-2/HO-1 pathway. Nutrition 2022, 99–100, 111673. [Google Scholar] [CrossRef]
- Tang, Q.; Yi, H.; Hong, W.; Wu, Q.; Yang, X.; Hu, S.; Xiong, Y.; Wang, L.; Jiang, Z. Comparative Effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on Growth Performance, Antioxidant Function, and Intestinal Immunity in Weaned Pigs. Front. Vet. Sci. 2021, 8, 728849. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, J.; Zhou, Q.; Peng, Y.; Huang, Y.; Zhou, Y.; Zheng, J.; Zhao, M.; Hu, C.; Lan, S. Effects of inactivated Lactobacillus rhamnosus on growth performance, serum indicators, and colonic microbiota and metabolism of weaned piglets. BMC Vet. Res. 2024, 20, 422. [Google Scholar] [CrossRef]
- Gao, L.M.; Liu, G.Y.; Wang, H.L.; Wassie, T.; Wu, X. Maternal pyrimidine nucleoside supplementation regulates fatty acid, amino acid and glucose metabolism of neonatal piglets. Anim. Nutr. 2022, 11, 309–321. [Google Scholar] [CrossRef]
- Yang, G.; Wang, L.; Yi, H.; Wang, Z.; Yang, X.; Gao, K.; Wen, X.; Jiang, Z. Effects of Lactobacillus reuteri LR1 on Serum Biochemical Indexes and mRNA Expressions of Intestinal Nutrient Transporters of Weaned Piglets. Chin. J. Anim. Nutr. 2018, 30, 4589–4600. [Google Scholar]
- Mi, M.; Shen, Z.; Hu, N.; Zhang, Q.; Wang, B.; Pan, L.; Qin, G.; Bao, N.; Zhao, Y. Effects of diets with different amino acid release characteristics on the gut microbiota and barrier function of weaned pigs. BMC Microbiol. 2023, 23, 18. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H.; Wang, S.; Zhang, W.; Wang, J.; Tian, H.; Wang, Y.; Ji, H. Fecal Microbiota and Its Correlation with Fatty Acids and Free Amino Acids Metabolism in Piglets After a Lactobacillus Strain Oral Administration. Front. Microbiol. 2019, 10, 785. [Google Scholar] [CrossRef]
- Tie, H.; Wu, P.; Jiang, W.; Liu, Y.; Kuang, S.; Zeng, Y.; Jiang, J.; Tang, L.; Zhou, X.; Feng, L. Dietary nucleotides supplementation affect the physicochemical properties, amino acid and fatty acid constituents, apoptosis and antioxidant mechanisms in grass carp (Ctenopharyngodon idellus) muscle. Aquaculture 2019, 502, 312–325. [Google Scholar] [CrossRef]
- Yoon, K.N.; Lee, H.G.; Yeom, S.J.; Kim, S.S.; Park, J.H.; Song, B.S.; Yi, S.W.; Do, Y.J.; Oh, B.; Oh, S.I.; et al. Lactiplantibacillus argentoratensis AGMB00912 alleviates salmonellosis and modulates gut microbiota in weaned piglets: A pilot study. Sci. Rep. 2024, 14, 15466. [Google Scholar] [CrossRef]
- Gao, T.; Li, R.; Hu, L.; Hu, Q.; Wen, H.; Zhou, R.; Yuan, P.; Zhang, X.; Huang, L.; Zhuo, Y.; et al. Probiotic Lactobacillus rhamnosus GG improves insulin sensitivity and offspring survival via modulation of gut microbiota and serum metabolite in a sow model. J. Anim. Sci. Biotechnol. 2024, 15, 89. [Google Scholar] [CrossRef]
- Wu, G. Metabolism of amino acids in the small intestine: A new perspective on animal nutrition. Feed Ind. 2011, 32, 51–54. [Google Scholar] [CrossRef]
- Hu, S.H.; Feng, Y.Y.; Yang, Y.X.; Ma, H.D.; Zhou, S.X.; Qiao, Y.N.; Zhang, K.H.; Zhang, L.; Huang, L.; Yuan, Y.Y.; et al. Amino acids downregulate SIRT4 to detoxify ammonia through the urea cycle. Nat. Metab. 2023, 5, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, S.; Ma, C.; Song, F.; Li, X.; Wang, H.; Wang, X. Research progress on the intestinal function and transport mechanism of glutamate. Mod. J. Anim. Husb. Vet. Med. 2025, 1, 91–96. [Google Scholar] [CrossRef]
- Ghosh, N.; Mahalanobish, S.; Sil, P.C. Reprogramming of urea cycle in cancer: Mechanism, regulation and prospective therapeutic scopes. Biochem. Pharmacol. 2024, 228, 116326. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Nazemi, M.; Yanes, B.; Martinez, M.L.; Walker, H.J.; Pham, K.; Collins, M.O.; Bard, F.; Rainero, E. The extracellular matrix supports breast cancer cell growth under amino acid starvation by promoting tyrosine catabolism. PLoS Biol. 2024, 22, e3002406. [Google Scholar] [CrossRef]
- Colombero, C.; Remy, D.; Antoine-Bally, S.; Mace, A.S.; Monteiro, P.; Elkhatib, N.; Fournier, M.; Dahmani, A.; Montaudon, E.; Montagnac, G.; et al. mTOR Repression in Response to Amino Acid Starvation Promotes ECM Degradation Through MT1-MMP Endocytosis Arrest. Adv. Sci. 2021, 8, e2101614. [Google Scholar] [CrossRef]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef]
- Liao, X.; Li, X.; Liu, R. Extracellular-matrix mechanics regulate cellular metabolism: A ninja warrior behind mechano-chemo signaling crosstalk. Rev. Endocr. Metab. Disord. 2023, 24, 207–220. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).