Nematicidal Effects and Cytotoxicity of Levamisole on Thelazia callipaeda
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Establishment of the T. callipaeda Viability Assessment System (TVAS)
2.3. Investigation of the Nematicidal Effect of Levamisole on T. callipaeda
2.3.1. Development of Animal Models for T. callipaeda Maintenance and Infection
2.3.2. In Vivo Levamisole Anthelmintic Experiment Against T. callipaeda
2.4. Cytotoxicity Assessment of Levamisole on RCECs
2.4.1. Standard Culture of RCECs
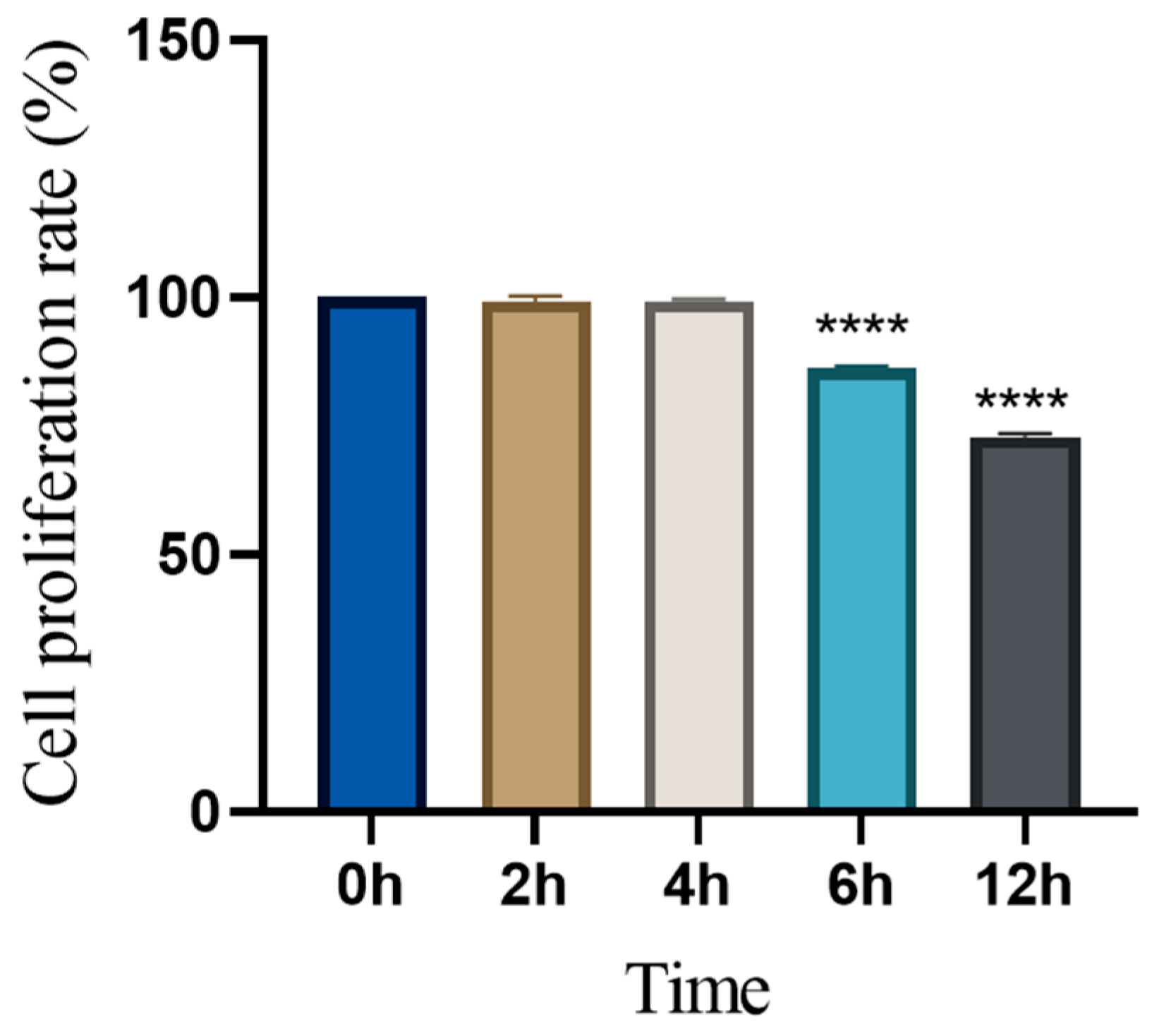
2.4.2. CCK-8 Assay
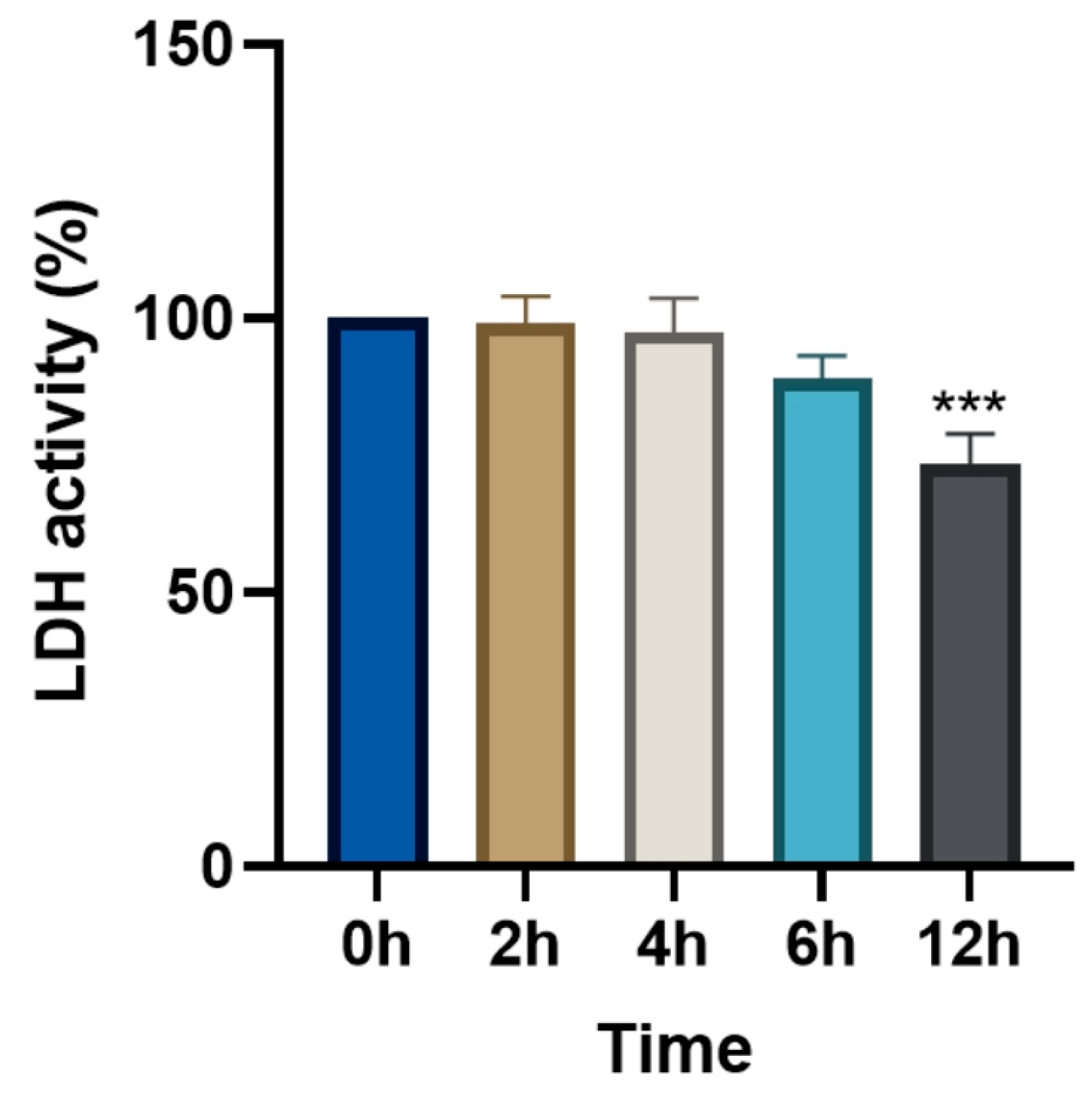
2.4.3. LDH Cytotoxicity Assay
2.5. Statistical Method
3. Results
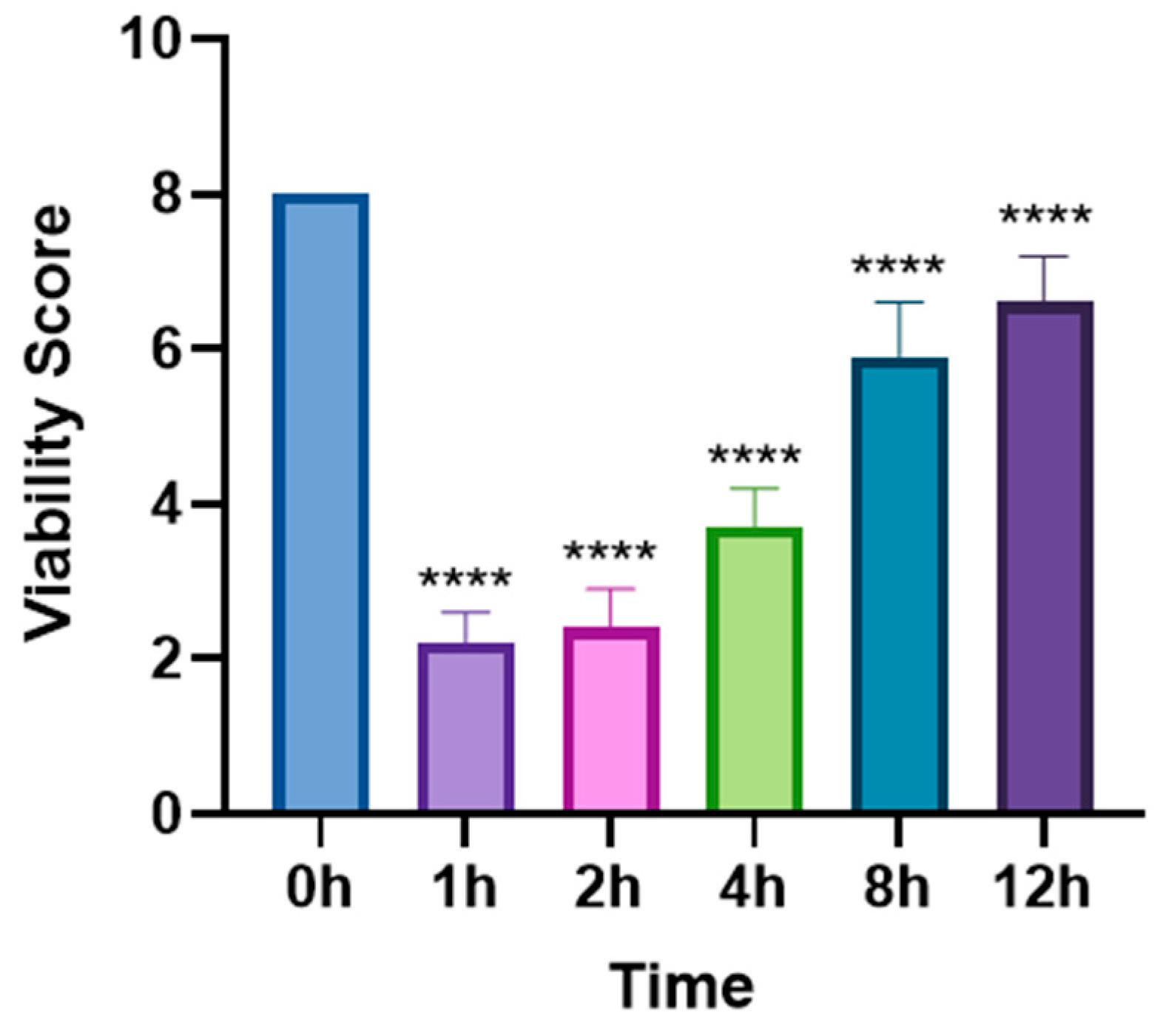
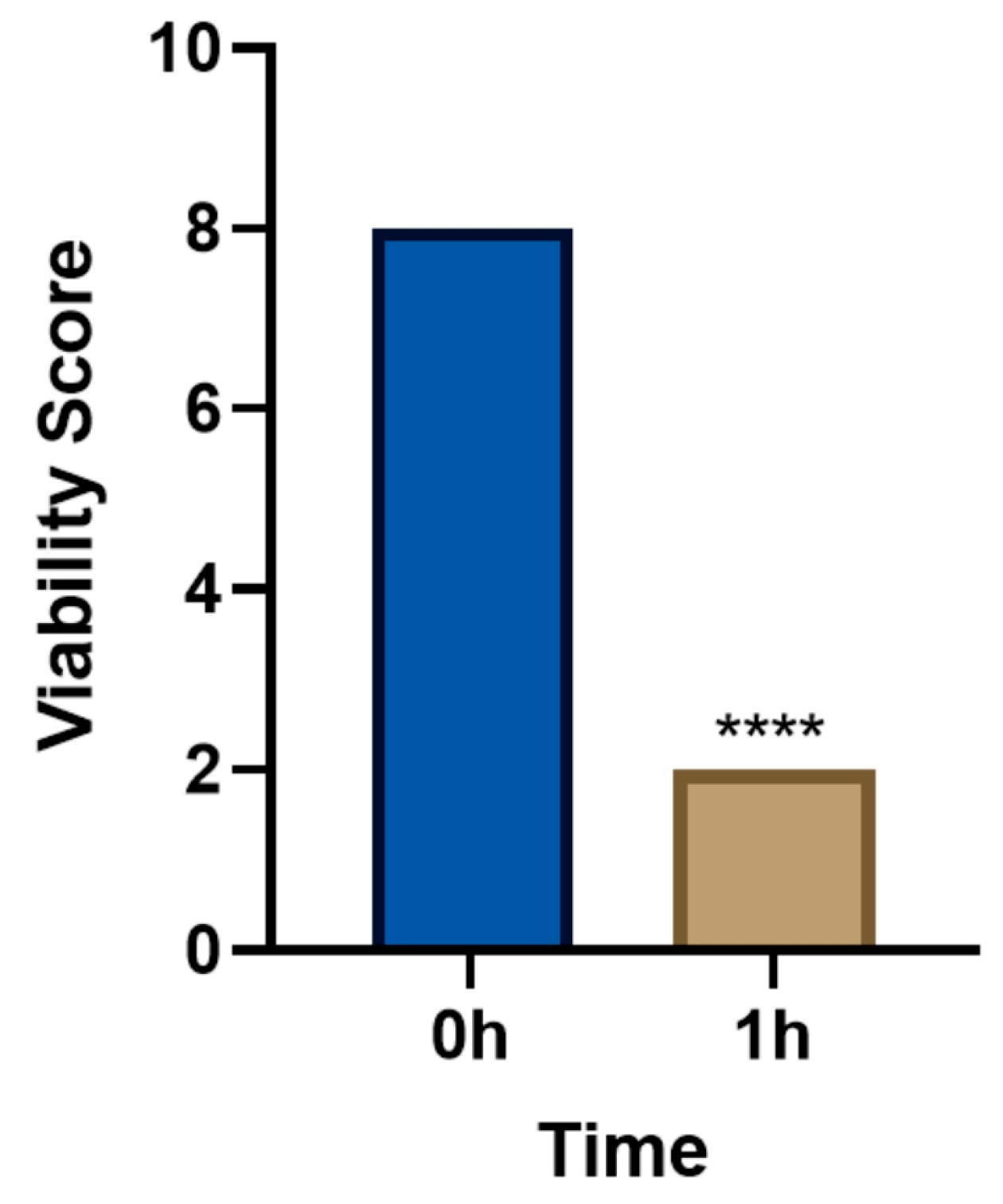
3.1. In Vivo Lethal Effect of Levamisole on T. callipaeda
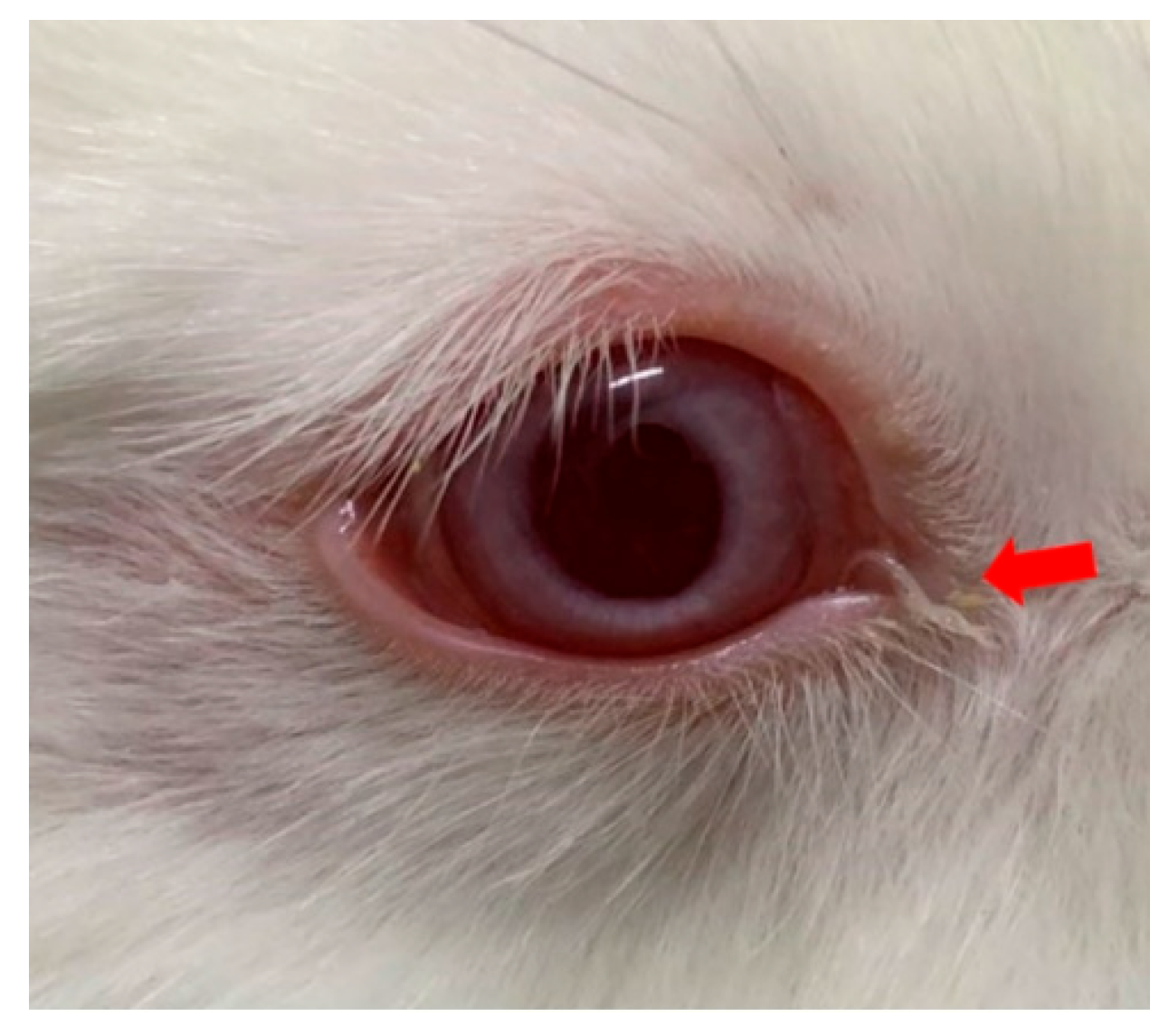
3.2. Ocular Symptoms Scoring in Experimental Animals Following Levamisole Administration
3.3. Hematological and Biochemical Assessments in Experimental Animals Following Levamisole Administration
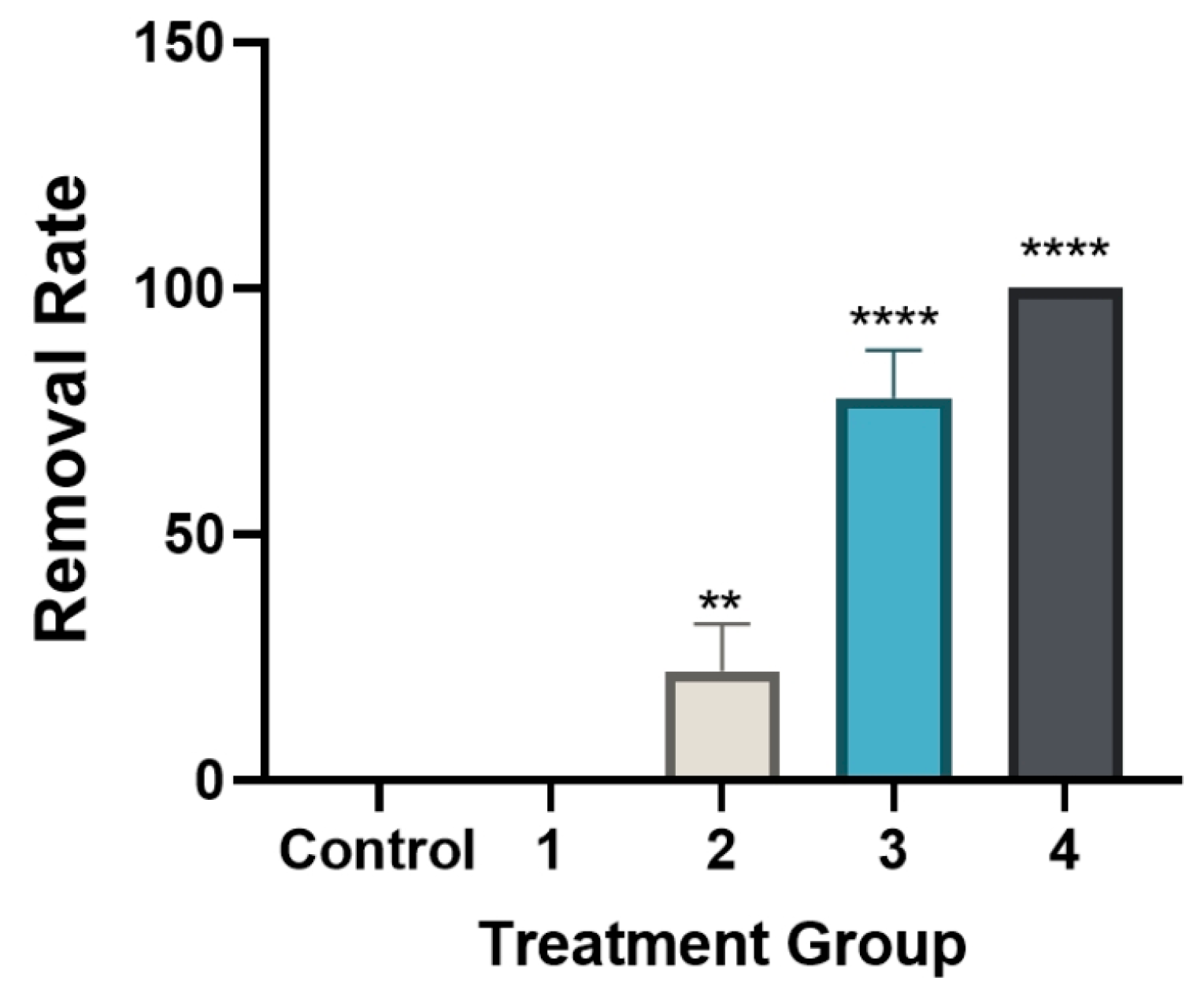
3.4. Time-Dependent Changes in Cell Proliferation Following Levamisole Treatment
3.5. Changes in Relative LDH Activity (%) Following Levamisole Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T. callipaeda | Thelazia callipaeda |
| RCEC | rabbit conjunctival epithelial cells |
| TVAS | Thelazia callipaeda viability assessment system |
| NS | normal saline |
| DW | distilled water |
| WBC | white blood cell count |
| NEU | neutrophil |
| LYM | lymphocyte |
| MONO | monocyte |
| EOS | eosinophil |
| BASO | basophil |
| RBC | red blood cell count |
| HGB | hemoglobin |
| HCT | hematocrit |
| MCV | mean corpuscular volume |
| MCH | mean corpuscular hemoglobin concentration |
| MCHC | mean corpuscular hemoglobin |
| RDW | red cell distribution width |
| PLT | platelet |
| PCT | thrombocytocrit |
| MPV | mean platelet volume |
| PDW | platelet distribution width |
| TP | total protein |
| ALB | albumin |
| GLB | globulin |
| A/G | albumin/globulin |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| ALP | alkaline phosphatase |
| CK | creatine kinase |
| GLU | glucose |
| BUN | blood urea nitrogen |
| CRE | creatinine |
| CHOL | cholesterol |
| TG | triglyceride |
| OD | optical density |
References
- Otranto, D.; Mendoza-Roldan, J.A.; Dantas-Torres, F. Thelazia callipaeda. Trends Parasitol. 2021, 37, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Bezerra-Santos, M.A.; Bernardini, I.; Lia, R.P.; Mendoza-Roldan, J.A.; Beugnet, F.; Pombi, M.; Otranto, D. Phortica Oldenbergi (Diptera: Drosophilidae): A New Potential Vector of the Zoonotic Thelazia callipaeda Eyeworm. Acta Trop. 2022, 233, 106565. [Google Scholar] [CrossRef]
- Doi, K.; Tokiwa, T.; Imoto, M.; Chou, S.; Yamasaki, F.; Kato, T.; Hayama, S. Molecular Characterization of Oriental Eyeworm (Thelazia callipaeda) Detected from Raccoon (Procyon lotor) and Japanese Raccoon Dog (Nyctereutes viverrinus) in Kanto Region, Japan. Parasit Vectors 2023, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Gasser, R.B.; Chu, D.; Wang, Z.; Yuan, X.; Cantacessi, C.; Otranto, D. Human Thelaziosis—A Neglected Parasitic Disease of the Eye. J. Parasitol. 2006, 92, 872–876. [Google Scholar] [CrossRef]
- Unterköfler, M.S.; Dengg, P.; Niederbacher, M.; Lindorfer, S.; Eberle, A.; Huck, A.; Staufer, K.; Zittra, C.; Wortha, L.N.; Hodžić, A.; et al. Occurrence of Thelazia callipaeda and Its Vector Phortica Variegata in Austria and South Tyrol, Italy, and a Global Comparison by Phylogenetic Network Analysis. Parasites Vectors 2023, 16, 294. [Google Scholar] [CrossRef]
- Do Vale, B.; Lopes, A.P.; Da Conceição Fontes, M.; Silvestre, M.; Cardoso, L.; Coelho, A.C. Systematic Review on Infection and Disease Caused by Thelazia callipaeda in Europe: 2001–2020. Parasite 2020, 27, 52. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, Z.; Wei, J.; Wen, Y.; He, N.; Tang, L.; Lin, D.; Lin, J. A First Report of Thelazia callipaeda Infection in Phortica Okadai and Wildlife in National Nature Reserves in China. Parasites Vectors 2021, 14, 13. [Google Scholar] [CrossRef]
- Choe, S.; Kim, S.; Nath, T.C.; Kim, J.-H. Eight Cases of Canine Thelaziosis Found in Two Localities in Korea. Parasites Hosts Dis. 2023, 61, 325–331. [Google Scholar] [CrossRef]
- Sangwan, A.K.; Ralte, L.; Kuotsu, N.; Epao, V. Canine Ocular Thelaziosis from North East India. Vet. Parasitol. Reg. Stud. Rep. 2021, 26, 100651. [Google Scholar] [CrossRef]
- Sobotyk, C.; Dietrich, J.; Verocai, G.G.; Maxwell, L.; Niedringhaus, K. Thelazia callipaeda Eyeworms in American Black Bear, Pennsylvania, USA, 2023. Emerg. Infect. Dis. 2024, 30, 1961. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, C.; Tan, X.; Chen, N.; Jin, Y. Epidemiology of Ocular Thelaziosis in Domestic Dogs in Beijing. Pathogens 2024, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Marino, V.; Montoya, A.; Mascuñan, C.; Domínguez, I.; Gálvez, R.; Hernández, M.; Zenker, C.; Checa, R.; Sarquis, J.; Barrera, J.P.; et al. Feline Thelaziosis (Thelazia callipaeda) in Spain: State-of-the-Art and First Prophylactic Trial in Cats. J. Feline Med. Surg. 2021, 23, 1117–1128. [Google Scholar] [CrossRef]
- Motta, B.; Schnyder, M.; Basano, F.S.; Nägeli, F.; Nägeli, C.; Schiessl, B.; Mallia, E.; Lia, R.P.; Dantas-Torres, F.; Otranto, D. Therapeutic Efficacy of Milbemycin Oxime/Praziquantel Oral Formulation (Milbemax®) against Thelazia callipaeda in Naturally Infested Dogs and Cats. Parasites Vectors 2012, 5, 85. [Google Scholar] [CrossRef]
- Otranto, D.; Solari Basano, F.; Pombi, M.; Capelli, G.; Nazzari, R.; Falsone, L.; Petry, G.; Pollmeier, M.G.; Lia, R.P. Effectiveness of the Spot-on Combination of Moxidectin and Imidacloprid (Advocate®) in the Treatment of Ocular Thelaziosis by Thelazia callipaeda in Naturally Infected Cats. Parasites Vectors 2019, 12, 25. [Google Scholar] [CrossRef]
- Bezerra-Santos, M.A.; Mendoza-Roldan, J.A.; Sgroi, G.; Lia, R.P.; Venegoni, G.; Solari Basano, F.; Nele, R.; Mahabir, S.P.; Borowski, S.; Geurden, T.; et al. Efficacy of a Formulation of Sarolaner/Moxidectin/Pyrantel (Simparica Trio®) for the Prevention of Thelazia callipaeda Canine Eyeworm Infection. Parasites Vectors 2022, 15, 370. [Google Scholar] [CrossRef]
- Di Cesare, A.; Zanet, S.; Traversa, D.; Colombo, M.; Tielemans, E.; Beugnet, F.; Ferroglio, E. Efficacy of a Combination of Esafoxolaner, Eprinomectin and Praziquantel (NexGard® Combo) against Thelazia callipaeda in Naturally Infected Cats. Parasite 2024, 31, 10. [Google Scholar] [CrossRef]
- Otranto, D.; Colella, V.; Crescenzo, G.; Solari Basano, F.; Nazzari, R.; Capelli, G.; Petry, G.; Schaper, R.; Pollmeier, M.; Mallia, E.; et al. Efficacy of Moxidectin 2.5% and Imidacloprid 10% in the Treatment of Ocular Thelaziosis by Thelazia callipaeda in Naturally Infected Dogs. Vet. Parasitol. 2016, 227, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Xiulan, L.; Guifang, Z.; Deshun, Y.; Xiqi, C. Diagnosis and treatment of Thelazia callipaeda in canine and feline. Chin. J. Zoonoses 1992, 8, 54. [Google Scholar]
- Wu, S. Treatment of 133 cases of Thelazia callipaeda in cattle and sheep with levamisole hydrochloride. Chin. J. Vet. Med. 1987, 13, 33. [Google Scholar]
- Liyuan, L.; Yue, Z.; Xinhao, T.; Xuewei, W.; Qian, K.; Zhijun, R.; Yuelam, Z.; Jianhua, Q. Diagnosis and management of a case of canine thelaziasis. Chin. J. Vet. Med. 2017, 53, 111. [Google Scholar]
- Meiling, W. Prevention and management of canine ocular thelaziasis. Chin. J. Anim. Husb. Vet. Med. 2014, 103–104. [Google Scholar]
- Haibo, F.; Guangneng, P. Diagnosis and treatment of two cases of canine Thelazia callipaeda disease. Chin. J. Vet. Med. 2013, 49, 58. [Google Scholar]
- Koopman, M.; Peter, Q.; Seinstra, R.I.; Perni, M.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P.J.; Nollen, E.A.A. Assessing Motor-Related Phenotypes of Caenorhabditis Elegans with the Wide Field-of-View Nematode Tracking Platform. Nat. Protoc. 2020, 15, 2071–2106. [Google Scholar] [CrossRef]
- Cho, J.; Lu, J.; Kim, D.; Park, Y. Determination of Health Status during Aging Using Bending and Pumping Rates at Various Survival Rates in Caenorhabditis Elegans. Sci. Rep. 2025, 15, 9057. [Google Scholar] [CrossRef]
- Sammi, S.R.; Jameson, L.E.; Conrow, K.D.; Leung, M.C.K.; Cannon, J.R. Caenorhabditis Elegans Neurotoxicity Testing: Novel Applications in the Adverse Outcome Pathway Framework. Front. Toxicol. 2022, 4, 826488. [Google Scholar] [CrossRef] [PubMed]
- Efron, N. Grading Scales for Contact Lens Complications. Ophthalmic Physiol. Opt. 1998, 18, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.J.; Robertson, A.P.; Buxton, S.K.; Beech, R.N.; Charvet, C.L.; Neveu, C. Levamisole Receptors: A Second Awakening. Trends Parasitol. 2012, 28, 289–296. [Google Scholar] [CrossRef]
- Xunlong, L.; Qingling, Y.; Bangcheng, L.; Guangzhong, D.; Weixian, W. Efficacy of Ivermectin, Levamisole and Imidacloprid & Permethrin against oriental eyeworm in canine. Taiwan Vet. J. 2008, 34, 233–238. [Google Scholar] [CrossRef]
- Campillo, J.T.; Eiden, C.; Boussinesq, M.; Pion, S.D.S.; Faillie, J.; Chesnais, C.B. Adverse Reactions with Levamisole Vary According to Its Indications and Misuse: A Systematic Pharmacovigilance Study. Br. J. Clin. Pharmacol. 2022, 88, 1094–1106. [Google Scholar] [CrossRef]
- López-Sánchez, C.; Rozas-Muñoz, E.; Mir-Bonafé, J.F. Levamisole-Induced Vasculopathy. JAMA Dermatol. 2021, 157, 338. [Google Scholar] [CrossRef]


| (1-1) | |||
| Score Items | Time | Scoring Method | |
| Light stimulation score | 0 s | T. callipaeda specimens were removed from a dark environment, images of the morphology immediately were immediately captured after exposure to light and after 30 s of exposure, and then scoring was performed based on the scoring criteria in Table 1(1-2). | |
| 30 s | |||
| Locomotion score | 0–30 s | The T. callipaeda specimens were transferred from a dark environment, and their locomotion within 30 s of light exposure was recorded via video. The scoring process was conducted following the criteria outlined in Table 1(1-2). | |
| (1-2) | |||
| Score Item | Score Value | Score Standard | Reference Diagram |
| Light stimulation score | 4 | The worm is tightly coiled in multiple loops or irregularly bent with over three bends, each less than 90°. | 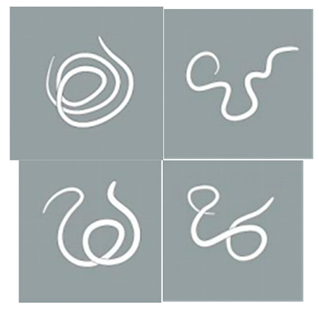 |
| 3 | The worm is partially coiled into a single loop, forming shapes that resemble the letters “W”, “E”, or “P” or appears serpentine with 2–3 bends, each less than 90° |  | |
| 2 | The worm exhibits partial coiling without forming a full circle or presents a single bend with an angle ≤ 160°. |  | |
| 1 | The worm exhibits slight bending or remains straight and rigid, with a curvature exceeding 160°. |  | |
| Locomotion score | 4 | The worm demonstrates more than 5 movements, including serpentine swimming, rolling, and coiling. | 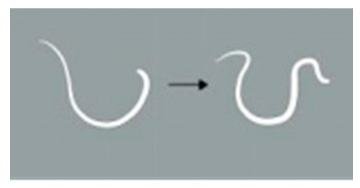 |
| 3 | The worm exhibits 3 to 5 movements, including serpentine swimming, rolling, and coiling. | 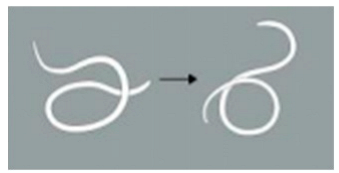 | |
| 2 | The worm exhibits 1 to 3 movements, including serpentine swimming, rolling, and coiling. | 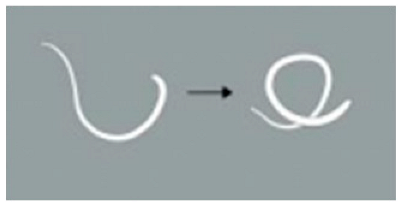 | |
| 1 | The worm remains static. | / | |
| Score Item | Score Value | Score Standard |
|---|---|---|
| Conjunctiva | 0 | Conjunctiva appears normal in color with vascularization and no signs of edema. |
| 1 | Conjunctiva is red with vascular congestion and mild edema. | |
| 2 | Conjunctiva appears dark red with edema leading to mild eyelid ectropion. | |
| 3 | Conjunctiva appears dark red with pronounced edema leading to partial eyelid closure. | |
| 4 | Conjunctiva is dark red with indistinct blood vessels and severe edema leading to almost complete eyelid closure. | |
| Discharge | 0 | No ocular discharge present. |
| 1 | Minimal ocular discharge present. | |
| 2 | Noticeable ocular discharge with moisture on the periocular area and eyelashes. | |
| 3 | Profuse discharge causing eyelid adhesion. |
| Number of Applications | Score Item | Score Value | |||||
|---|---|---|---|---|---|---|---|
| Control | 1 h | 2 h | 4 h | 8 h | 12 h | ||
| 0 | Conjunctiva | 0 | 0 | 0 | 0 | 0.7 ± 0.6 * | 1 ** |
| Discharge | 0 | 0 | 0 | 0.3 ± 0.6 | 0.3 ± 0.6 | 0.3 ± 0.6 | |
| 1 | Conjunctiva | 0 | 0 | 0 | 0 | 1.3 ± 0.6 *** | 1 ** |
| Discharge | 0 | 0 | 0 | 0 | 1.3 ± 0.6 ** | 1.3 ± 0.6 ** | |
| 2 | Conjunctiva | 0 | 0 | 0 | 0 | 0.7 ± 0.6 * | 1 ** |
| Discharge | 0 | 0 | 0 | 0 | 0.3 ± 0.6 | 0.3 ± 0.6 | |
| 3 | Conjunctiva | 0 | 0 | 0 | 0 | 0 | 0.7 ± 0.6 * |
| Discharge | 0 | 0 | 0 | 0 | 0.3 ± 0.6 | 0.7 ± 0.6 | |
| 4 | Conjunctiva | 0 | 0 | 0 | 0 | 0 | 0 |
| Discharge | 0 | 0 | 0 | 0 | 0 | 0 | |
| Item | Unit | After 24 h of Administration | Before Administration | t-Test | ||
|---|---|---|---|---|---|---|
| n | ± SD | n | ± SD | p-Value | ||
| WBC | 109 count·L−1 | 6 | 7.82 ± 2.13 | 6 | 7.86 ± 1.23 | p = 0.34 |
| NEU | % | 6 | 45.92 ± 11.32 | 6 | 45.52 ± 11.12 | p = 0.62 |
| LYM | % | 6 | 7.82 ± 10.13 | 6 | 7.42 ± 9.21 | p = 0.15 |
| MONO | % | 6 | 2.62 ± 1.17 | 6 | 2.42 ± 1.02 | p = 0.24 |
| EOS | % | 6 | 0.12 ± 0.05 | 6 | 0.12 ± 0.10 | p = 0.52 |
| BASO | % | 6 | 1.12 ± 0.65 | 6 | 1.14 ± 0.45 | p = 0.87 |
| RBC | 1012 count·L−1 | 6 | 5.48 ± 0.95 | 6 | 5.49 ± 0.85 | p = 0.12 |
| HGB | g·L−1 | 6 | 109.38 ± 11.05 | 6 | 109.28 ± 10.01 | p = 0.86 |
| HCT | % | 6 | 33.32 ± 5.03 | 6 | 33.22 ± 4.09 | p = 0.27 |
| MCV | fL | 6 | 62.49 ± 1.52 | 6 | 63.49 ± 1.63 | p = 0.53 |
| MCH | pg | 6 | 21.03 ± 1.10 | 6 | 20.13 ± 1.09 | p = 0.58 |
| MCHC | g·L−1 | 6 | 314.21 ± 10.65 | 6 | 314.23 ± 10.21 | p = 0.36 |
| RDW | % | 6 | 12.42 ± 0.41 | 6 | 12.35 ± 0.19 | p = 0.50 |
| PLT | 109 count·L−1 | 6 | 142.41 ± 24.35 | 6 | 146.31 ± 22.15 | p = 0.89 |
| PCT | % | 6 | 0.19 ± 0.05 | 6 | 0.21 ± 0.06 | p = 0.12 |
| MPV | fL | 6 | 5.02 ± 0.92 | 6 | 4.98 ± 0.22 | p = 0.37 |
| PDW | fL | 6 | 15.33 ± 1.02 | 6 | 15.23 ± 1.12 | p = 0.62 |
| Item | Unit | After 24 h of Administration | Before Administration | t-Test | ||
|---|---|---|---|---|---|---|
| n | ± SD | n | ± SD | p-Value | ||
| TP | g·L−1 | 6 | 66.22 ± 4.03 | 6 | 65.23 ± 4.10 | p = 0.46 |
| ALB | g·L−1 | 6 | 42.32 ± 4.22 | 6 | 43.52 ± 3.12 | p = 0.67 |
| GLB | g·L−1 | 6 | 27.32 ± 3.23 | 6 | 26.89 ± 0.43 | p = 0.74 |
| A/G | % | 6 | 1.63 ± 0.13 | 6 | 1.70 ± 0.09 | p = 0.45 |
| ALT | U·L−1 | 6 | 1.42 ± 0.25 | 6 | 1.51 ± 0.03 | p = 0.67 |
| AST | U·L−1 | 6 | 45.32 ± 15.25 | 6 | 45.40 ± 13.85 | p = 0.23 |
| ALP | U·L−1 | 6 | 96.28 ± 31.25 | 6 | 96.25 ± 30.13 | p = 0.47 |
| CK | U·L−1 | 6 | 1049.32 ± 431.25 | 6 | 1049.59 ± 420.32 | p = 0.99 |
| GLU | mmol L−1 | 6 | 7.32 ± 0.53 | 6 | 7.31 ± 0.42 | p = 0.36 |
| BUN | mmol L−1 | 6 | 8.39 ± 1.32 | 6 | 8.28 ± 1.25 | p = 0.38 |
| CRE | mmol L−1 | 6 | 111.32 ± 21.50 | 6 | 113.42 ± 22.01 | p = 0.43 |
| Ca | mmol L−1 | 6 | 3.31 ± 0.75 | 6 | 3.36 ± 0.43 | p = 0.24 |
| p | mmol L−1 | 6 | 1.52 ± 0.31 | 6 | 1.57 ± 0.03 | p = 0.66 |
| CHOL | mmol L−1 | 6 | 1.39 ± 0.45 | 6 | 1.43 ± 0.39 | p = 0.57 |
| TG | mmol L−1 | 6 | 0.89 ± 0.45 | 6 | 0.95 ± 0.02 | p = 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Zhong, Y.; Chen, N.; Liu, Z.; Yuan, Z.; Jin, Y. Nematicidal Effects and Cytotoxicity of Levamisole on Thelazia callipaeda. Animals 2025, 15, 1551. https://doi.org/10.3390/ani15111551
Han Z, Zhong Y, Chen N, Liu Z, Yuan Z, Jin Y. Nematicidal Effects and Cytotoxicity of Levamisole on Thelazia callipaeda. Animals. 2025; 15(11):1551. https://doi.org/10.3390/ani15111551
Chicago/Turabian StyleHan, Zhengxuan, Yipeng Zhong, Ni Chen, Zichen Liu, Zhankui Yuan, and Yipeng Jin. 2025. "Nematicidal Effects and Cytotoxicity of Levamisole on Thelazia callipaeda" Animals 15, no. 11: 1551. https://doi.org/10.3390/ani15111551
APA StyleHan, Z., Zhong, Y., Chen, N., Liu, Z., Yuan, Z., & Jin, Y. (2025). Nematicidal Effects and Cytotoxicity of Levamisole on Thelazia callipaeda. Animals, 15(11), 1551. https://doi.org/10.3390/ani15111551






