When Antlers Grow Abnormally: A Hidden Disease Behind Common Cervid Trophy Deformities, Introducing Pedunculitis Chronica Deformans
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Anomalous and Normal Trophies
2.2. Macroscopic Examinations
2.3. Analysis of Pedicle Ellipticity
2.4. Microscopic Examinations
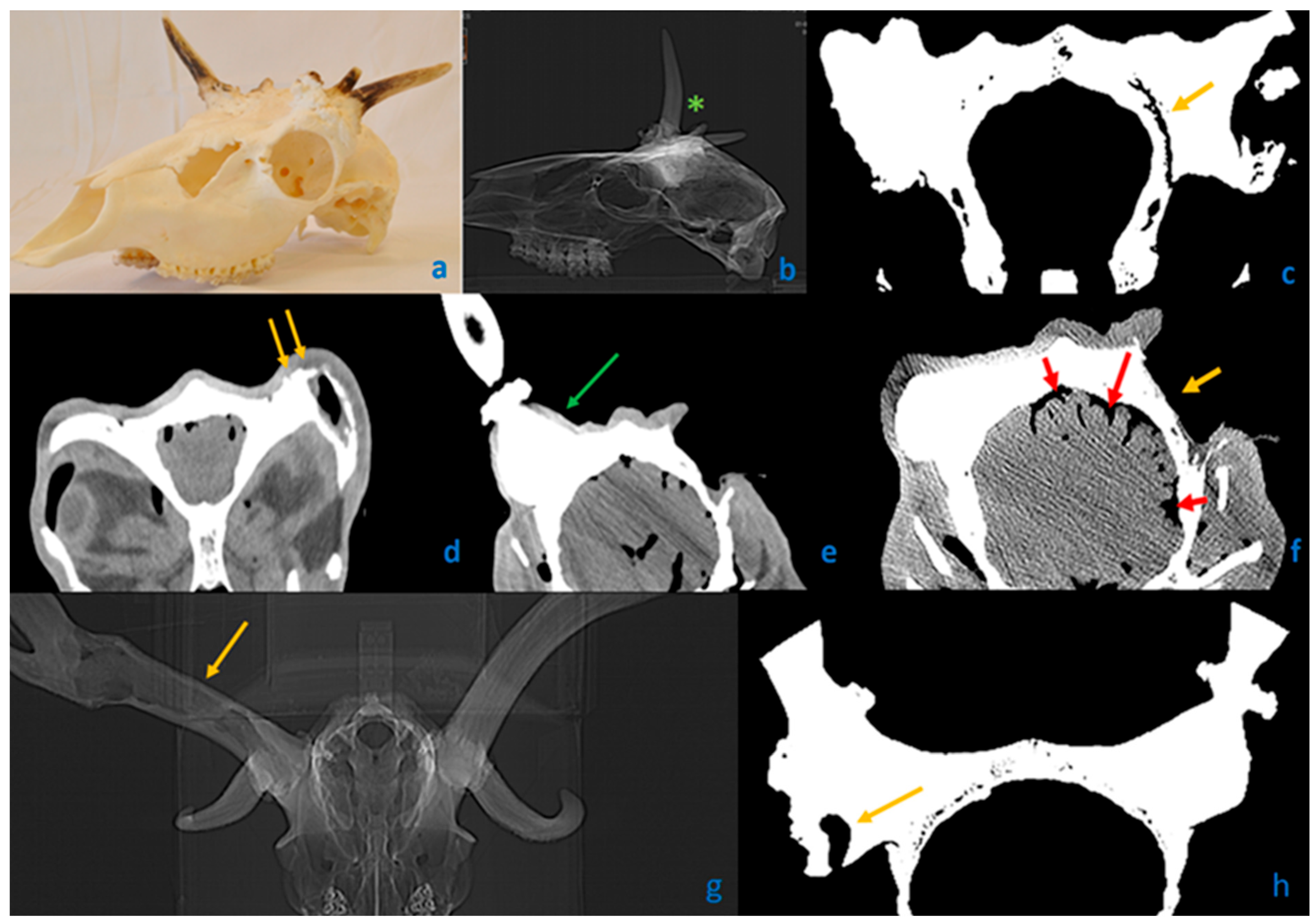
2.5. Radiological Examinations
2.6. Statistical Analysis
3. Results and Discussion
3.1. Epidemiology of Cervidae with Aberrant Trophies
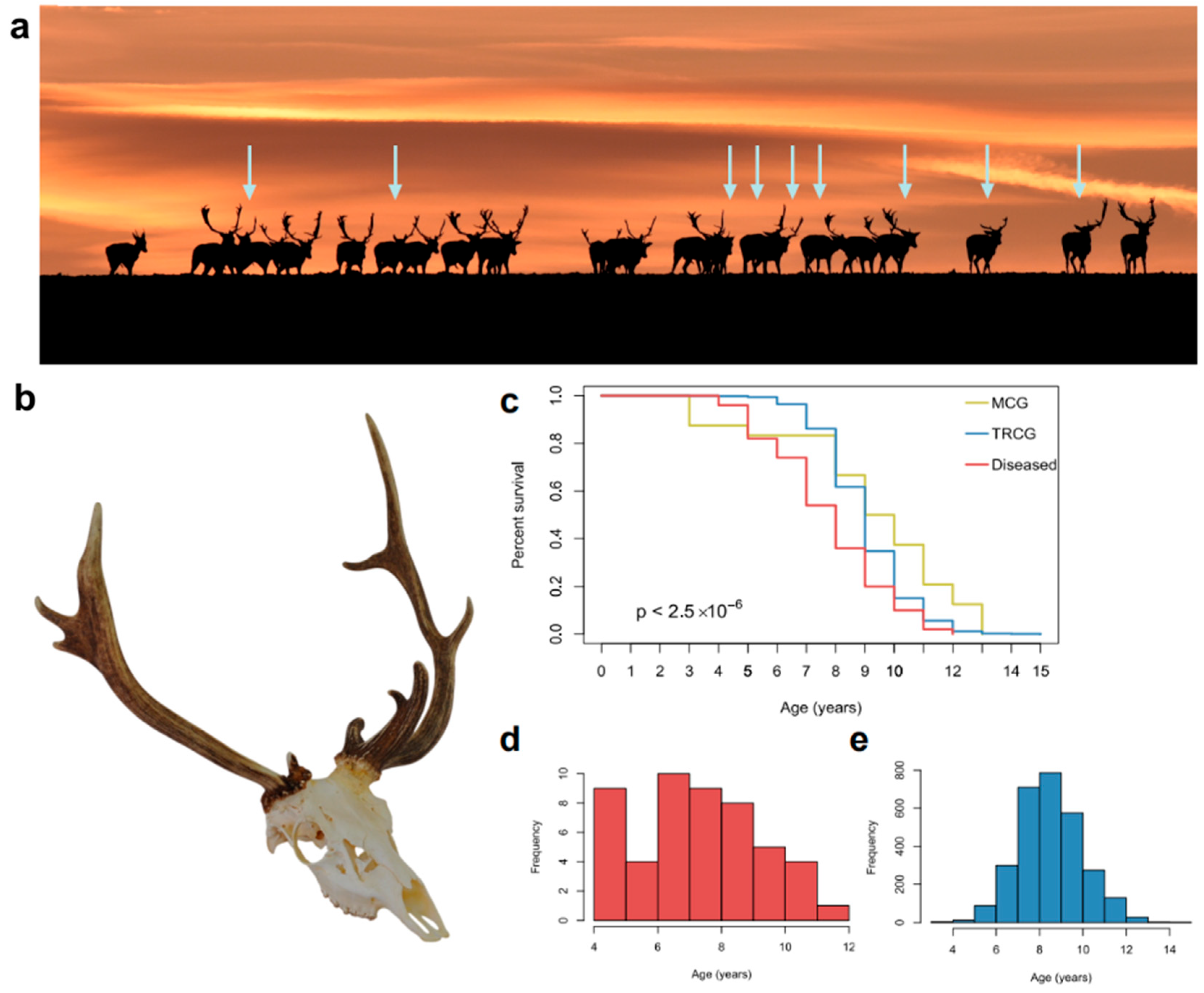
3.2. Health Condition of Fallow Deer (Dama dama) with Aberrant Trophies
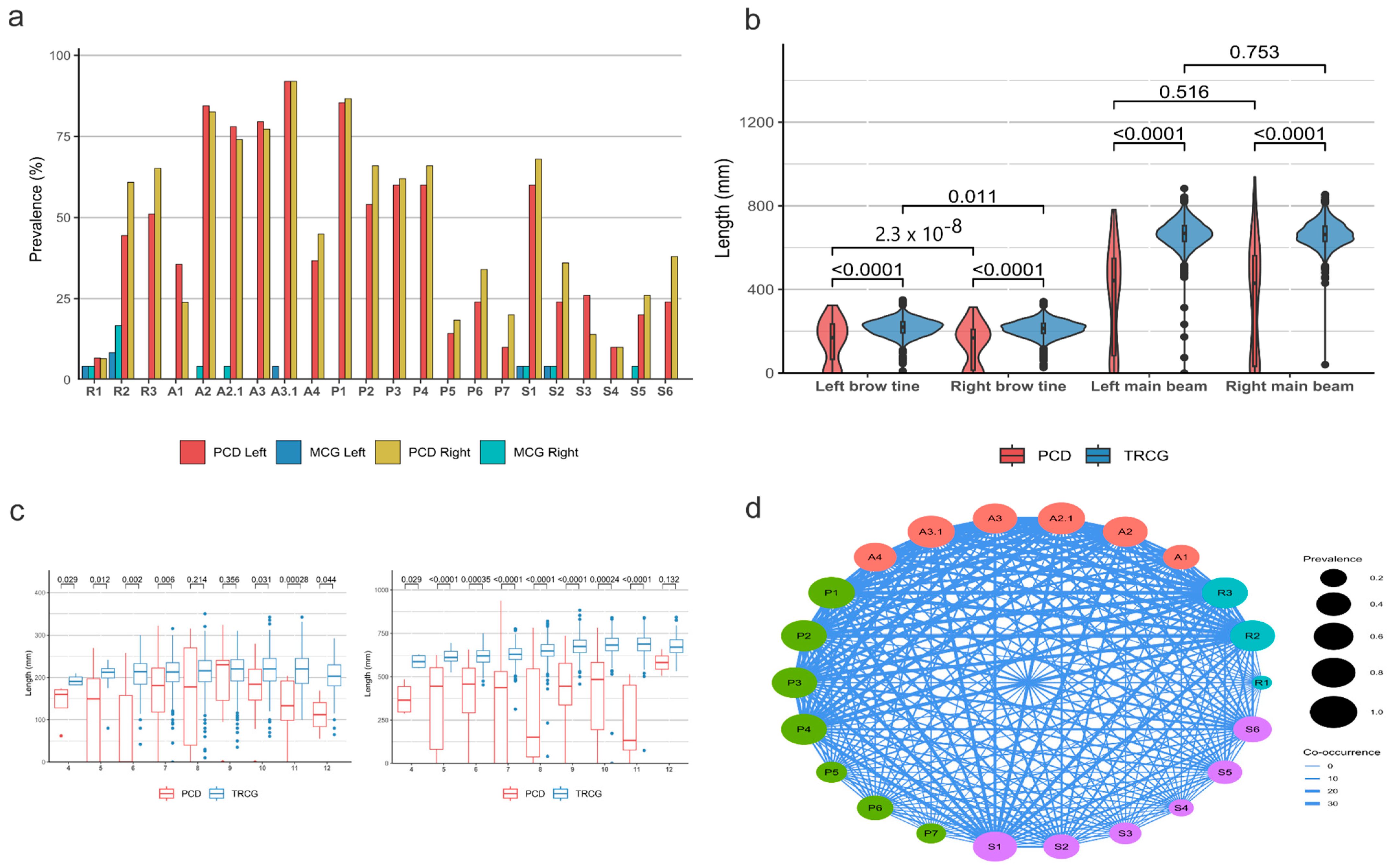
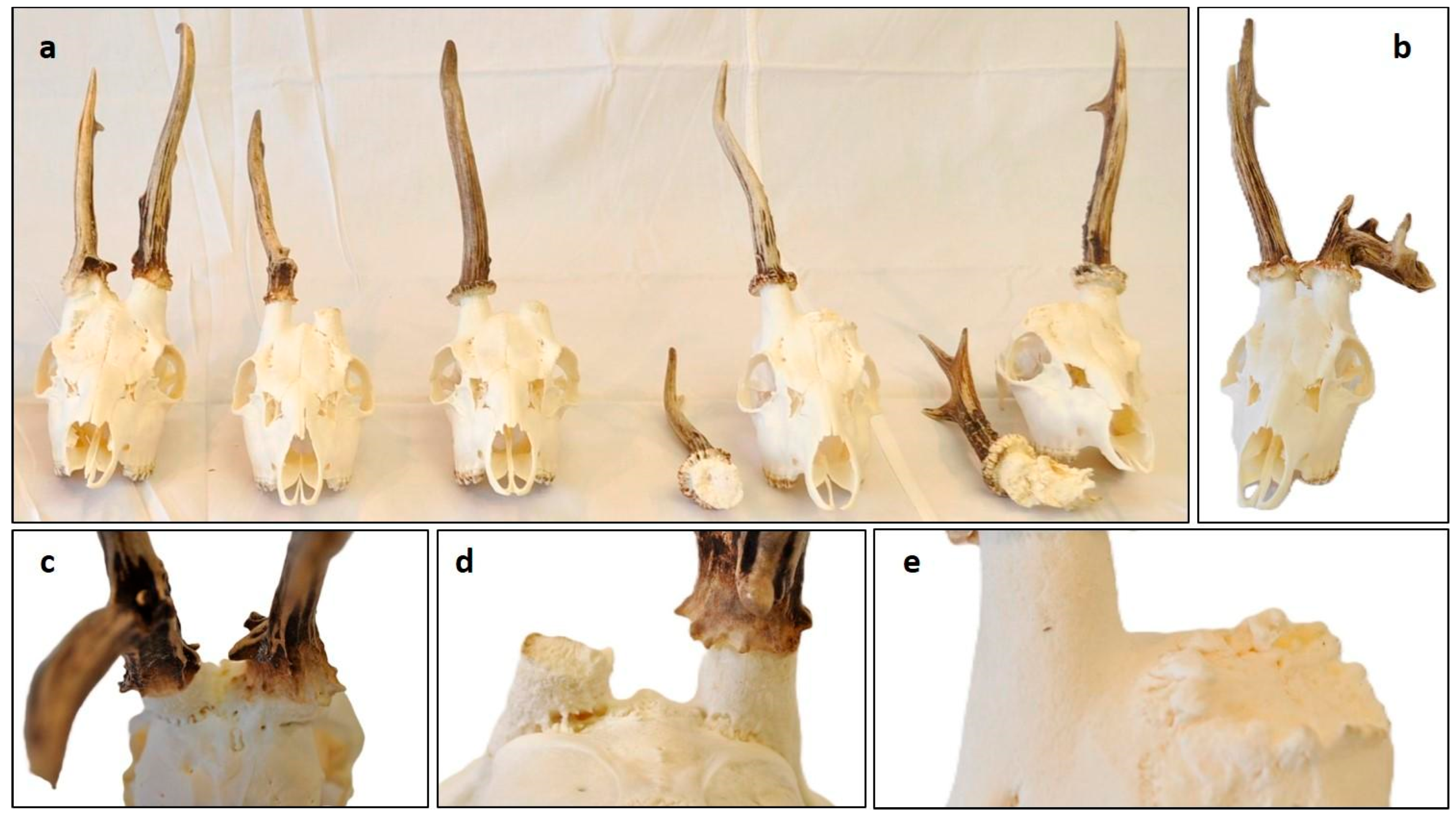
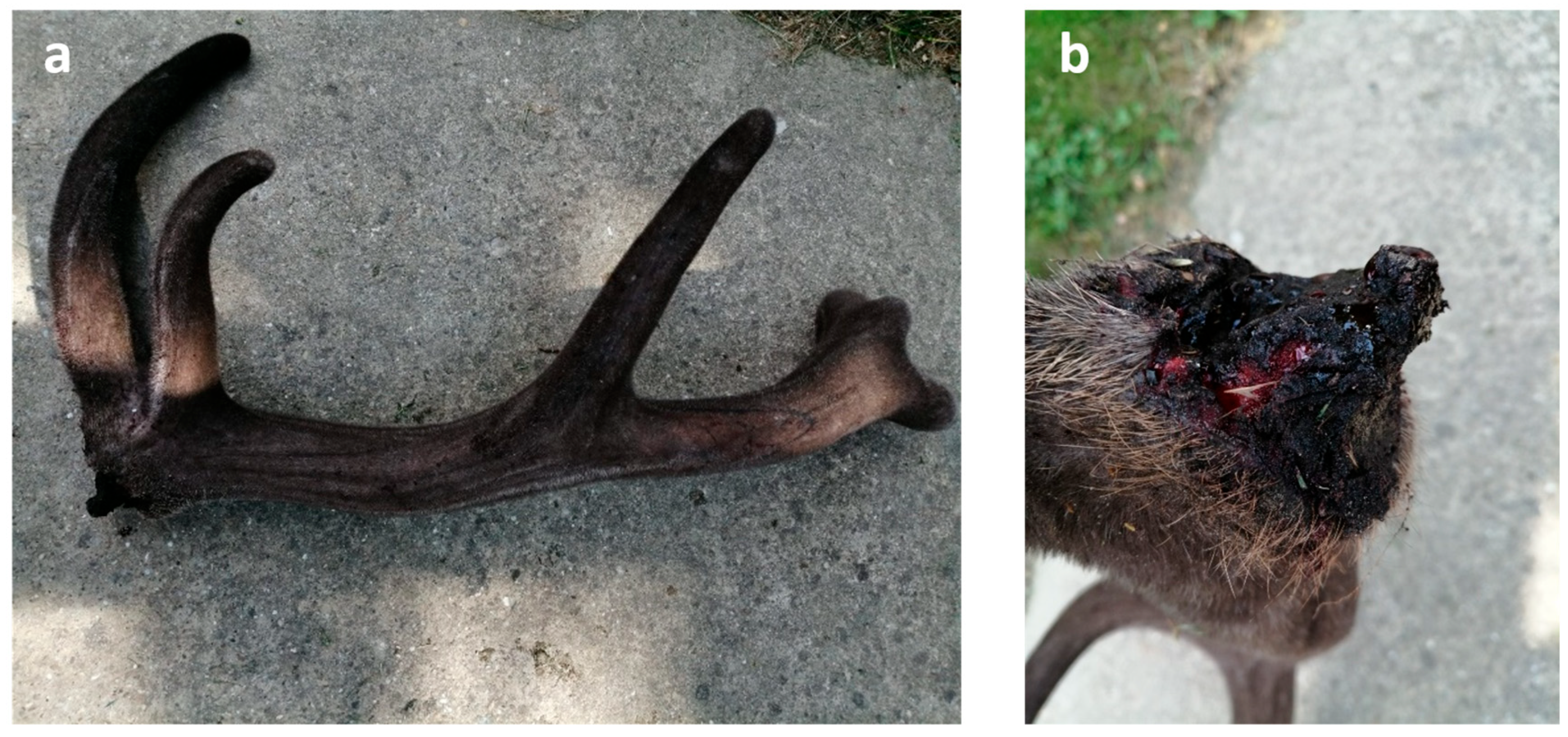
3.3. Characteristics of Fallow Deer (Dama dama) Aberrant Trophies
3.4. Antler Anomalies in Roe Deer (Capreolus capreolus) Trophies
3.5. Occurrence of RAPS Features in Red Deer (Cervus elaphus)
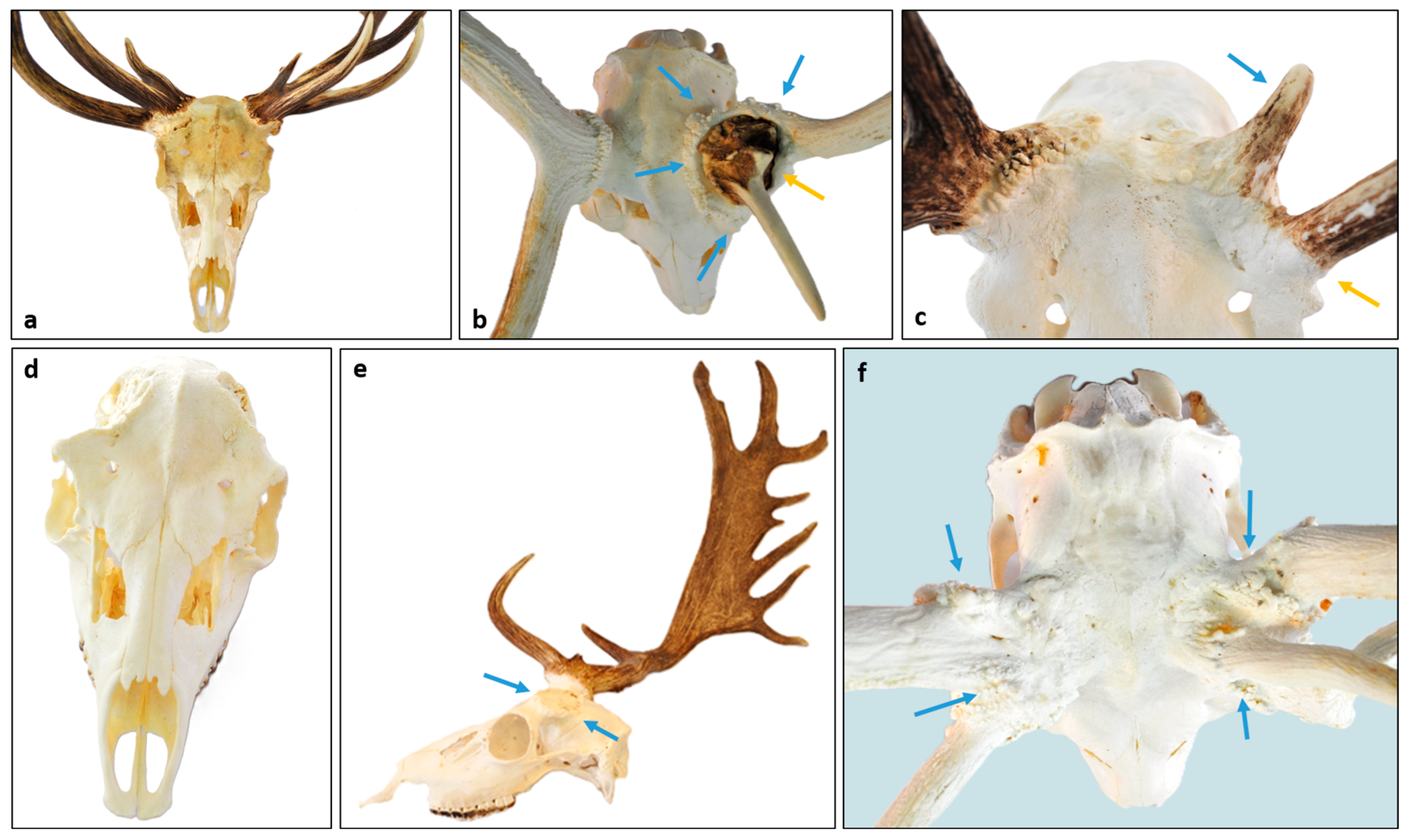
3.6. Considerations for RAPS Score Assessment
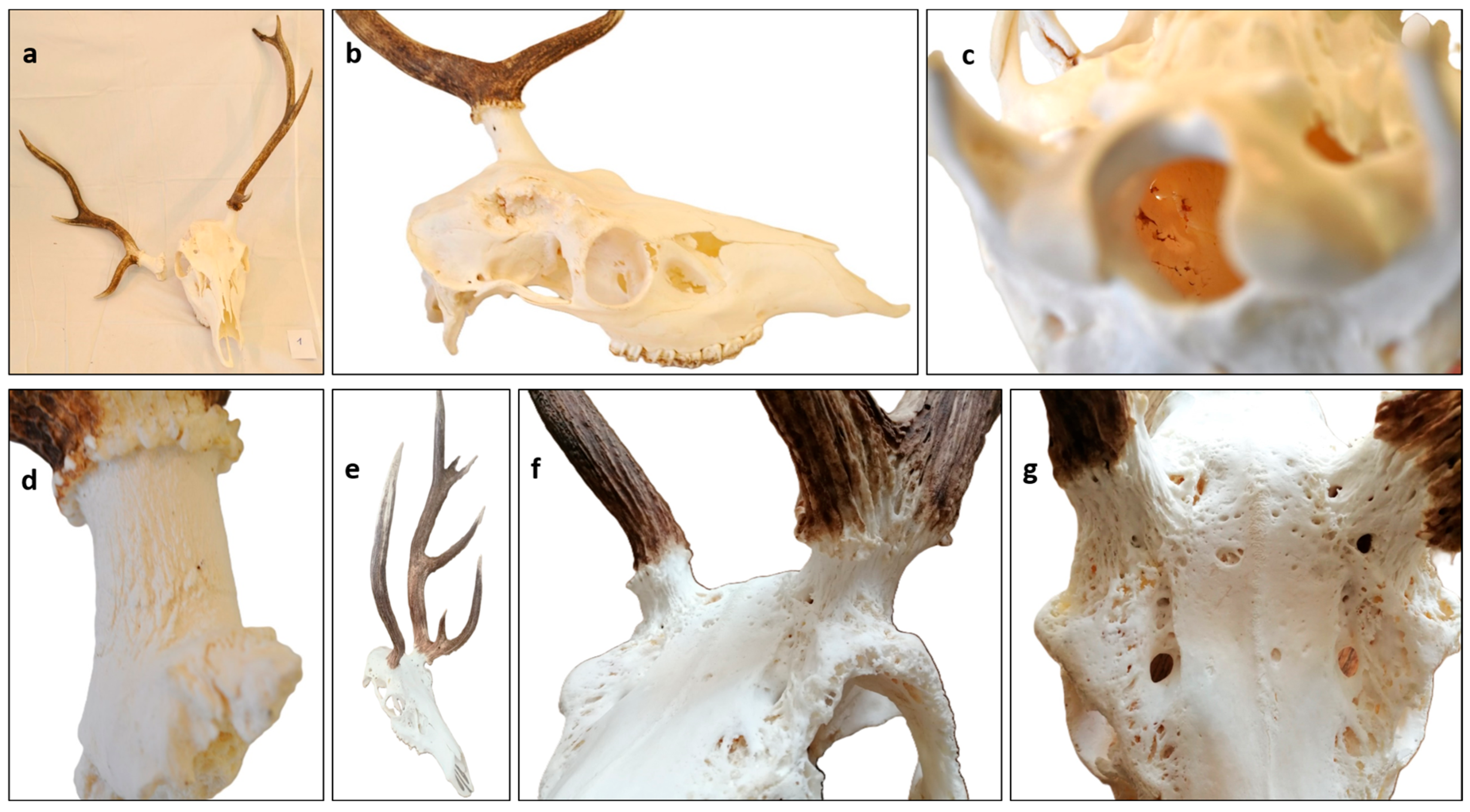
3.7. Healing of the Casting Wound
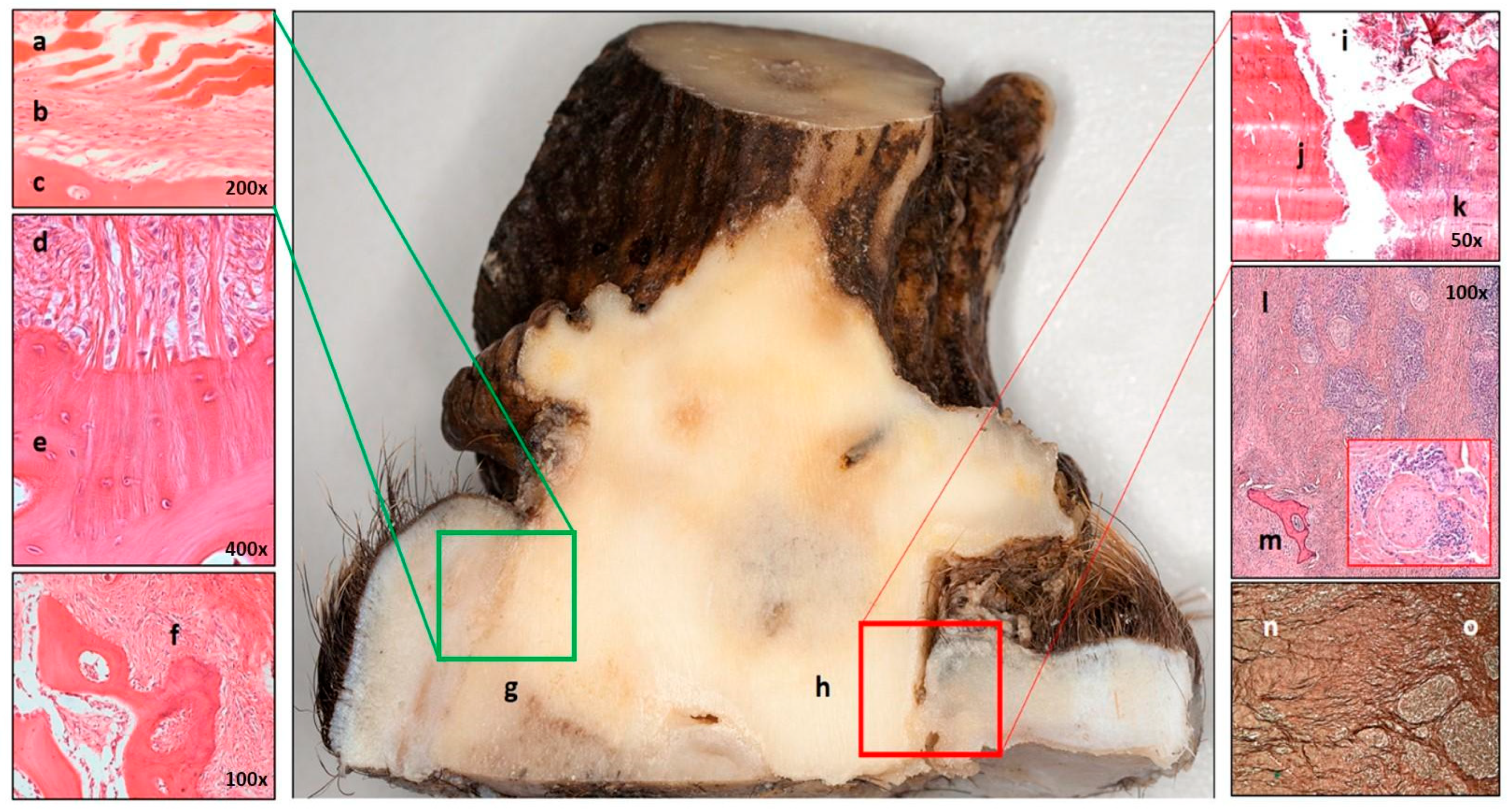
3.8. The Pedunculitis Chronica Deformans (PCD) Is a Consequence of Impaired Wound Healing
3.9. Peduncular–Dermal Junction (PDJ); Structural and Pathological Insights
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAB | aberrant antler beams |
| ASC | antlerogenic stem cell |
| B&C | Boone and Crockett Club |
| CC | Capreolus capreolus (roe deer) |
| CE | Cervus elaphus (red deer) |
| CIC | International Council for Game and Wildlife Conservation |
| CT | computed tomography |
| DD | Dama dama (fallow deer) |
| HTR | Hungarian trophy register |
| MCG | morphology control group |
| PCD | Pedunculitis Chronica Deformans |
| PDJ | peduncular–dermal junction |
| RAPS | rose, antler, pedicle, skull |
| SOOS | spike-on-one-side |
| TRCG | trophy register control group |
References
- Goss, R.J. Deer Antlers: Regeneration, Function, and Evolution; Academic Press: Cambridge, MA, USA, 1983; Available online: http://www.sciencedirect.com:5070/book/9780122930805/deer-antlers (accessed on 18 May 2025).
- Landete-Castillejos, T.; Currey, J.; Estevez, J.; Fierro, Y.; Calatayud, A.; Ceacero, F.; Garcia, A.; Gallego, L. Do drastic weather effects on diet influence changes in chemical composition, mechanical properties and structure in deer antlers? Bone 2010, 47, 815–825. [Google Scholar] [CrossRef] [PubMed]
- ‘One in a Million’ Three-Antler Deer Spotted in US-BBC News. Available online: https://www.bbc.com/news/world-us-canada-50440102 (accessed on 12 September 2022).
- Rörig, A. Über Geweihentwickelung und Geweihbildung. Arch. Für Entwicklungsmechanik Org. 1900, 10, 618–644. [Google Scholar] [CrossRef]
- Robinette, W.L.; Jones, D.A. Antler Anomalies of Mule Deer. J. Mammal. 1959, 40, 96–108. [Google Scholar] [CrossRef]
- Rachlow, J.L.; Lee, R.M.; Riley, R.K. Abnormal Antlers and Pedicles on Rocky Mountain Elk in Northern Arizona. Southwest. Nat. 2003, 48, 147–153. [Google Scholar] [CrossRef]
- Karns, G.R.; Ditchkoff, S.S. Trauma-induced malformed antler development in male white-tailed deer. Wildl. Soc. Bull. 2013, 37, 832–837. [Google Scholar] [CrossRef]
- Karns, G.R.; Ditchkoff, S.S. Antler Breakage Patterns in White-tailed Deer. J. Southeast. Assoc. Fish Wildl. Agencies 2012, 66, 114–119. [Google Scholar]
- Kierdorf, U.; Flohr, S.; Gomez, S.; Landete-Castillejos, T.; Kierdorf, H. The structure of pedicle and hard antler bone in the European roe deer (Capreolus capreolus): A light microscope and backscattered electron imaging study. J. Anat. 2013, 223, 364–384. [Google Scholar] [CrossRef]
- Henshaw, J. Antlers—The Unbrittle Bones of Contention. Nature 1971, 231, 469. [Google Scholar] [CrossRef]
- Currey, J.D.; Landete-Castillejos, T.; Estevez, J.; Ceacero, F.; Olguin, A.; Garcia, A.; Gallego, L. The mechanical properties of red deer antler bone when used in fighting. J. Exp. Biol. 2009, 212, 3985–3993. [Google Scholar] [CrossRef]
- Kaufman, D.W.; Kaufman, G.A. Extreme Antler Malformation in a Mule Deer (Odocoileus hemionus) in Northcentral Kansas. Trans. Kans. Acad. Sci. 2019, 122, 275–278. [Google Scholar] [CrossRef]
- Faragó, S.; Köller, J.; Zoltán, A. Természeti-vadászati örökségünk. In A Legkiválóbb Vadásztrófeák; Nimród Vadászújság: Budapest, Hungary, 2009. [Google Scholar]
- Nimród-Mi Lesz Veled, Lábodi Dám?-Napjaink Dámgazdálkodási Problémái a SEFAG Zrt. Területén-Nimród-What Will Happen to You, the Fallow Deer of Labod?-The Problems of Fallow Deer Management in the Area of SEFAG LTD. Available online: https://nimrod.hu/hirek/milesz (accessed on 3 October 2023).
- Gál, J.; Janosi, K.; Marosan, M.; Sugár, L. Purulent inflammation of the basis of antler in fallow deer (Dama dama) caused by Staphylococcus xylosus. Magy. Allatorvosok Lapja 2011, 133, 161–164. [Google Scholar]
- Baumann, C.D.; Davidson, W.R.; Roscoe, D.E.; Beheler-Amass, K. Intracranial abscessation in white-tailed deer of North America. J. Wildl. Dis. 2001, 37, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.S.; Belser, E.H.; Killmaster, C.H.; Bowers, J.W.; Irwin, B.J.; Yabsley, M.J.; Miller, K.V. Epizootiology of Cranial Abscess Disease in White-Tailed Deer (Odocoileus virginianus) of Georgia, USA. J. Wildl. Dis. 2015, 51, 609–618. [Google Scholar] [CrossRef]
- Timmons, J.; Shaub, M.; Scherer, L.; Gereg, I.; Maxwell, L.; Potts, L.; Stevens, M.; Vile, M.; Miller, E.A.; Niedringhaus, K.D. Pathologic Findings of Cranial Abscesses Involving the Pituitary Gland in Free-Ranging White-Tailed Deer (Odocoileus virginianus) in Pennsylvania. Animals 2025, 15, 409. [Google Scholar] [CrossRef]
- Karns, G.R.; Lancia, R.A.; Deperno, C.S.; Conner, M.C.; Stoskopf, M.K. Intracranial Abscessation as a Natural Mortality Factor for Adult Male White-Tailed Deer (Odocoileus virginianus) in Kent County, Maryland, USA. J. Wildl. Dis. 2009, 45, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R.J.; Drew, K.R.; Baskin, L.M. (Eds.) Wildlife Production Systems: Economic Utilisation of Wild Ungulates, 1st ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Behaviour and Management of European Ungulates: Professor Marco Apollonio and Professor Rory Putman: 978-184995-122-7-Whittles Publishing. Available online: https://www.whittlespublishing.com/Behaviour_and_Management_of_European_Ungulates (accessed on 6 May 2025).
- National Game Management Database. Available online: http://www.ova.info.hu/index-en.html (accessed on 10 May 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 5 February 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Use R! Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Kassambara, A. ggpubr: “ggplot2” Based Publication Ready Plots; R Package Version 0.6.0; 2023. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 5 February 2025).
- Pedersen, T.L.; RStudio. ggraph: An Implementation of Grammar of Graphics for Graphs and Networks; R Package Version 2.2.1; 2024. Available online: https://cran.r-project.org/web/packages/ggraph/index.html (accessed on 5 February 2025).
- Kolde, R. pheatmap: Pretty Heatmaps; R Package Version 1.0.12; 2019. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 5 February 2025).
- Kierdorf, U.; Kierdorf, H.; Konjević, D. Pathological fracture of a red deer antler secondary to purulent inflammation—A case report. Vet. Arh. 2013, 83, 347–356. [Google Scholar]
- Davidson, W.R.; Nettles, V.F.; Hayes, L.E.; Howerth, E.W.; Couvillion, C.E. Epidemiologic features of an intracranial abscessation/suppurative meningoencephalitis complex in white-tailed deer. J. Wildl. Dis. 1990, 26, 460–467. [Google Scholar] [CrossRef]
- Cierny, G.; Mader, J.T.; Penninck, J.J. A clinical staging system for adult osteomyelitis. Clin. Orthop. 2003, 414, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, A.; Hofmann, G.O.; Krukemeyer, M.G.; Krenn, V.; Langwald, S. Histopathological Osteomyelitis Evaluation Score (HOES)—An innovative approach to histopathological diagnostics and scoring of osteomyelitis. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2014, 3, Doc08. [Google Scholar] [CrossRef]
- Shed Antlers: Different Pedicle Types|Every Spring Deer Shed Their Antlers and Many Avid Sportsmen Take to the Woods to Find Sheds. Here We Take an In-Depth Look at the Different Shapes of...|By MSU Deer LabFacebook|Facebook. Available online: https://www.facebook.com/msu.deerlab/videos/shed-antlers-different-pedicle-types/188785065871222/ (accessed on 2 June 2024).
- Bubenik, A.B. Epigenetical, Morphological, Physiological, and Behavioral Aspects of Evolution of Horns, Pronghorns, and Antlers. In Horns, Pronghorns, and Antlers: Evolution, Morphology, Physiology, and Social Significance; Bubenik, G.A., Bubenik, A.B., Eds.; Springer: New York, NY, USA, 1990; pp. 3–113. [Google Scholar] [CrossRef]
- Kierdorf, U.; Stoffels, E.; Stoffels, D.; Kierdorf, H.; Szuwart, T.; Clemen, G. Histological studies of bone formation during pedicle restoration and early antler regeneration in roe deer and fallow deer. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 273, 741–751. [Google Scholar] [CrossRef]
- Li, C.; Yang, F.; Sheppard, A. Adult stem cells and mammalian epimorphic regeneration-insights from studying annual renewal of deer antlers. Curr. Stem Cell Res. Ther. 2009, 4, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Sybenga, A.B.; Jupiter, D.C.; Speights, V.O.; Rao, A. Diagnosing Osteomyelitis: A Histology Guide for Pathologists. J. Foot Ankle Surg. 2020, 59, 75–85. [Google Scholar] [CrossRef]
- Li, C. Residual antler periosteum holds the potential to partially regenerate lost antler tissue. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2021, 335, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Suttie, J.M.; Clark, D.E. Morphological observation of antler regeneration in red deer (Cervus elaphus). J. Morphol. 2004, 262, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Agriculture and Natural Resources Marketing. Deer Ecology & Management Lab. Available online: https://www.msudeer.msstate.edu/ (accessed on 8 January 2025).
- Kierdorf, U.; Kierdorf, H. Deer antlers—A model of mammalian appendage regeneration: An extensive review. Gerontology 2011, 57, 53–65. [Google Scholar] [CrossRef]
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 1998, 176, 26S–38S. [Google Scholar] [CrossRef]
- Jonathan Sherratt’s Research: Scar Formation. Available online: https://www.macs.hw.ac.uk/~jas/researchinterests/scartissueformation.html (accessed on 18 May 2025).
- Cumming, B.D.; McElwain, D.L.S.; Upton, Z. A mathematical model of wound healing and subsequent scarring. J. R. Soc. Interface 2009, 7, 19–34. [Google Scholar] [CrossRef]
- Li, C.; Suttie, J.M.; Clark, D.E. Histological examination of antler regeneration in red deer (Cervus elaphus). Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2005, 282A, 163–174. [Google Scholar] [CrossRef]
- Landete-Castillejos, T.; Kierdorf, H.; Gomez, S.; Luna, S.; García, A.; Cappelli, J.; Pérez-Serrano, M.; Pérez-Barbería, J.; Gallego, L.; Kierdorf, U. Antlers-Evolution, development, structure, composition, and biomechanics of an outstanding type of bone. Bone 2019, 128, 115046. [Google Scholar] [CrossRef]
- Newman, B.A.; D’Angelo, G.J. A Review of Cervidae Visual Ecology. Animals 2024, 14, 420. [Google Scholar] [CrossRef]
- Széchenyi, Z. A Szarvas Selejtezése (Selective Culling of Red Deer). Régi Vadászkönyvek. Available online: https://www.vadaszkonyv.com/szechenyi-zsigmond-a-szarvas-selejtezese/ (accessed on 3 January 2025).
- Pathology Outlines-Osteomyelitis Overview. Available online: https://www.pathologyoutlines.com/topic/mandiblemaxillaosteomyelitis.html (accessed on 1 July 2024).
- Kierdorf, U.; Kierdorf, H. Roe and red deer antlers as bioindicators of pollution of deer. Vet Arh. 2006, 76, S117–S129. [Google Scholar]
- Ludolphy, C.; Kierdorf, U.; Kierdorf, H. Lead concentrations in antlers of European roe deer (Capreolus capreolus) from an agricultural area in Northern Germany over a 119-year period—A historical biomonitoring study. Environ. Sci. Pollut. Res. 2021, 28, 56069–56078. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, I.; Babarczi, B.; Molnár, Z.; Tóth, A.; Skoda, G.; Horváth, G.F.; Horváth, A.; Tóth, D.; Sükösd, F.; Szemethy, L.; et al. First Results on the Presence of Mycotoxins in the Liver of Pregnant Fallow Deer (Dama dama) Hinds and Fetuses. Animals 2024, 14, 1039. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A.; Bosshardt, D.D. Structure of periodontal tissues in health and disease. Periodontology 2000 2006, 40, 11–28. [Google Scholar] [CrossRef]
- Harris, H.A. Bone Formation and the Osteoblast. Lancet 1928, 212, 489–493. [Google Scholar] [CrossRef]
- Li, C.; Suttie, J.M. Tissue collection methods for antler research. Eur. J. Morphol. 2003, 41, 23–30. [Google Scholar] [CrossRef]
- Li, C.; Yang, F.; Li, G.; Gao, X.; Xing, X.; Wei, H.; Deng, X.; Clark, D.E. Antler regeneration: A dependent process of stem tissue primed via interaction with its enveloping skin. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2007, 307, 95–105. [Google Scholar] [CrossRef]

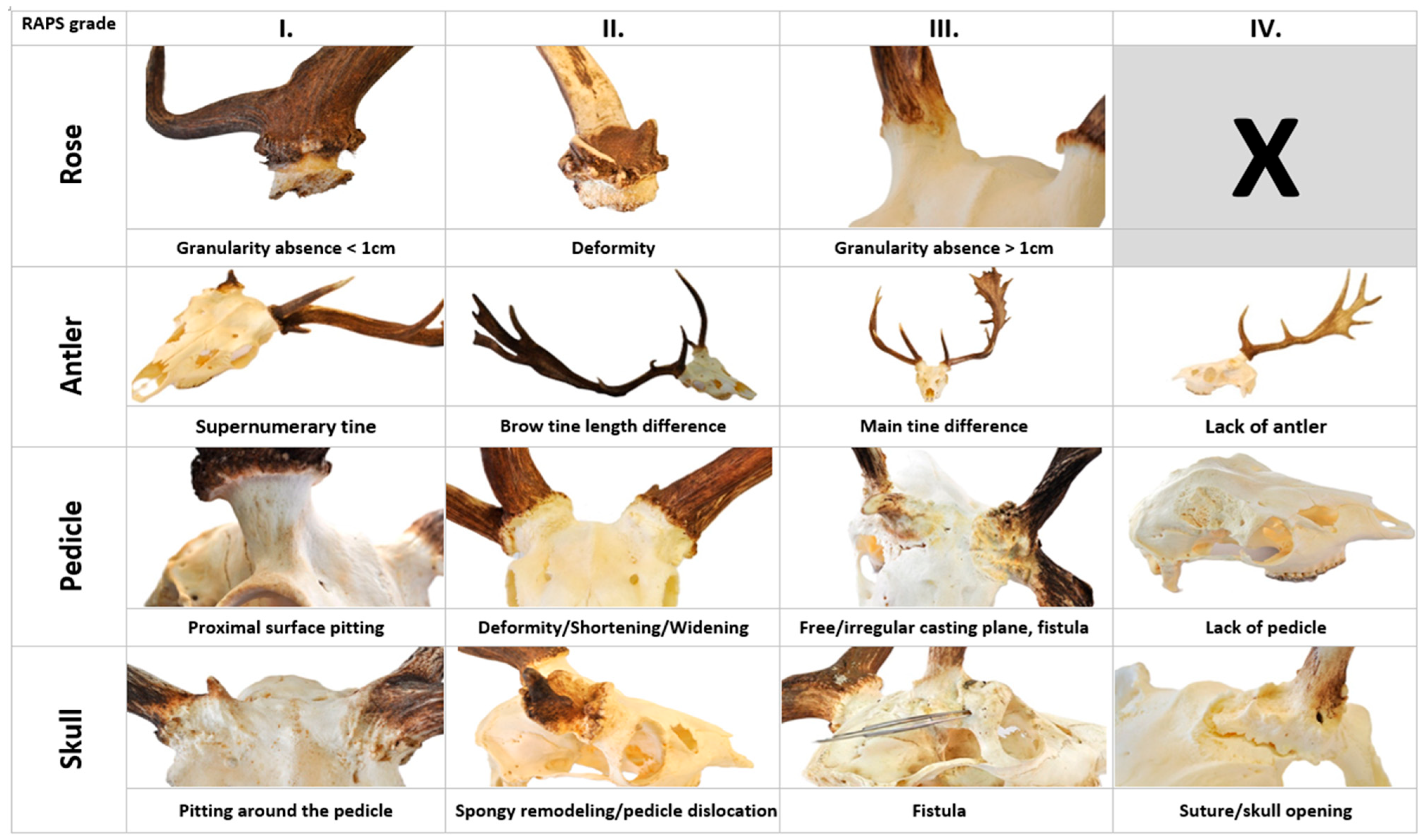

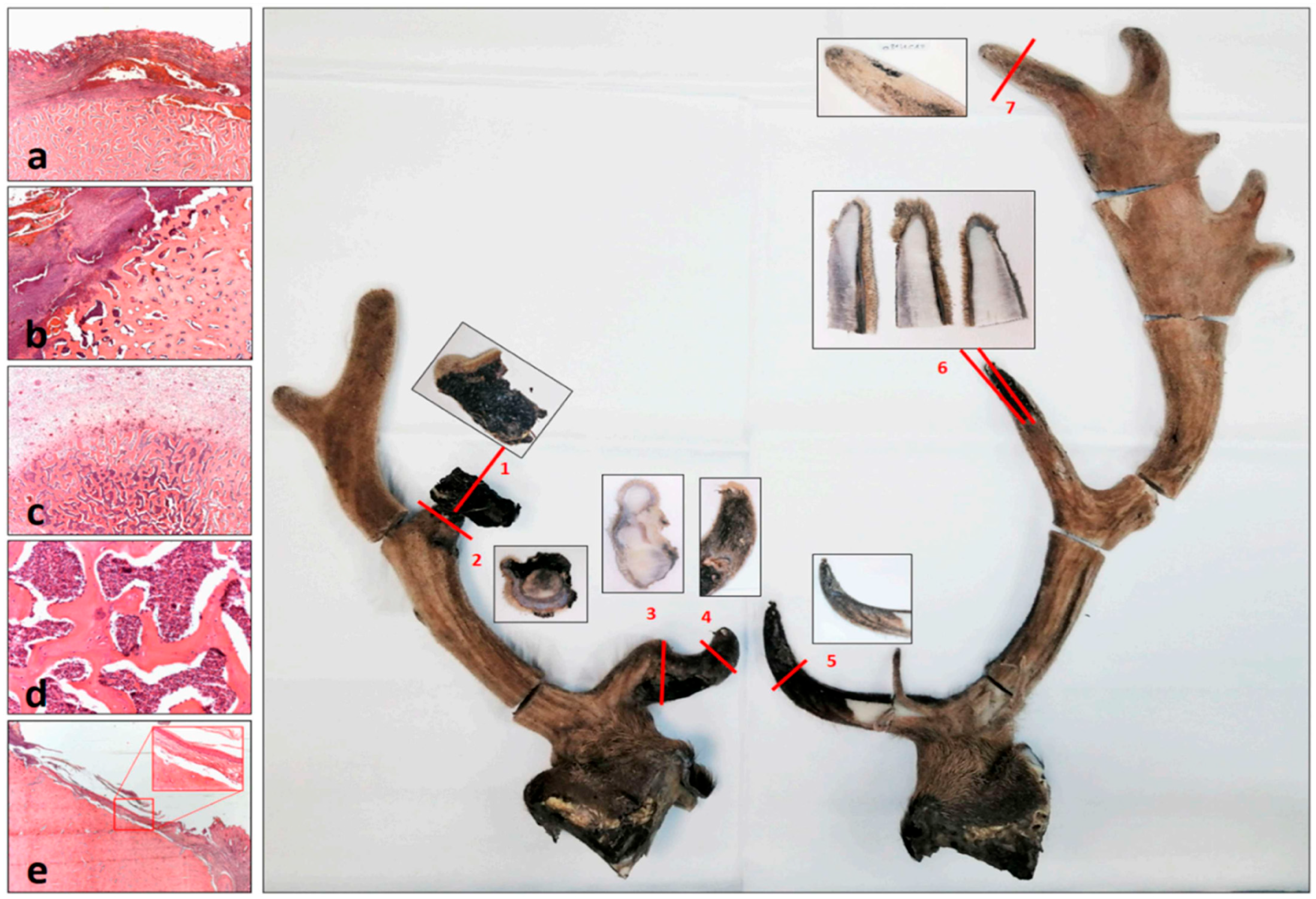

| Rose | R1 | Lack of continuity in the grain of the rose is longer than 5 mm but less than 1 cm. |
| R2 | Rose deformity (also visible on cast antlers). | |
| R3 | Loss of rose granularity is greater than 1 cm. | |
| Antler | A1 | Supernumerary tine originating from the rose can be a sign of an extra antler bud. |
| A2 | Visually assessed length asymmetry between the left and right brow tines. | |
| A2.1 | Difference from trophy register control group (TRCG), which is derived from data of the Hungarian Trophy Register after exclusions of cases marked abnormal. | |
| A3 | Visually determined asymmetry in the main beam length between the two sides. | |
| A3.1 | Difference relative to the TRCG. | |
| A4 | Absence of antlers or aberrant antler beams. | |
| Pedicle | P1 | Pitting on the proximal surface of the pedicle. |
| P2 | The pedicle deformity characteristic of PCD in Fallow deer (Dama dama) is the deviation from the regular circle diameter more than 4 mm, also visible with the eye. | |
| P3 | Shortening of the pedicle more than physiological. | |
| P4 | Widening of the pedicle beyond the normal extent. | |
| P5 | Peduncle fistula. | |
| P6 | Free casting plane from which does not protrude any antler–bony protuberance. | |
| P7 | More than fifty percent of the casting plane of the pedicle is in or below the plane of the skull, which is considered to be the absence of it. | |
| Skull | S1 | The pitting extends on the skull surface. |
| S2 | Spongy remodelation of skull bones where the cavities are larger than at intertrabecular spaces in spongious bone. | |
| S3 | Discohesion along the interosseous suture. | |
| S4 | Separation of the cortical bones is an independent sign of bone displacement. | |
| S5 | A fistula mouth opens distant from the pedicle on the skull. | |
| S6 | The center of the pedicle has shifted significantly, mostly towards the brow ridge. |
| Grade I | R1, A1, P1, S1 |
| Grade II | P2, R2, A2, A2.1, P2, P3, P4, S2, S6 |
| Grade III | R3, A3, A3.1, P5, P6, S4 |
| Grade IV | A4, P7, S3, S4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sükösd, F.; Lakatos, I.; Ürmös, Á.; Karkas, R.; Sükösd, Á.; Palánki, G.; Arany Tóth, A.; Erdélyi, K.; Misó, M.; Gőbölös, P.; et al. When Antlers Grow Abnormally: A Hidden Disease Behind Common Cervid Trophy Deformities, Introducing Pedunculitis Chronica Deformans. Animals 2025, 15, 1530. https://doi.org/10.3390/ani15111530
Sükösd F, Lakatos I, Ürmös Á, Karkas R, Sükösd Á, Palánki G, Arany Tóth A, Erdélyi K, Misó M, Gőbölös P, et al. When Antlers Grow Abnormally: A Hidden Disease Behind Common Cervid Trophy Deformities, Introducing Pedunculitis Chronica Deformans. Animals. 2025; 15(11):1530. https://doi.org/10.3390/ani15111530
Chicago/Turabian StyleSükösd, Farkas, István Lakatos, Ádám Ürmös, Réka Karkas, Ákos Sükösd, Gábor Palánki, Attila Arany Tóth, Károly Erdélyi, Mihály Misó, Péter Gőbölös, and et al. 2025. "When Antlers Grow Abnormally: A Hidden Disease Behind Common Cervid Trophy Deformities, Introducing Pedunculitis Chronica Deformans" Animals 15, no. 11: 1530. https://doi.org/10.3390/ani15111530
APA StyleSükösd, F., Lakatos, I., Ürmös, Á., Karkas, R., Sükösd, Á., Palánki, G., Arany Tóth, A., Erdélyi, K., Misó, M., Gőbölös, P., Posta, K., Kovács, F., Ferenczi, S., Horváth, G., Szemethy, L., & Szőke, Z. (2025). When Antlers Grow Abnormally: A Hidden Disease Behind Common Cervid Trophy Deformities, Introducing Pedunculitis Chronica Deformans. Animals, 15(11), 1530. https://doi.org/10.3390/ani15111530







