Levels of Mineral Elements in Different Organs of Dogs from the Ionian-Etnean Volcanic Area
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling Area
2.2. Sample Collection
2.3. Mineralization ICP-MS and DMA-80 Analysis
2.4. Calibration Procedure
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gorain, B.; Choudhury, H.; Roychoudhury, S.; Sengupta, P. Environmental and occupational exposure of metals and female reproductive health. Environ. Sci. Pollut. Res. Int. 2022, 29, 62067–62092. [Google Scholar] [CrossRef] [PubMed]
- López-Alonso, M.; Miranda, M.; García-Partida, P.; Cantero, F.; Hernández, J.; Benedito, J.L. Use of dogs as indicators of metal exposure in rural and urban habitats in NW Spain. Sci. Total Environ. 2007, 372, 668–675. [Google Scholar] [CrossRef]
- Vigneri, R.; Malandrino, P.; Gianì, F.; Russo, M.; Vigneri, P. Heavy metals in the volcanic environment and thyroid cancer. Mol. Cell. Endocrinol. 2017, 457, 73–80. [Google Scholar] [CrossRef]
- Calabrese, S.; Aiuppa, A.; Allard, P.; Bagnato, E.; Bellomo, S.; Brusca, L.; D’Alessandro, W.; Parello, F. Atmospheric sources and sinks of volcanogenic elements in a basaltic volcano (Etna, Italy). Geochim. Cosmochim. Acta 2011, 75, 7401–7425. [Google Scholar] [CrossRef]
- Aiuppa, A.; Allard, P.; D’Alessandro, W.; Giammanco, S.; Parello, F.; Valenza, M. Magmatic Gas Leakage at Mount Etna (Sicily, Italy): Relationships with the Volcano-Tectonic Structures, the Hydrological Pattern and the Eruptive Activity. In Mt. Etna: Volcano Laboratory; AGU: Washington, DC, USA, 2004; pp. 129–145. [Google Scholar]
- Giammanco, S.; Ottaviani, M.; Valenza, M.; Veschetti, E.; Principio, E.; Giammanco, G.; Pignato, S. Major and Trace Elements Geochemistry in the Ground Waters of a Volcanic Area: Mount Etna (sicily, Italy). Water Res. 1998, 32, 19–30. [Google Scholar] [CrossRef]
- Cimino, G.; Ziino, M. Heavy metal pollution, Part VII. Emissions from Mount Etna Volcano. Geophys. Res. Lett. 1983, 10, 31–34. [Google Scholar] [CrossRef]
- Aiuppa, A.; Allard, P.; D’Alessandro, W.; Michel, A.; Parello, F.; Treuil, M.; Valenza, M. Mobility and fluxes of major, minor and trace metals during basalt weathering and groundwater transport at Mt. Etna volcano (Sicily). Geochim. Cosmochim. Acta 2000, 64, 1827–1841. [Google Scholar] [CrossRef]
- Varrica, D.; Tamburo, E.; Dongarrà, G.; Sposito, F. Trace elements in scalp hair of children chronically exposed to volcanic activity (Mt. Etna, Italy). Sci. Total Environ. 2014, 470–471, 117–126. [Google Scholar] [CrossRef]
- Malandrino, P.; Russo, M.; Ronchi, A.; Minoia, C.; Cataldo, D.; Regalbuto, C.; Giordano, C.; Attard, M.; Squatrito, S.; Trimarchi, F.; et al. Increased thyroid cancer incidence in a basaltic volcanic area is associated with non-anthropogenic pollution and biocontamination. Endocrine 2016, 53, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Aiuppa, A.; Bellomo, S.; Brusca, L.; D’Alessandro, W.; Federico, C. Natural and anthropogenic factors affecting groundwater quality of an active volcano (Mt. Etna, Italy). Appl. Geochem. 2003, 18, 863–882. [Google Scholar] [CrossRef]
- Nicoletti, A.; Vasta, R.; Venti, V.; Mostile, G.; Lo Fermo, S.; Patti, F.; Scillieri, R.; De Cicco, D.; Volanti, P.; Marziolo, R.; et al. The epidemiology of amyotrophic lateral sclerosis in the Mount Etna region: A possible pathogenic role of volcanogenic metals. Eur. J. Neurol. 2016, 23, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Boumediene, F.; Vasta, R.; Rascunà, C.; Lo Fermo, S.; Volanti, P.; Marziolo, R.; Patti, F.; Ferrante, M.; Preux, P.M.; Marin, B.; et al. Amyotrophic lateral sclerosis spatial epidemiology in the Mount Etna region, Italy. Eur. J. Neurol. 2019, 26, e90–e91. [Google Scholar] [CrossRef]
- Boffetta, P.; Memeo, L.; Giuffrida, D.; Ferrante, M.; Sciacca, S. Exposure to emissions from Mount Etna (Sicily, Italy) and incidence of thyroid cancer: A geographic analysis. Sci. Rep. 2020, 10, 21298. [Google Scholar] [CrossRef]
- Gianì, F.; Masto, R.; Trovato, M.A.; Malandrino, P.; Russo, M.; Pellegriti, G.; Vigneri, P.; Vigneri, R. Heavy Metals in the Environment and Thyroid Cancer. Cancers 2021, 13, 4052. [Google Scholar] [CrossRef]
- Nava, V.; Licata, P.; Biondi, V.; Catone, G.; Gugliandolo, E.; Pugliese, M.; Passantino, A.; Crupi, R.; Aragona, F. Horse Whole Blood Trace Elements from Different Sicily Areas: Biomonitoring of Environmental Risk. Biol. Trace Elem. Res. 2024, 202, 3086–3096. [Google Scholar] [CrossRef]
- Bryan, J.N.; Maitz, C.A. Translational History and Hope of Immunotherapy of Canine Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2024, 30, 4272–4285. [Google Scholar] [CrossRef]
- Bruno, F.; Nava, V.; Fazio, F.; Sansotta, C.; Bruschetta, G.; Licata, P.; Parrino, V. Heavy Metals Bioaccumulation in Mytilus galloprovincialis and Tapes decussatus from Faro Lake (Messina), Italy. Biol. Trace Elem. Res. 2024, 202, 5762–5770. [Google Scholar] [CrossRef]
- Bruno, F.; Nava, V.; Zappalà, S.; Costa, G.L.; Fazio, F.; Parrino, V.; Licata, P. Mineral composition in mussel Mytilus galloprovincialis and clam Tapes decussatus from Faro Lake of Messina: Risk assessment for human health. Front. Toxicol. 2024, 6, 1494977. [Google Scholar] [CrossRef]
- Messina, L.; Licata, P.; Bruno, F.; Litrenta, F.; Costa, G.L.; Ferrantelli, V.; Peycheva, K.; Panayotova, V.; Fazio, F.; Bruschetta, G.; et al. Occurrence and health risk assessment of mineral composition and aflatoxin M1 in cow milk samples from different areas of Sicily, Italy. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2024, 85, 127478. [Google Scholar] [CrossRef] [PubMed]
- Löpez-Alonso, M.; Miranda, M.; García-Partida, P.; Mendez, A.; Castillo, C.; Benedito, J.L. Toxic and trace metal concentrations in liver and kidney of dogs: Influence of diet, sex, age, and pathological lesions. Biol. Trace Elem. Res. 2007, 116, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; De Roma, A.; Maglio, P.; Sansone, D.; Picazio, G.; Bianco, R.; De Martinis, C.; Rosato, G.; Baldi, L.; Gallo, P. Heavy metals in organs of stray dogs and cats from the city of Naples and its surroundings (Southern Italy). Environ. Sci. Pollut. Res. Int. 2019, 26, 3473–3478. [Google Scholar] [CrossRef] [PubMed]
- Serpe, F.P.; Russo, R.; Simone, A.D.; Florio, S.; Esposito, M.; Severino, L. Levels of heavy metals in liver and kidney of dogs from urban environment. Open Vet. J. 2012, 2, 15–18. [Google Scholar] [CrossRef]
- Cho, J.; Kim, D.; Lee, S.; Lee, Y. Cobalt Chloride-Induced Estrogen Receptor α Down-Regulation Involves Hypoxia-Inducible Factor-1α in MCF-7 Human Breast Cancer Cells. Mol. Endocrinol. 2005, 19, 1191–1199. [Google Scholar] [CrossRef]
- Wise, J.P., Jr.; Young, J.L.; Cai, J.; Cai, L. Current understanding of hexavalent chromium [Cr(VI)] neurotoxicity and new perspectives. Environ. Int. 2022, 158, 106877. [Google Scholar] [CrossRef]
- Romaniuk, A.; Lyndin, M.; Sikora, V.; Lyndina, Y.; Romaniuk, S.; Sikora, K. Heavy metals effect on breast cancer progression. J. Occup. Med. Toxicol. 2017, 12, 32. [Google Scholar] [CrossRef]
- Aquino, N.B.; Sevigny, M.B.; Sabangan, J.; Louie, M.C. The role of cadmium and nickel in estrogen receptor signaling and breast cancer: Metalloestrogens or not? J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2012, 30, 189–224. [Google Scholar] [CrossRef]
- Amundson, L.A.; Kirn, B.N.; Swensson, E.J.; Millican, A.A.; Fahey, G.C. Copper metabolism and its implications for canine nutrition. Transl. Anim. Sci. 2024, 8, txad147. [Google Scholar] [CrossRef]
- Cedeño, Y.; López-Alonso, M.; Miranda, M. Hepatic concentrations of copper and other metals in dogs with and without chronic hepatitis. J. Small Anim. Pract. 2016, 57, 703–709. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Wilk, A.; Wiszniewska, B.; Szypulska-Koziarska, D.; Kaczmarek, P.; Romanowski, M.; Różański, J.; Słojewski, M.; Ciechanowski, K.; Marchelek-Myśliwiec, M.; Kalisińska, E. The Concentration of Vanadium in Pathologically Altered Human Kidneys. Biol. Trace Elem. Res. 2017, 180, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Defourny, S.V.P.; Caioni, G.; Bellocci, M.; Melai, V.; Scortichini, G.; Salini, R.; Martino, M.; Di Teodoro, G.; Cocco, A.; Cantelmi, M.C.; et al. Domestic dogs as environmental sentinel in comparative toxicologic pathology: Assessment of metals and rare earth elements concentrations in healthy and neoplastic mammary glands. One Health 2024, 18, 100749. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.S.; Rana, B.; Swami, B.; Venu, V.; Chatterjee, M. Vanadium mediated apoptosis and cell cycle arrest in MCF7 cell line. Chem. -Biol. Interact. 2006, 163, 239–247. [Google Scholar] [CrossRef]
- Bishayee, A.; Oinam, S.; Basu, M.; Chatterjee, M. Vanadium chemoprevention of 7,12-dimethylbenz(a)anthracene-induced rat mammary carcinogenesis: Probable involvement of representative hepatic phase I and II xenobiotic metabolizing enzymes. Breast Cancer Res. Treat. 2000, 63, 133–145. [Google Scholar] [CrossRef]
- Passlack, N.; Mainzer, B.; Lahrssen-Wiederholt, M.; Schafft, H.; Palavinskas, R.; Breithaupt, A.; Zentek, J. Concentrations of strontium, barium, cadmium, copper, zinc, manganese, chromium, antimony, selenium, and lead in the liver and kidneys of dogs according to age, gender, and the occurrence of chronic kidney disease. J. Vet. Sci. 2015, 16, 57–66. [Google Scholar] [CrossRef]
- Fieten, H.; Hooijer-Nouwens, B.D.; Biourge, V.C.; Leegwater, P.A.; Watson, A.L.; van den Ingh, T.S.; Rothuizen, J. Association of dietary copper and zinc levels with hepatic copper and zinc concentration in Labrador Retrievers. J. Vet. Intern. Med. 2012, 26, 1274–1280. [Google Scholar] [CrossRef]
- Pereira, A.M.; Maia, M.R.G.; Fonseca, A.J.M.; Cabrita, A.R.J. Zinc in Dog Nutrition, Health and Disease: A Review. Animals 2021, 11, 978. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale. Attuazione Della Direttiva 2013/39/UE, che Modifica le Direttive 2000/60/CE per Quanto Riguarda le Sostanze Prioritarie nel Settore Della Politica Delle Acque; (15G00186); DL (2015/172); Decreto Legislativo 13 ottobre 2015, n. 172; Gazzetta Ufficiale: Rome, Italy, 2015. [Google Scholar]
| Breed | Age | Sex | Total Animals | Weight (kg) * |
|---|---|---|---|---|
| Mixed-breed | 7 | m | 6 | 22–25 |
| Mixed-breed | 8 | f | 7 | 13–18 |
| Mixed-breed | 10 | f | 5 | 20–25 |
| Czechoslovakian Shepherd | 10 | m | 8 | 26–39 |
| Mixed-breed | 8 | f | 6 | 15–20 |
| Rottweiler | 2 | f | 4 | 35–40 |
| Golden Retriever | 6 | f | 3 | 25–30 |
| Mixed-breed | 8 | m | 7 | 24–34 |
| Mixed-breed | 12 | f | 6 | 7–18 |
| German Shepherd | 12 | m | 4 | 30–35 |
| Pitt Bull | 5 | m | 5 | 16–30 |
| Mixed-breed | 15 | m | 8 | 23–27 |
| Chihuahua | 16 | m | 6 | 1.2–3 |
| Mixed-breed | 6 | m | 5 | 3–8 |
| Element | LOD (mg/L) | LOQ (mg/L) | R2 | ERMBB184 Bovine Muscle (%) |
|---|---|---|---|---|
| Bi | 0.0015 | 0.005 | 0.9995 | 96.50 ± 0.75 ** |
| Cd | 0.001 | 0.003 | 0.9999 | 102.70 ± 1.43 |
| Co | 0.002 | 0.007 | 0.9996 | 97.00 ± 0.80 ** |
| Cr | 0.0015 | 0.005 | 0.9996 | 95.55 ± 0.46 ** |
| Cu | 0.003 | 0.01 | 0.9995 | 98.75 ± 1.48 |
| Fe | 0.003 | 0.01 | 0.9995 | 97.25 ± 0.78 |
| Hg * | 0.300 * | 1.000 * | 0.9997 | 98.65 ± 0.55 |
| Ni | 0.0018 | 0.006 | 0.9998 | 97.15 ± 0.40 ** |
| Pb | 0.001 | 0.003 | 0.9999 | 101.50 ± 1.70 ** |
| V | 0.003 | 0.01 | 0.9995 | 96.00 ± 0.45 ** |
| Zn | 0.03 | 0.10 | 0.9997 | 98.50 ± 0.86 |
| Metals | Organs | ||||
|---|---|---|---|---|---|
| Liver | Kidney | Heart | Muscle | Lung | |
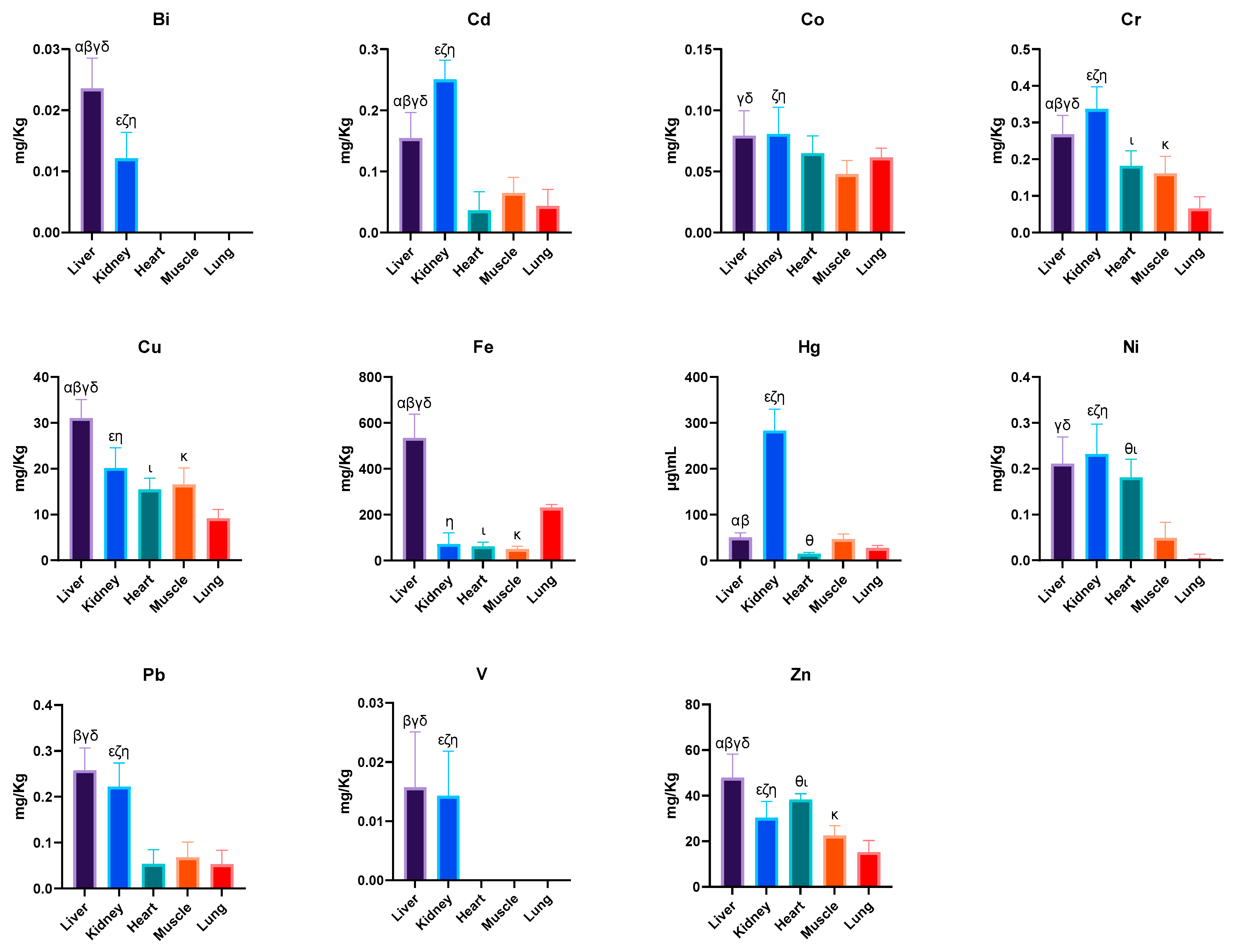
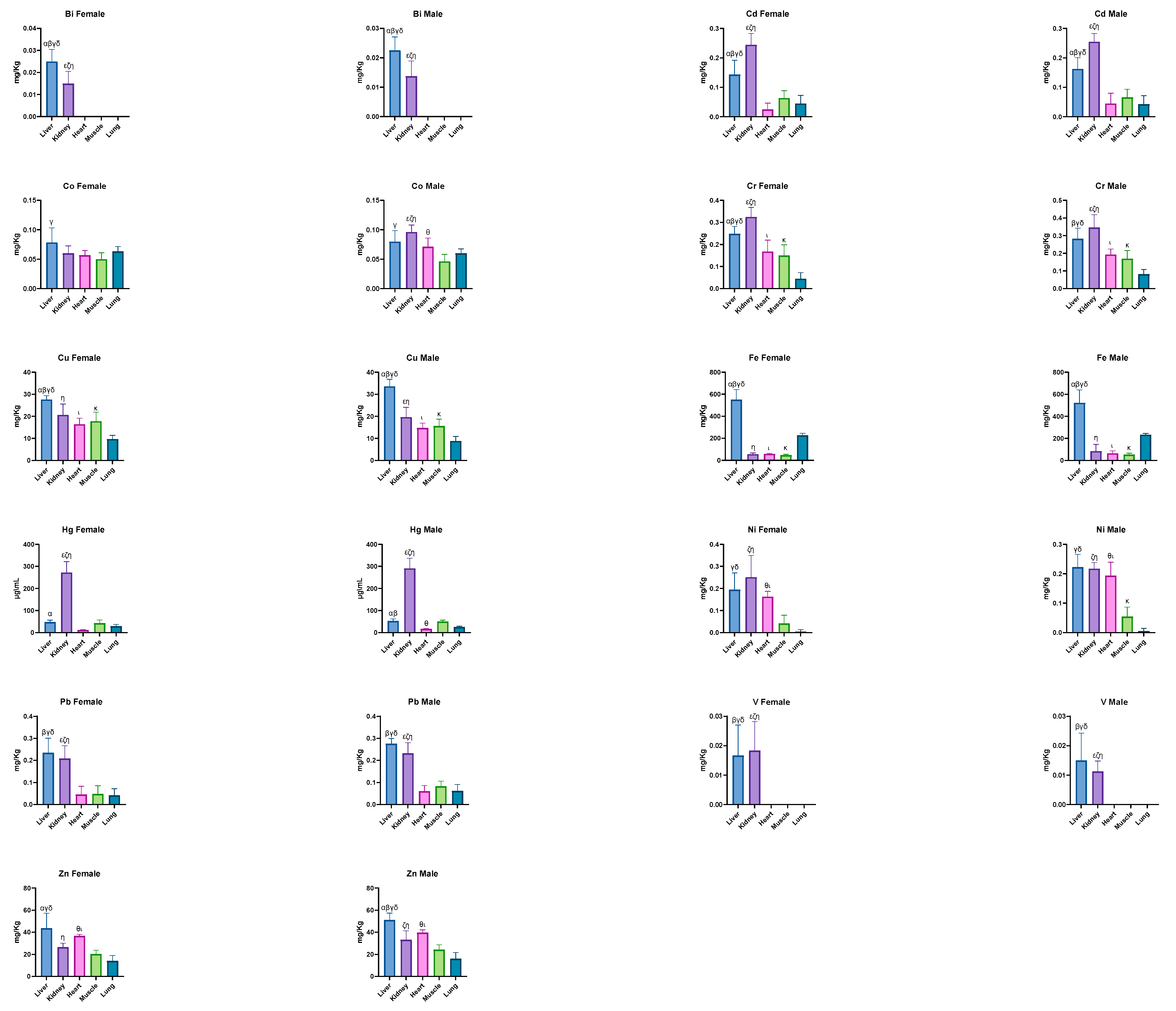
| Bi (mg/kg) | 0.024 ± 0.005 α,β,γ,δ | 0.012 ± 0.004 ε,ζ,η | <LOQ | <LOQ | <LOQ |
| Cd (mg/kg) | 0.154 ± 0.042 α,β,γ,δ | 0.251 ± 0.031 ε,ζ,η | 0.036 ± 0.031 | 0.065 ± 0.025 | 0.044 ± 0.027 |
| Co (mg/kg) | 0.079 ± 0.021 γ,δ | 0.081 ± 0.022 ζ,η | 0.065 ± 0.014 | 0.048 ± 0.011 | 0.061 ± 0.008 |
| Cr (mg/kg) | 0.268 ± 0.052 α,β,γ,δ | 0.337 ± 0.061 ε,ζ,η | 0.182 ± 0.042 ι | 0.161 ± 0.046 κ | 0.066 ± 0.032 |
| Cu (mg/kg) | 31.040 ± 3.998 α,β,γ,δ | 20.110 ± 4.465 ε,η | 15.470 ± 2.495 ι | 16.560 ± 3.600 κ | 9.181 ± 1.932 |
| Fe (mg/kg) | 534.400 ± 104.000 α,β,γ,δ | 72.510 ± 47.550 η | 62.080 ± 17.500 ι | 50.790 ± 11.020 κ | 230.900 ± 14.340 |
| Hg (μg/kg) | 50.590 ± 9.491 α,β | 283.000 ± 46.780 ε,ζ,η | 14.940 ± 2.830 θ | 47.280 ± 10.420 | 27.400 ± 5.705 |
| Ni (mg/kg) | 0.211 ± 0.058 γ,δ | 0.232 ± 0.065 ε,ζ,η | 0.181 ± 0.040 θ,ι | 0.049 ± 0.034 | 0.005 ± 0.009 |
| Pb (mg/kg) | 0.258 ± 0.049 β,γ,δ | 0.222 ± 0.051 ε,ζ,η | 0.054 ± 0.031 | 0.068 ± 0.033 | 0.053 ± 0.030 |
| V (mg/kg) | 0.012 ± 0.013 β,γ,δ | 0.010 ± 0.011 ε,ζ,η | 0.072 ± 0.197 | 0.001 ± 0.004 | <LOQ |
| Zn (mg/kg) | 47.870 ± 10.360 α,β,γ,δ | 30.290 ± 7.242 ε,ζ,η | 38.400 ± 2.522 θ,ι | 22.500 ± 4.342 κ | 15.250 ± 5.130 |
| Heavy Metals (μg/L) | ||||||
|---|---|---|---|---|---|---|
| As | Cd | Cr | Hg | Ni | Pb | |
| Water | <LOD | 0.66 ± 0.02 | 2.30 ± 0.22 | 18.02 ± 1.14 | 0.92 ± 0.12 | <LOD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, F.; Miller, A.; Bruschetta, G.; Nava, V.; Rifici, C.; Zappalà, S.; Licata, P. Levels of Mineral Elements in Different Organs of Dogs from the Ionian-Etnean Volcanic Area. Animals 2025, 15, 1545. https://doi.org/10.3390/ani15111545
Bruno F, Miller A, Bruschetta G, Nava V, Rifici C, Zappalà S, Licata P. Levels of Mineral Elements in Different Organs of Dogs from the Ionian-Etnean Volcanic Area. Animals. 2025; 15(11):1545. https://doi.org/10.3390/ani15111545
Chicago/Turabian StyleBruno, Fabio, Anthea Miller, Giuseppe Bruschetta, Vincenzo Nava, Claudia Rifici, Sebastiano Zappalà, and Patrizia Licata. 2025. "Levels of Mineral Elements in Different Organs of Dogs from the Ionian-Etnean Volcanic Area" Animals 15, no. 11: 1545. https://doi.org/10.3390/ani15111545
APA StyleBruno, F., Miller, A., Bruschetta, G., Nava, V., Rifici, C., Zappalà, S., & Licata, P. (2025). Levels of Mineral Elements in Different Organs of Dogs from the Ionian-Etnean Volcanic Area. Animals, 15(11), 1545. https://doi.org/10.3390/ani15111545







