When Sardines Disappear: Tracking Common Dolphin, Delphinus delphis, Distribution Responses Along the Western Iberian Coast
Simple Summary
Abstract
1. Introduction
- The Null Hypothesis (H0): There is no statistically significant difference in the distance of the common dolphin from the coast across different time periods (annually; four-year period; before and after the Sardine Ban)
- Alternative Hypothesis (H1): There is a statistically significant difference in the distance of the common dolphin from the coast across different time periods (annually; four-year period; before and after the Sardine Ban)
- The Null Hypothesis (H0): There is no statistically significant relationship between sardine biomass levels and the distance of the common dolphin from the coast.
- Alternative Hypothesis (H1): There is a statistically significant relationship between sardine biomass and the distance of the common dolphin from the coast.
2. Materials and Methods
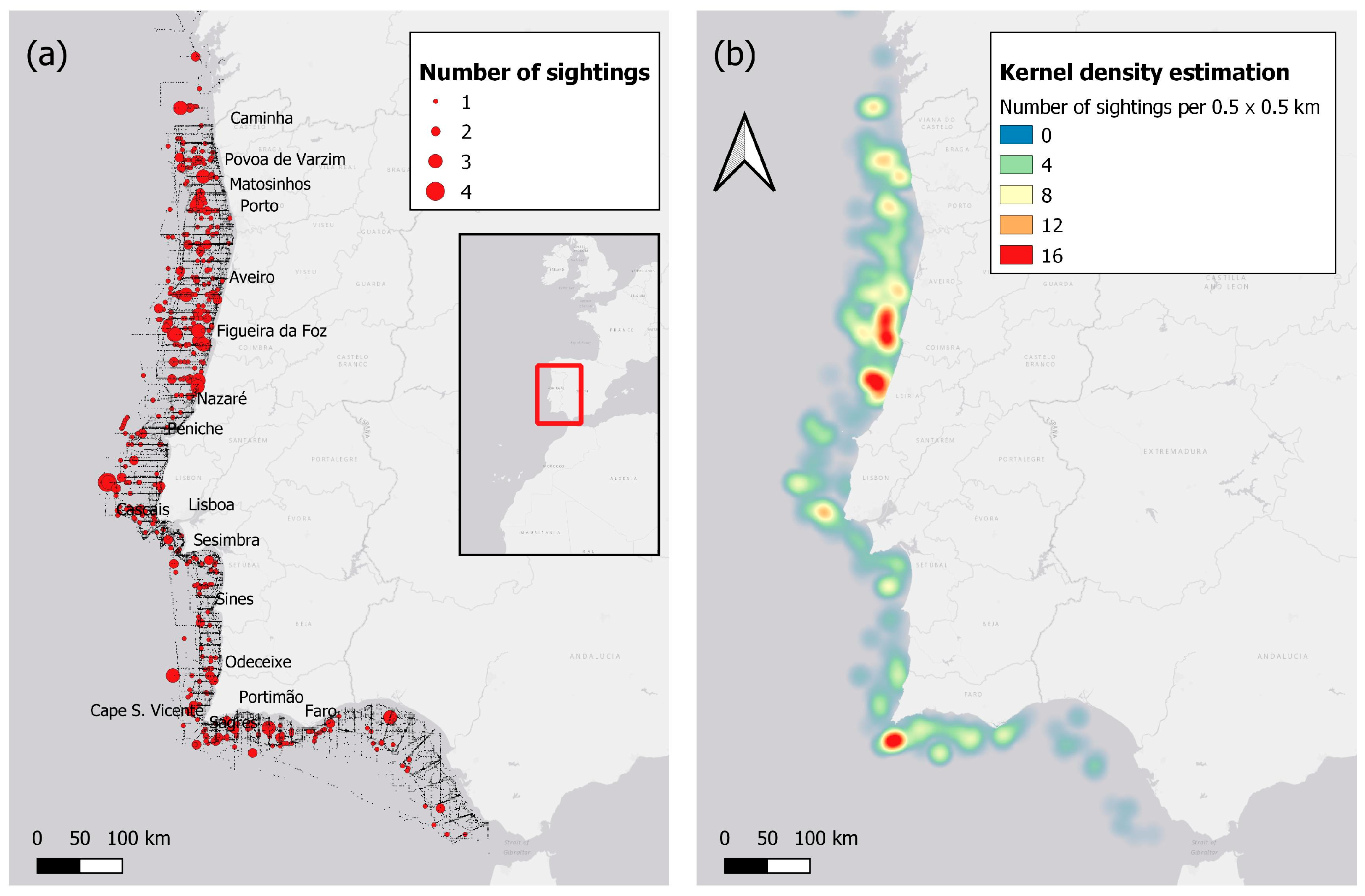
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
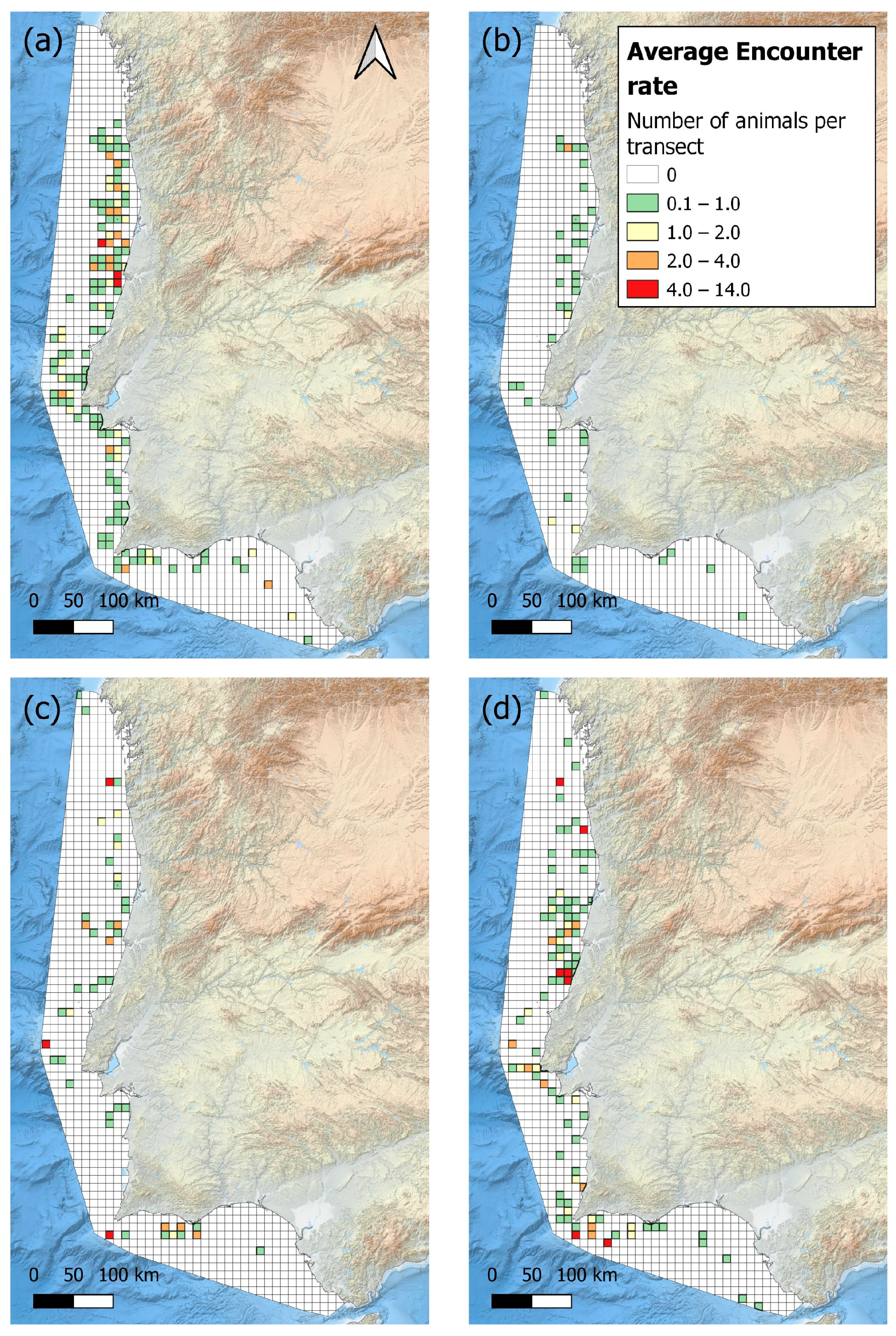
2.3.1. Temporal Stratification
- (i)
- Every year;
- (ii)
- Every four years;
- (iii)
- Before and after the Sardine Ban.
2.3.2. Sightings and Grid Maps
2.3.3. Distance to Coast
- E[distance] represents the expected value of distance of common dolphins’ distance from the coast;
- β0 is the intercept value;
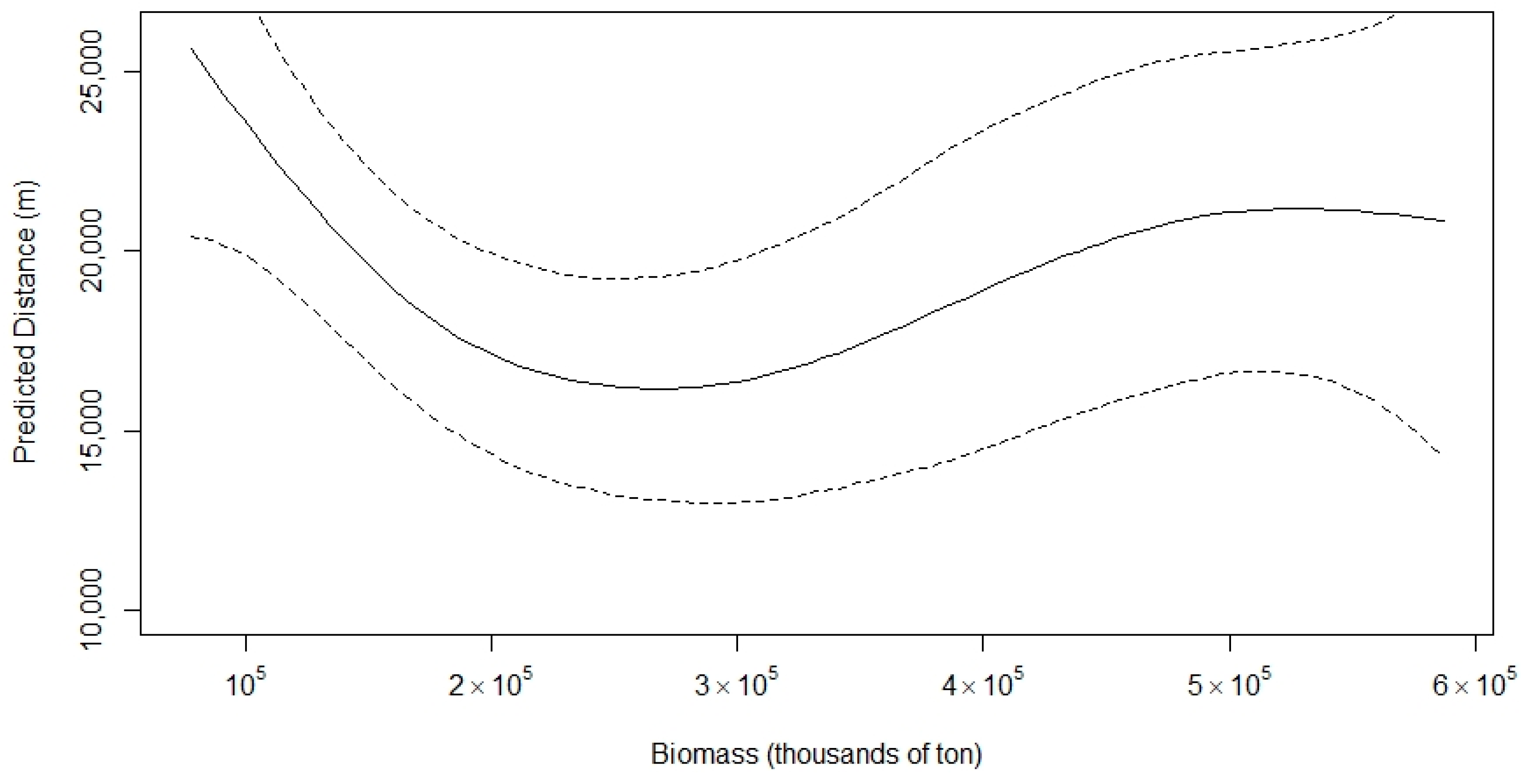
- s(biomass2) is a smooth function (spline) of sardine biomass.
3. Results
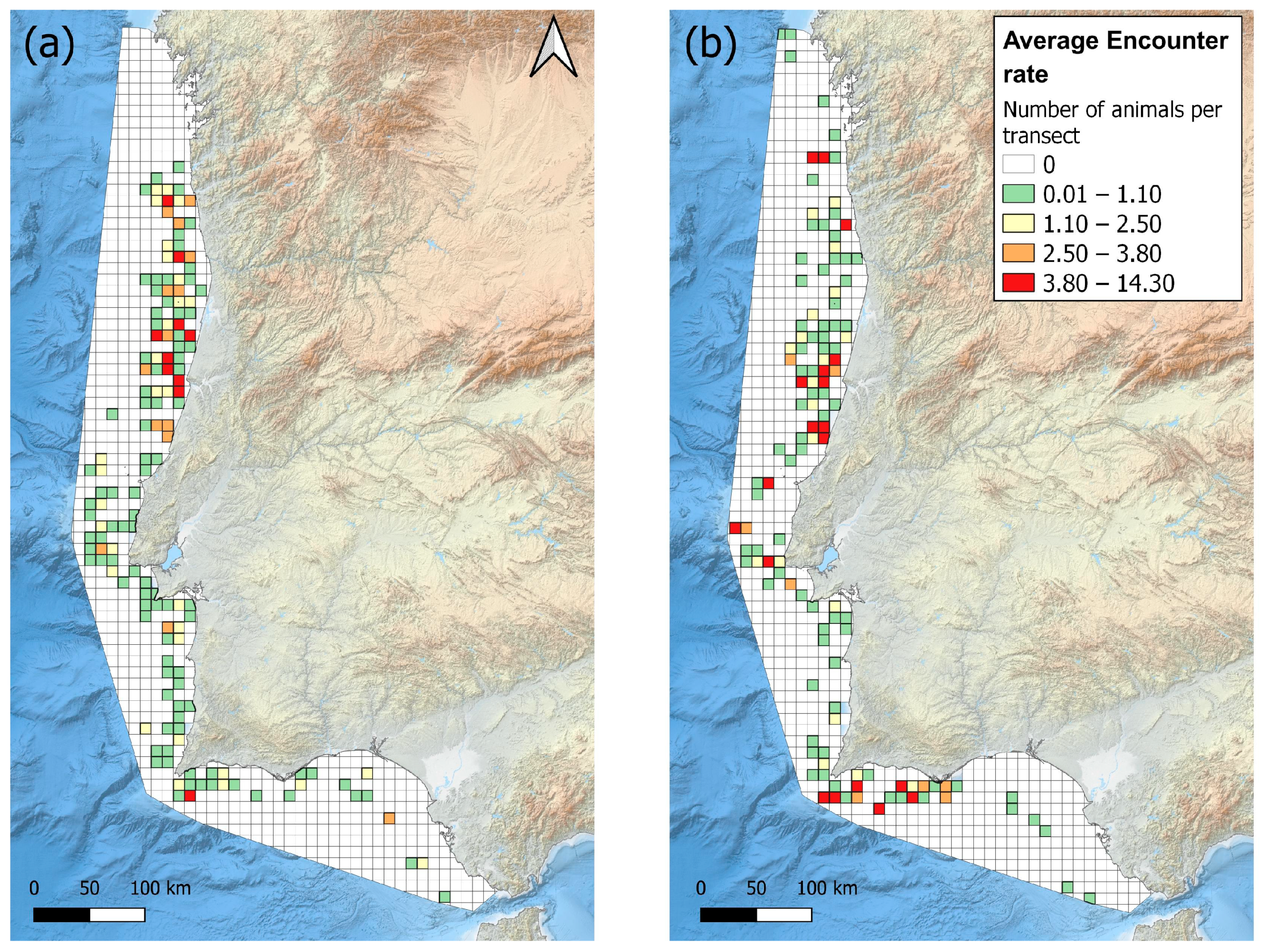
3.1. Sighting and Grid Maps
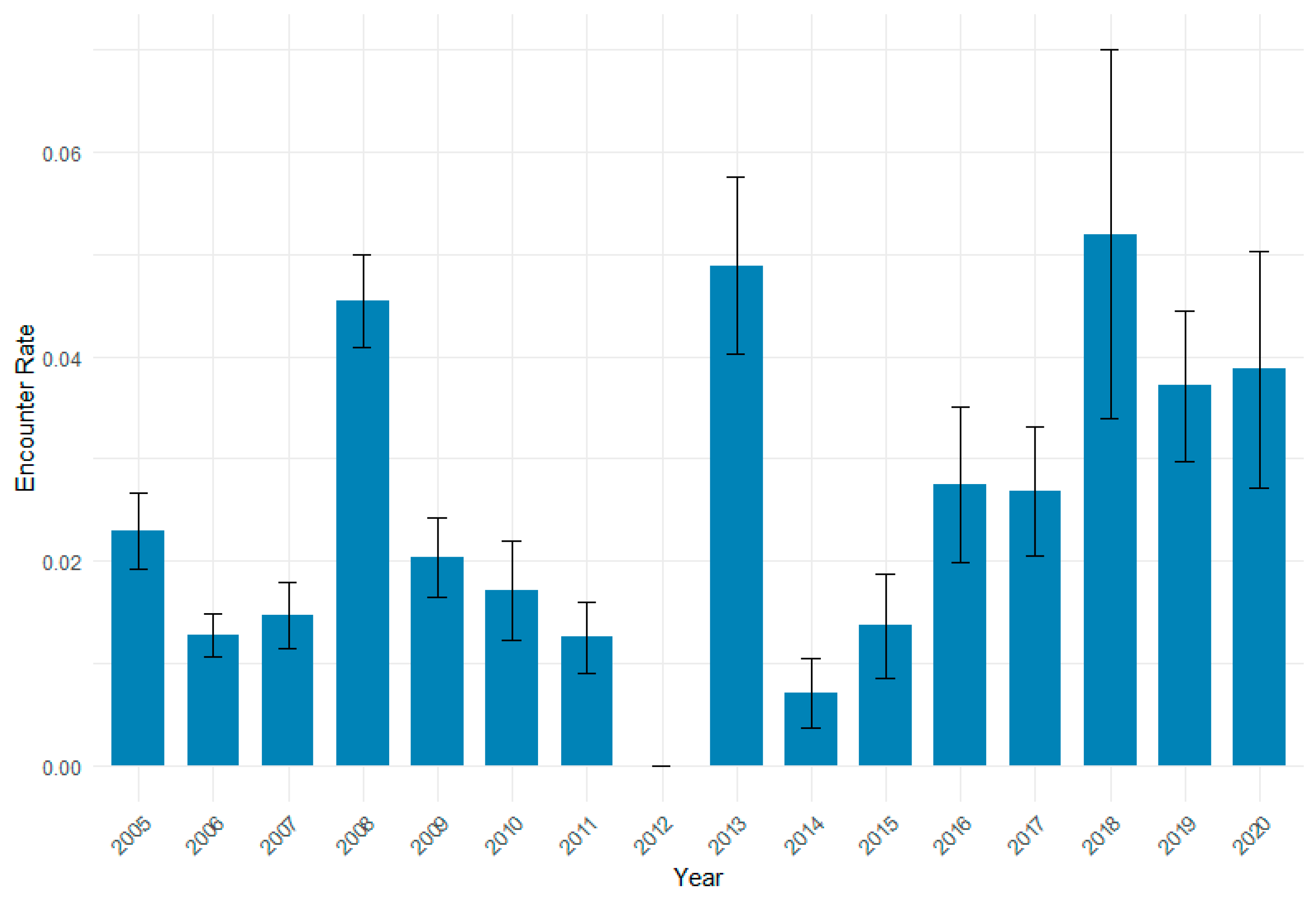
3.2. Encounter Rate
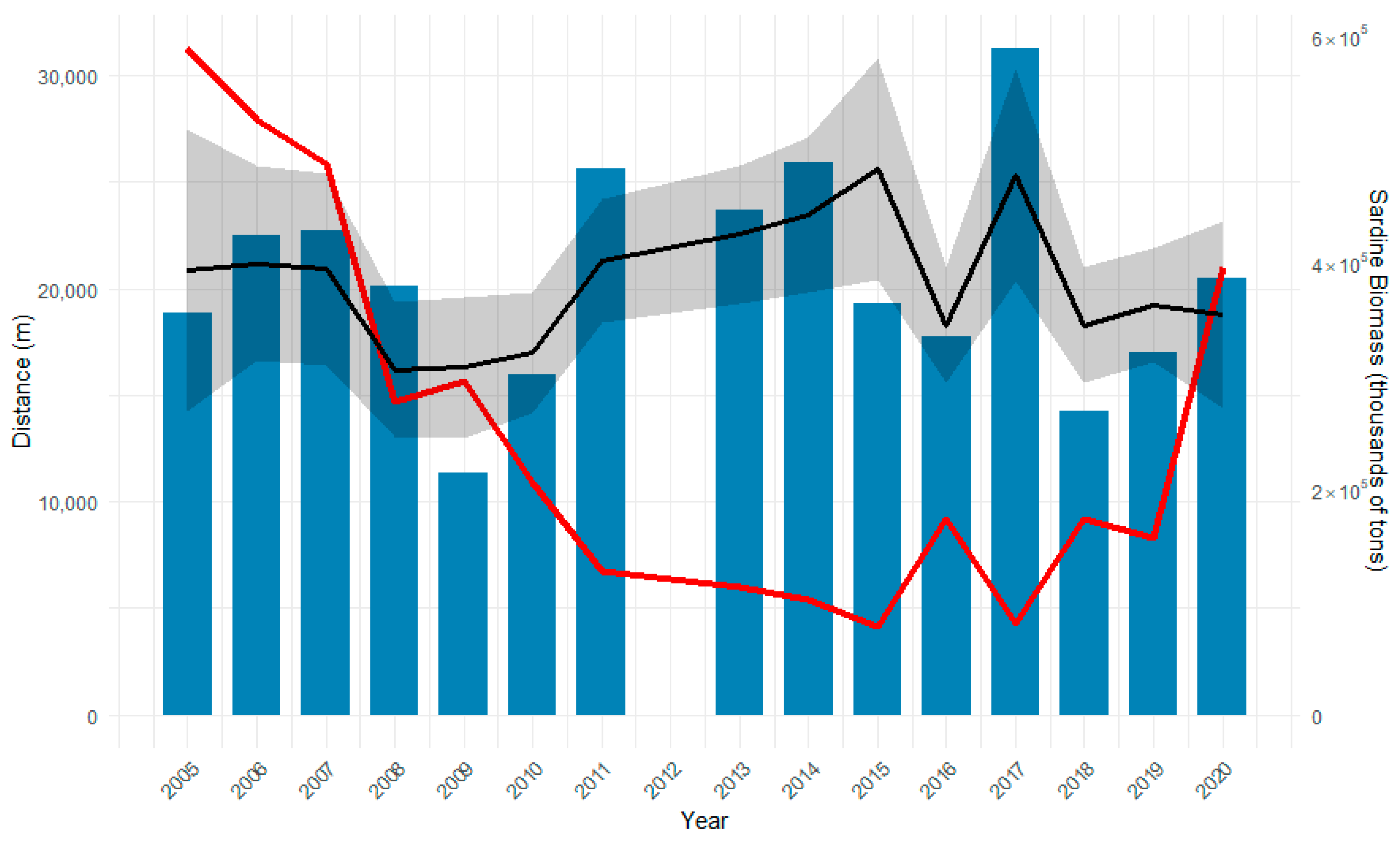
3.3. Distance from the Coast
- 2005–2008 (μ = 20.67 ± 10.86 km) and 2013–2016 (μ = 22.16 ± 12.30 km);
- 2009–2012 (μ = 16.69 ± 10.86 km) and 2013–2016;
- 2013–2016 and 2017–2020 (μ = 19.54 ± 12.30 km).
4. Discussion
4.1. Overview of the Study
4.2. Common Dolphin Encounter Rate and Distribution Associated with the Sardine Ban
4.3. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BLim | Biological Limits |
| CV | Coefficient of Variation |
| DGRM | Direção-Geral de Recursos Naturais, Segurança e Serviços Marítimos |
| EU | European Union |
| ESAS | European Seabird at Sea Methodology |
| GAM | Generalised Additive Model |
| ICES | International Council for the Exploration of the Sea |
| IPMA | Portuguese Institute for the Sea and the Atmosphere |
| MPA | Marine Protected Area |
| MSFD | Marine Strategy Framework Directive |
| MSP | Marine Spatial Planning |
| PPSF | Portuguese Purse Seine Fishery |
| SDM | Species Distribution Model |
| SPEA | Portuguese Society for the Study of Birds |
| WGHANSA | Working Group of Southern Horse Mackerel, Anchovy, and Sardine |
References
- Agardy, T. Information needs for Marine Protected Areas: Scientific and Societal. Bull. Mar. Sci. 2000, 66, 875–888. Available online: https://www.ingentaconnect.com/content/umrsmas/bullmar/2000/00000066/00000003/art00025 (accessed on 26 April 2025).
- Hunt, T.N.; Allen, S.J.; Bejdar, L.; Parra, G.J. Identifying priority habitat conservation and management of Australian humpback dolphins within a marine protected area. Sci. Rep. 2020, 10, 14366. [Google Scholar] [CrossRef] [PubMed]
- Passadore, C.; Moller, L.M.; Diaz-Aguirre, F.; Parra, G.J. Modelling Dolphin Distribution to Inform Future Spatial Conservation Decisions in a Marine Protected Area. Sci. Rep. 2018, 8, 15659. [Google Scholar] [CrossRef] [PubMed]
- Parson, E.C.M.; Baulch, S.; Bechshoft, T.; Bellazzi, G.; Bouchet, P.; Cosentino, A.M.; Godard-Codding, C.A.J.; Gulland, F.; Hoffmann-Kuhnt, M.; Hoyt, E.; et al. Key research questions of global importance for cetacean conservation. Endanger. Species Res. 2015, 27, 113–118. [Google Scholar] [CrossRef]
- Pompa, S.; Ehrich, P.R.; Ceballos, G. Global distribution and conservation of marine mammals. Biol. Sci. 2011, 108, 13600–13605. [Google Scholar] [CrossRef]
- Marshal, C.E.; Glegg, G.A.; Howell, K.L. Species Distribution modelling to support marine conservation spatial planning: The next steps. Mar. Policy 2014, 45, 330–332. [Google Scholar] [CrossRef]
- Bosso, L.; Panzuto, R.; Balestrieri, R.; Smeraldo, S.; Chiusano, M.L.; Raffini, F.; Gili, C. Integrating citizen science and spatial ecology to inform management and conservation of the Italian seahorses. Ecol. Inform. 2024, 79, 102402. [Google Scholar] [CrossRef]
- Karp, M.A.; Cimino, M.; Craig, J.K.; Crear, D.P.; Haak, C.; Hazen, E.L.; Woodworth-Jefcoats, P.A. Applications of species distribution modeling and future needs to support marine resource management. ICES J. Mar. Sci. 2025, 82, fsaf024. [Google Scholar] [CrossRef]
- Bosso, L.; Raffini, F.; Ambrosino, L.; Panzuto, R.; Gili, C.; Chiusano, M.L.; Miralto, M. Geoportals in marine spatial planning: State of the art and future perspectives. Ocean Coast. Manag. 2025, 266, 107688. [Google Scholar] [CrossRef]
- Galparsoro, I.; Montero, N.; Mandiola, G.; Menchaca, I.; Borja, Á.; Flannery, W.; Stelzenmüller, V. Assessment tool addresses implementation challenges of ecosystem-based management principles in marine spatial planning processes. Commun. Earth Environ. 2025, 6, 55. [Google Scholar] [CrossRef]
- Jog, K.; Sutaria, D.; Diedrich, A.; Grech, A.; Marsh, H. Marine mammal interactions with fisheries: Review of research and management trends across commercial and small-scale fisheries. Front. Mar. Sci. 2022, 9, 16. [Google Scholar] [CrossRef]
- Pennino, M.G.; Arcangeli, A.; Fonseca, V.P.; Campana, I.; Pierce, G.J.; Rotta, A.; Bellido, J.M. A spatially explicit risk assessment approach: Cetaceans and marine traffic in the Pelagos Sanctuary (Mediterranean Sea). PLoS ONE 2017, 12, e0179686. [Google Scholar] [CrossRef] [PubMed]
- Erbe, C.; Marley, S.A.; Schoeman, R.P.; Smith, J.N.; Triggs, L.E.; Embling, C.B. The Effects of Ship Noise on Marine Mammals- A Review. Impacts Shipp. Mar. Fauna 2019, 6, 606. [Google Scholar] [CrossRef]
- Desjonqueres, C.; Villen-Perez, S.; deMarco, P.; Maruez, R.; Beltran, J.F.; Llusia, D. Acoustic species distribution models (aSDMs): A framework to forecast shifts in calling behaviour under climate change. Methods Ecol. Evol. 2022, 13, 2275–2288. [Google Scholar] [CrossRef]
- Kavanagh, A.S.; Nykänen, M.; Hunt, W.; Richardson, N.; Jesson, M.J. Seismic surveys reduce cetacean sightings across a large marine ecosystem. Sci. Rep. 2019, 9, 10. [Google Scholar] [CrossRef]
- Parsons, E.C.M. The negative Impacts of Whale-watching. J. Mar. Biol. 2012, 1, 807294. [Google Scholar] [CrossRef]
- Bailey, H.; Senior, B.; Simmons, D.; Rusin, J.; Picken, G.; Thompson, P.M. Assessing underwater noise levels during pile-driving at an offshore windfarm and its potential effects on marine mammals. Mar. Pollut. Bull. 2010, 60, 888–897. [Google Scholar] [CrossRef]
- Nichols, C.; Bejder, L.; Green, L.; Johnson, C.; Keeling, L.; Noren, D.; Van der Hoop, J.; Simmonds, M. Anthropogenic threats to wild Cetacean Welfare and a Tool to Inform Policy in This Area. Front. Vet. Sci. 2020, 7, 57. [Google Scholar] [CrossRef]
- Sahri, A.; Herwata Putra, M.I.; Kusuma Mustika, P.L.; Kreb, D.; Murk, A.J. Cetacean habitat modelling to inform conservation management, marine spatial planning, and as a basis for anthropogenic threat mitigation in Indonesia. Ocean Coast. Manag. 2021, 205, 105555. [Google Scholar] [CrossRef]
- Wade, P.R.; Long, K.J.; Francis, T.B.; Punt, A.E.; Hammond, P.S.; Heinemann, D.; Moore, J.E.; Reeves, R.R.; Sepúlveda, M.; Sullaway, G.; et al. Best practices for assessing and managing bycatch of marine mammals. Front. Mar. Sci. 2021, 8, 757330. [Google Scholar] [CrossRef]
- Russo, D.; Sgammato, R.; Bosso, L. First sighting of the humpback whale Megaptera novaeangliae in the Tyrrhenian Sea and a mini-review of Mediterranean records. Hystrix 2016, 27, 219. [Google Scholar] [CrossRef]
- Di Sciara, G.N.; Hoyt, E.; Reeves, R.; Ardron, J.; Marsh, H.; Vongraven, D.; Barr, B. Place-based approaches to marine mammal conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 85–100. [Google Scholar] [CrossRef]
- Kiszka, J.J.; Woodstock, M.W.; Heitaus, M.R. Functional Roles and Ecological Importance of Small Cetaceans in Aquatic Ecosystems. Front. Mar. Sci. 2022, 9, 7. [Google Scholar] [CrossRef]
- Lettrich, M.D.; Asaro, M.J.; Borggaard, D.L.; Dick, D.M.; Griffis, R.B.; Litz, J.A.; Orphanides, C.D.; Palka, D.L.; Pendleton, D.E.; Soldevilla, M.S. A Method for Assessing the Vulnerability of Marine Mammals to a Changing Climate; NOAA Technical Memo. NMFS-F/SPO-196; NOAA: Washington, DC, USA, 2019; Volume 73. [Google Scholar] [CrossRef]
- Zacharias, M.; Roff, J. Use of focal species in marine conservation and management: A review and critique. Aquat. Conserv. Mar. Freshw. Ecosyst. 2001, 11, 59–76. [Google Scholar] [CrossRef]
- Pace, D.S.; Tizzi, R.; Mussi, B. Cetaceans Value and Conservation in the Mediterranean Sea. J. Biodivers. Endanger. Species 2015, S1, 4. [Google Scholar] [CrossRef]
- Hammond, P.S.; Lacey, C.; Gilles, A.; Viquerat, S.; Börjesson, P.; Herr, H.; Macleod, K.; Ridoux, V.; Santos, M.B.; Scheidat, M.; et al. Estimates of Cetacean Abundance in European Atlantic Waters in Summer 2016 from the SCANS-III Aerial and Shipboard Surveys; Sea Mammal Research Unit: St Andrews, UK, 2017. [Google Scholar]
- Evans, W.E. Common dolphin, Delphinus delphis, Linnaeus, 1758. In Handbook of Marine Mammals; Ridgway, S.H., Harrison, R., Eds.; University Press: London, UK, 1994; pp. 191–224. [Google Scholar]
- Murphy, S.; Pinn, E.; Jepson, P. The short-beaked common dolphin (Delphinus delphis) in the Northeastern Atlantic: Distribution, ecology, management and conservation status. In Oceanography and Marine Biology; Hughes, R.N., Hughes, D.J., Smith, I.P., Eds.; CRC Press: Boca Raton, FL, USA, 2013; Volume 51, pp. 193–280. [Google Scholar] [CrossRef]
- Gilles, A.; Authier, M.; Ramirez-Martinez, N.C.; Araújo, H.; Blanchard, A.; Carlström, J.; Eira, C.; Dorémus, G.; Fernández, M.C.; Geelhoed, S.C.V.; et al. Estimates of Cetacean Abundance in European Atlantic Waters in Summer 2023 from the SCAN-IV Aerial and Shipboard Survey. 2023, p. 64. Available online: http://doi.org/10.13140/RG.2.2.34873.95845 (accessed on 26 April 2025).
- Silva, M.A.; Sequeira, M. Patterns in the mortality of common dolphins (Delphinus delphis) on the Portuguese coast, using stranding records, 1975–1998. Aquat. Mamm. 2003, 29, 88–98. [Google Scholar] [CrossRef]
- Marçalo, A.; Katara, I.; Feijo, D.; Araújo, H.; Oliveira, I.; Santos, J.; Ferreira, M.; Monteiro, S.; Pierce, G.J.; Silva, A.; et al. Quantification of interactions between the Portuguese sardine purse-seine fishery and cetaceans. ICES J. Mar. Sci. 2015, 72, 2438–2449. [Google Scholar] [CrossRef]
- Marçalo, A.; Nicolau, L.; Giménez, J.; Ferreira, M.; Santos, J.; Araújo, H.; Silva, A.; Vingada, J.; Pierce, G.J. Feeding ecology of the common dolphin (Delphinus delphis) in Western Iberian waters: Has the decline in sardine (Sardina pilchardus) affected dolphin diet. Mar. Biol. 2018, 165, 44. [Google Scholar] [CrossRef]
- Vingada, J.; Eira, C. Conservação de Cetáceos e Aves Marinhas em Portugal Continental. Aveiro: Life+ MarPro. 2018. Available online: www.marprolife.org (accessed on 26 April 2025).
- Dias, I.C.; Marçalo, A.; Feijó, D.; Domingos, I.; Silva, A.A. Interactions between the common dolphin, Delphinus delphis, and the Portuguese purse seine fishery over a period of 15 years (2003–2018). Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 1351–1364. [Google Scholar] [CrossRef]
- ICES. Working Group on Bycatch of Protected Species (WGBYC). ICES Sci. Rep. 2024, 5, 334. [Google Scholar] [CrossRef]
- Silva, M.A. Diet of common dolphins, Delphinus delphis, off the Portuguese continental coast. J. Mar. Biol. 1999, 79, 531–540. [Google Scholar] [CrossRef]
- Spitz, J.; Mourocq, E.; Ridoux, V. Prey selection by the common dolphin: Fulfilling high energy requirements with high quality food. J. Exp. Mar. Biol. 2010, 390, 73–77. [Google Scholar] [CrossRef]
- Young, D.D.; Cockcroft, V.G. Diet of common dolphins (Delphinus delphis) of the south-east coast of southern Africa: Opportunism or specialization? J. Zool. 1994, 234, 41–53. [Google Scholar] [CrossRef]
- Santos, M.B.; Pierce, G.J.; Reid, R.J.; Ross, H.M.; Patterson, I.A.P.; Reid, D.G.; Peach, K. Variability in the diet of harbour porpoises (Phocoena phocoena) in Scottish waters 1992–2003. Mar. Mammal Sci. 2004, 20, 27. [Google Scholar] [CrossRef]
- Santos, M.B.; German, I.; Correia, D.; Read, F.L.; Cedeira, J.M.; Caldas, M.; Lopez, A.; Velasco, F.; Pierce, G.J. Long-term variation in common dolphin diet in relation to prey abundance. Mar. Ecol. Prog. Ser. 2013, 481, 24–268. [Google Scholar] [CrossRef]
- Santos, M.B.; Saavedra, C.; Pierce, G.J. Quantifying the predation on sardine and hake by cetaceans in the Atlantic waters of the Iberian Peninsula. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 106, 232–244. [Google Scholar] [CrossRef]
- Charnov, E.L. Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 1976, 9, 129–136. [Google Scholar] [CrossRef]
- Fiuza, A.F.G. Upwelling patterns of Portugal. In Coastal Upwelling. Its Sediment Records (Part A); Suess, E., Thiede, J., Eds.; Plenum: New York, NY, USA, 1983; pp. 85–98. [Google Scholar] [CrossRef]
- Peliz, A.; Rosa, T.L.; Santos, M.P.; Pissarra, J.L. Fronts, jets and counter flows in the Western Iberian upwelling system. J. Mar. Syst. 2002, 35, 61–77. [Google Scholar] [CrossRef]
- Borges, M.F.; Santos, A.M.; Crato, N.; Mendes, H.; Mota, B. Sardine regime shifts off Portugal: A time series analysis of catches and wind conditions. Sci. Mar. 2003, 67, 235–244. [Google Scholar] [CrossRef]
- Santos, M.B.; González-Quirós, R.; Riveiro, I.; Cabanas, J.M.; Porteiro, C.; Pierce, G.J. Cycles, trends, and residual variation in the Iberian sardine (Sardina pilchardus) recruitment series and their relationship with the environment. ICES J. Mar. Sci. 2012, 69, 739–750. [Google Scholar] [CrossRef]
- Lemos, R.T. The upwelling regime of the Portuguese coast, 1941–2000. Clim. Res. 2004, 24, 511–524. [Google Scholar] [CrossRef]
- Leitão, F.; Maharaj, R.R.; Vieira, V.M.N.C.S.; Teodósio, A.; Cheung, W.W.L. The effect of regional sea surface temperature rise on fisheries along the Portuguese Iberian Atlantic coast. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 1351–1359. [Google Scholar] [CrossRef]
- Silva, A.; Moreno, A.; Riveiro, I.; Santos, B.; Pita, C.; Garcia Rodrigues, J.; Villasante, S.; Pawlowski, L.; Duhamel, E. Research for Pech Committee—Sardine Fisheries: Resource Assessment and Social and Economic Situation; Directorate-General for Internal Policies IP/B/PECH/IC/2015_133; European Parliament’s PECH Committee: Brussel, Belgium, 2015. [Google Scholar]
- Teixeira, C.M.; Gamito, R.; Leitão, F.; Murta, A.G.; Cabral, H.N.; Erzini, K.; Costa, M.J. Environmental influence on commercial fishery landings of small pelagic fish in Portugal. Reg. Environ. Change 2016, 16, 709–716. [Google Scholar] [CrossRef]
- Silva, A.; Garrido, S.; Ibaibarriaga, L.; Pawlowski, L.; Riveiro, I.; Marques, V.; Ramos, F.; Duhamel, E.; Iglesias, M.; Bryère, P.; et al. Adult-mediated connectivity and spatial population structure of sardine in the Bay of Biscay and Iberian coast. Deep Sea Res. Part II 2018, 159, 62–74. [Google Scholar] [CrossRef]
- Monteiro, P.V. The purse seine fishing of sardine in Portuguese waters: A difficult compromise between fish stock sustainability and fishing effort. Rev. Fish. Sci. Aquac. 2017, 25, 218–229. [Google Scholar] [CrossRef]
- ICES, Working Group on Southern Horse Mackeral, Anchovy and Sardine (WGHANSA). ICES Sci. Rep. 2020, 2, 655. [CrossRef]
- Wise, L.; Silva, A.; Ferreira, M.; Silva, M.; Sequeira, W. Interactions between small cetaceans and the purse seine fishery in western Portuguese waters. Sci. Mar. 2007, 71, 405–412. [Google Scholar] [CrossRef][Green Version]
- Wise, L.; Galego, C.; Katara, I.; Marçalo, A.; Meirinho, A.; Monteiro, S.S.; Oliveira, N.; Santos, J.; Rodrigues, P.; Araújo, H.; et al. Portuguese purse seine fishery spatial and resource overlap with top predators. Mar. Ecol. Prog. Ser. 2018, 617–618, 183–198. [Google Scholar] [CrossRef]
- Marçalo, A.; Carvalho, F.; Frade, M.; Bentes, L.; Monteiro, P.; Pontes, J.; Alexandre, S.; Oliveira, F.; Kingston, A.; Erzini, K.; et al. Reducing cetaceans interactions with bottom set-nets and purse seining using acoustic deterrent devices in southern Iberia. Auatic Conserv. Mar. Freshw. Ecosyst. 2025, 35, 15. [Google Scholar] [CrossRef]
- ICES. The Workshop for the evaluation of the Iberian sardine HCR (WKSARHCR). ICES Sci. Rep. 2021, 3, 115. [Google Scholar] [CrossRef]
- Serra, N.; Ambar, I. Eddy Generation in the Mediterranean Undercurrent. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 4225–4243. [Google Scholar] [CrossRef]
- Álvarez-Salgado, X.A.; Figueiras, F.G.; Pérez, F.F.; Groom, S.; Nogueira, E.; Borges, A.V.; Chou, L.; Castro, C.G.; Moncoiffé, G.; Ríos, A.F.; et al. The Portugal coastal counter current off NW Spain: New insight on its biogeochemical variability. Prog. Oceanogr. 2003, 56, 281–321. [Google Scholar] [CrossRef]
- ICES. Report of the Working Group on Southern Horse Mackerel, Anchovy and Sardine (WGHANSA), Azores (Horta), Portugal. ICES CM. 2012, ICES CM 2012/ACOM:16, 544. Available online: https://ices-library.figshare.com/articles/report/Report_of_the_Working_Group_on_Southern_Horse_Mackerel_Anchovy_and_Sardine_WGHANSA_/19281419?file=34239803 (accessed on 26 April 2025).
- Conde, A.; Angélico, M.M.; Carrera, P.; Feijó, D.; Henriques, E.; Mendes, H.; Oliveira, P.B.; Oliveira, N.; Pastor, J.; Rodriguez, S.; et al. Relatório da Campanha “PELAGO23” Série PNAB/DCF—PELAGO (Primavera). Relatórios Camp. 2023, 63p. Available online: http://ipma.pt (accessed on 26 April 2025).
- Tasker, M.L.; Jones, P.H.; Dixon, T.; Blake, B.F. Counting seabirds at sea from ships: A review of methods employed and a suggestion for a standardized approach. Auk 1984, 101, 567–577. [Google Scholar] [CrossRef]
- Camphuysen, K.C.J.; Fox, T.A.D.; Leopold, M.M.F.; Peterson, I.K. Towards Standardised Seabirds at Sea Census Techniques in Connection with Environmental Impact Assessments for Offshore Wind Farms in the U.K.; COWRIE, Crown Estate: London, UK, 2004. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 26 April 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 26 April 2025).
- QGIS. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2019. Available online: http://qgis.org (accessed on 26 April 2025).
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. (B) 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Hammond, P.S.; Macleod, K.; Gillespie, D.; Swift, R.; Winship, A.; Canadas, A. Cetacean Offshore Distribution and Abundance in the European Atlantic (CODA); Microsoft Word—CODA Final Report 11-2-09.doc; University of St. Andrews: St Andrews, UK, 2009; p. 43. [Google Scholar]
- Silva, A.A.; Castro, J.; Cid, A.; Jesus, S.M.; Matos, F.L. Influence of Dolphin-Watching Tourism Vessels on the Whistle Emission Pattern of Common Dolphins and Bottlenose Dolphins. Oceans 2024, 5, 770–784. [Google Scholar] [CrossRef]
- Rodríguez-Climent, S.; Angélico, M.M.; Marques, V.; Oliveira, P.; Wise, L.; Silva, A. Essential habitat for sardine juveniles in Iberian water. Sci. Mar. 2017, 81, 351–360. [Google Scholar] [CrossRef]
- Ryan, J.; Chavez, F.; Bellingham, J. Physical-biological coupling in Monterey Bay, California: Topographic influences on phytoplankton ecology. Mar. Ecol. Prog. Ser. 2005, 287, 23–32. [Google Scholar] [CrossRef][Green Version]
- Salgado Kent, C.; Bouchet, P.; Wellard, R.; Parnum, I.; Fouda, L.; Erbe, C. Seasonal productivity drives aggregations of killer whales and other cetaceans over submarine canyons of the Bremer Sub-Basin, southwestern Australia. Aust. Mammal. 2021, 43, 168. [Google Scholar] [CrossRef]
- Vieira, N.; Carvalho, I.; Brito, C. Occurrence and Relative Abundance of Common Dolphins in Three Sites of the Portuguese Shore; IWC-SC/61/SM16; International Whaling Commission IWC: Cambridge, UK, 2009; p. 7. [Google Scholar]
- Certain, G.; Ridoux, V.; van Canneyt, O.; Bretagnolle, V. Delphinid spatial distribution and abundance estimates over the shelf of the Bay of Biscay. ICES J. Mar. Sci. 2008, 65, 656–666. [Google Scholar] [CrossRef]
- De la Cruz, A.; Ramos, F.; Navarro, G.; Cózar, A.; Bécares, J.; Arroyo, G.M. Drivers for spatial modelling of a critically endangered seabird on a dynamic ocean area: Balearic shearwaters are non-vegetarian. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 31, 1700–1714. [Google Scholar] [CrossRef]
- Lin, C.; Lin, H.; Suen, J.; Chou, L. Association between estuary characteristics and activities of the critically endangered Indo-Pacific humpback dolphin (Sousa chinensis). Front. Mar. Sci. 2021, 8, 577976. [Google Scholar] [CrossRef]
- García-García, L.M.; Ruiz-Villarreal, M.; Bernal, M. A biophysical model for simulating early life stages of sardine in the Iberian Atlantic stock. Fish. Res. 2016, 173, 250–272. [Google Scholar] [CrossRef]
- Bearzi, G.; Azzellino, A.; Politi, E.; Costa, M.; Bastianini, M. Influence of seasonal forcing on habitat use by bottlenose dolphins Tursiops truncatus in the northern Adriatic Sea. Ocean Sci. J. 2008, 43, 175–182. [Google Scholar] [CrossRef]
- Maze, K.S. Wursig Bottlenose dolphins of San Luis Pass, texas: Occurrence patterns, site-fidelity, and habitat use. Aquat. Mamm. 1999, 25, 91–103. [Google Scholar] [CrossRef]
- Directorate-General for Natural Resources (DGRM). Sardine Fishery Management Plan—(2012–2015); Fishery Management Group: Portaria, Greece, 2012; Available online: https://www.dgrm.pt/documents/20143/48027/Sardine+fishery+management+Plan.pdf/12f5d5e1-8982-8103-9b2d-3a85de07b15b (accessed on 26 April 2025).
- Szalaj, D.; Torres, M.A.; Veiga-Malta, T.; Angélico, M.M.; Sobrinho-Gonçalves, L.; Chaves, C.; Alcoforado, B.; Garrido, S.; Ré, P.; Cabral, H.; et al. Food-web dynamics in the Portuguese continental shelf ecosystem between 1986 and 2017: Unravelling drivers of sardine decline. Estuar. Coast. Shelf Sci. 2021, 251, 107259. [Google Scholar] [CrossRef]
- Bearzi, G.; Politi, E.; Agazzi, S.; Azzellino, A. Prey depletion caused by overfishing and the decline of marine megafauna in eastern Ionian Sea coastal waters (central Mediterranean). Biol. Conserv. 2006, 127, 373–382. [Google Scholar] [CrossRef]
- DRGM. Multiannual Management and Recovery Plan for the Iberian Sardine (2018–2023); República Portuguesa, Gobierno de España, 2018. Available online: https://www.dgrm.pt/documents/20143/46478/PGSARDINHA2021-2026.pdf/d36a1df9-7353-32a4-a06e-c72852e73cb0 (accessed on 26 April 2025).
- ICES. Report of the Working Group on Southern Horse Mackerel, Anchovy and Sardine (WGHANSA), Bilbao, Spain, 17. ICES CM: 2017/ACOM, 47, 2017. Available online: https://core.ac.uk/outputs/95151175/ (accessed on 26 April 2025).
- Feijó, D.; Marçalo, A.; Wise, L.; Bentos, T.; Barra, J.; Correia, M.; Pinto, D.; Lechuga, R.; Felíco, M.; Dinis, D.; et al. The Portuguese purse seine fishery (2006–2016): What has changed in 10 years? Fish. Mediterr. Environ. 2018, 11, 4. [Google Scholar]
- Nottestad, L.; Kraft, B.; Anthonypillai, V.; Bernasconi, M.; Langard, L.; Mork, H.L.; Fernao, A. Recent changes in distribution and relative abundance of cetaceans in the Norwegian Sea and their relationship with potential prey. Front. Ecol. Evol. 2015, 2, 83. [Google Scholar] [CrossRef]
- Grémillet, D.; Boulinier, T. Spatial ecology and conservation of seabirds facing global climate change: A review. Mar. Ecol. Prog. Ser. 2009, 391, 121–137. [Google Scholar] [CrossRef]
- Gonzalvo, J.; Valls, M.; Cardona, L.; Aguilar, A. Factors determining the interaction between common bottlenose dolphins and bottom trawlers off the Balearic Archipelago (western Mediterranean Sea). J. Exp. Mar. Biol. Ecol. 2008, 367, 47–52. [Google Scholar] [CrossRef]
- Ward, T.M.; Ivey, A.; Carroll, J. Code of practice for reducing accidental mortality of dolphins in purse-seine fisheries. Mar. Policy 2018, 87, 203–211. [Google Scholar] [CrossRef]
- Hammond, P.; Macleod, K.; Berggren, P.; Borchers, D.; Burt, L.; Cañadas, A.; Desportes, G.; Donovan, G.; Gilles, A.; Gillespie, D.; et al. Cetacean abundance and distribution in European Atlantic shelf waters to inform conservation and management. Biol. Conserv. 2013, 164, 107–122. [Google Scholar] [CrossRef]
- Van Weelden, C.; Towers, J.R.; Bosker, T. Impacts of climate change on cetacean distribution, habitat and migration. Clim. Change 2021, 1, 100009. [Google Scholar] [CrossRef]
- Hammond, P.S.; Francis, T.B.; Heinemann, D.; Long, K.J.; Moore, J.E.; Punt, A.E.; Reeves, R.R.; Sepúlveda, M.; Sigurðsson, G.M.; Siple, M.C.; et al. Estimating the abundance of marine mammal populations. Front. Mar. Sci. 2021, 8, 735770. [Google Scholar] [CrossRef]
- Ross, G.J.B.; Cockcroft, V.G.; Butterworth, D.S. Offshore distribution of bottlenose dolphins in Natal coastal waters and Algoa Bay, Eastern Cape, South African. J. Zool. 1987, 22, 50–56. [Google Scholar] [CrossRef]
- Hastie, G.D.J.; Swift, R.S.; Slesser, G.; Thompson, P.M.; Turrell, W.R. Environmental models for predicting oceanic dolphin habitat in the Northeast Atlantic. ICES J. Mar. Sci. 2005, 62, 760–770. [Google Scholar] [CrossRef]
- Davis, R.W.; Fargion, G.S.; May, N.; Leming, T.D.; Baumgartner, M.; Evans, W.E.; Mullin, K. Physical habitat of cetaceans along the continental slope in the northcentral and western Gulf of Mexico. Mar. Mammal Sci. 1998, 14, 490–507. [Google Scholar] [CrossRef]
- Becker, E.A.; Foley, D.G.; Forney, K.A.; Barlow, J.; Redfern, J.V.; Gentemann, C.L. Forecasting cetacean abundance patterns to enhance management decisions. Endanger. Species Res. 2012, 16, 97–112. [Google Scholar] [CrossRef]
- Becker, A.; Carretta, J.V.; Forney, K.A.; Barlow, J.; Brodie, S.; Hoopes, R.; Jacox, M.G.; Maxwell, S.M.; Redfern, J.V.; Sisson, N.B.; et al. Performance evaluation of cetacean species distribution models developed using Generalized Additive Models and boosted regression trees. Ecol. Evol. 2020, 10, 5759–5784. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brouder, S.; Marques, T.A.; Oliveira, N.; Monteiro, P.; Gonçalves, J.M.S.; Marçalo, A. When Sardines Disappear: Tracking Common Dolphin, Delphinus delphis, Distribution Responses Along the Western Iberian Coast. Animals 2025, 15, 1552. https://doi.org/10.3390/ani15111552
Brouder S, Marques TA, Oliveira N, Monteiro P, Gonçalves JMS, Marçalo A. When Sardines Disappear: Tracking Common Dolphin, Delphinus delphis, Distribution Responses Along the Western Iberian Coast. Animals. 2025; 15(11):1552. https://doi.org/10.3390/ani15111552
Chicago/Turabian StyleBrouder, Sarah, Tiago A. Marques, Nuno Oliveira, Pedro Monteiro, Jorge M. S. Gonçalves, and Ana Marçalo. 2025. "When Sardines Disappear: Tracking Common Dolphin, Delphinus delphis, Distribution Responses Along the Western Iberian Coast" Animals 15, no. 11: 1552. https://doi.org/10.3390/ani15111552
APA StyleBrouder, S., Marques, T. A., Oliveira, N., Monteiro, P., Gonçalves, J. M. S., & Marçalo, A. (2025). When Sardines Disappear: Tracking Common Dolphin, Delphinus delphis, Distribution Responses Along the Western Iberian Coast. Animals, 15(11), 1552. https://doi.org/10.3390/ani15111552





