Circadian Rhythm of Body Color Change in the Juvenile Chinese Giant Salamander (Andrias davidianus) Under Different Photoperiods
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experiments
2.3. Statistical Analysis
3. Results
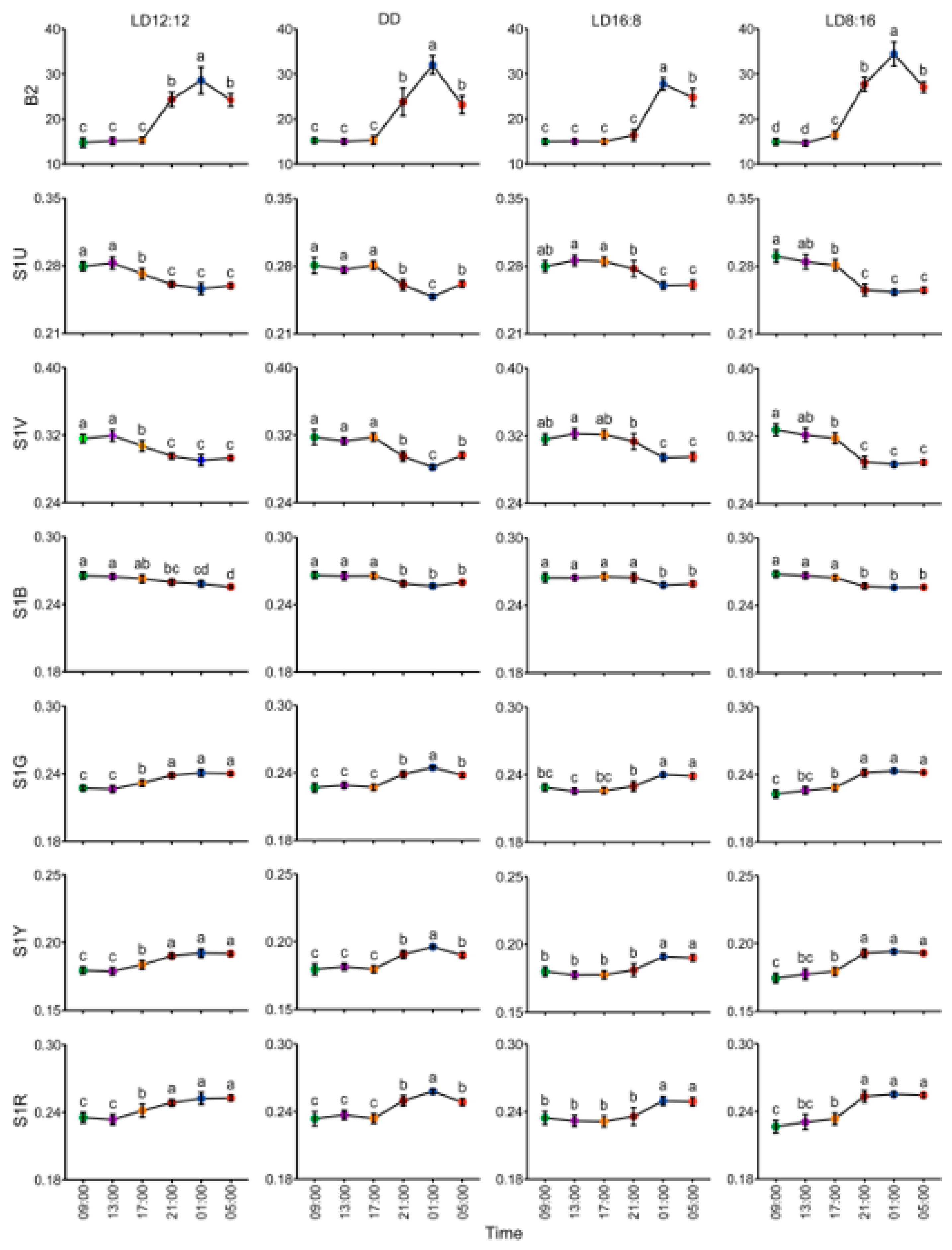
3.1. Spectral Characteristics of Dorsal Skin of Juvenile A. davidianus Under Different Photoperiods
3.2. Body Color Change in Juvenile A. davidianus Under Different Photoperiods
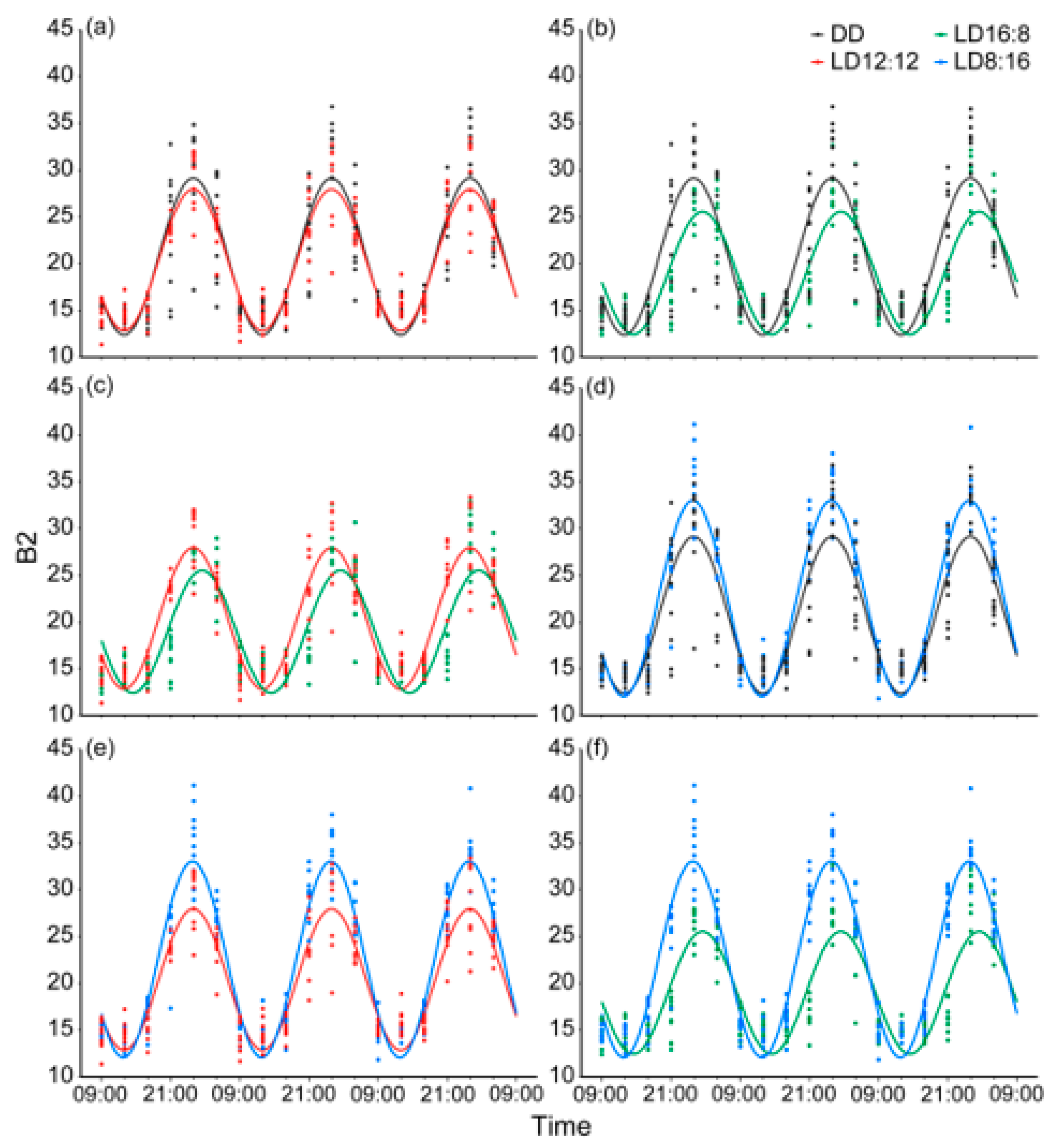
3.3. Circadian Rhythm and Differences in Body Color Change in Juvenile A. davidianus Under Different Photoperiods
4. Discussion
4.1. Characteristics and Mechanisms of Circadian Rhythm of Body Color Change in Juvenile A. davidianus
4.2. Function of Circadian Rhythm of Body Color Changes in Juvenile A. davidianus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauer, R.T. Coloration, Color Change, and Camouflage. In Shrimps: Their Diversity, Intriguing Adaptations and Varied Lifestyles; Springer International Publishing: Cham, Switzerland, 2023; pp. 247–285. [Google Scholar] [CrossRef]
- Shook, E.N.; Barlow, G.T.; Garcia-Rosales, D.; Gibbons, C.J.; Montague, T.G. Dynamic skin behaviors in cephalopods. Curr. Opin. Neurobiol. 2024, 86, 102876. [Google Scholar] [CrossRef] [PubMed]
- Sköld, H.N.; Aspengren, S.; Cheney, K.L.; Wallin, M. Chapter Four-Fish Chromatophores—From Molecular Motors to Animal Behavior. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: San Diego, CA, USA, 2016; Volume 321, pp. 171–219. [Google Scholar]
- Vissio, P.G.; Darias, M.J.; Di Yorio, M.P.; Pérez Sirkin, D.I.; Delgadin, T.H. Fish skin pigmentation in aquaculture: The influence of rearing conditions and its neuroendocrine regulation. General. Comp. Endocrinol. 2021, 301, 113662. [Google Scholar] [CrossRef] [PubMed]
- Rojas, B.; Lawrence, J.P.; Márquez, R. Amphibian Coloration: Proximate Mechanisms, Function, and Evolution. In Evolutionary Ecology of Amphibians; CRC Press: Boca Raton, FL, USA, 2023; pp. 219–258. [Google Scholar]
- Goda, M.; Kuriyama, T. Physiological and Morphological Color Changes in Teleosts and in Reptiles. In Pigments, Pigment Cells and Pigment Patterns; Hashimoto, H., Goda, M., Futahashi, R., Kelsh, R., Akiyama, T., Eds.; Springer: Singapore, 2021; pp. 387–423. [Google Scholar] [CrossRef]
- Darnell, M.Z. Ecological physiology of the circadian pigmentation rhythm in the fiddler crab Uca panacea. J. Exp. Mar. Biol. Ecol. 2012, 426–427, 39–47. [Google Scholar] [CrossRef]
- Vaissi, S.; Parto, P.; Sharifi, M. Ontogenetic changes in spot configuration (numbers, circularity, size and asymmetry) and lateral line in Neurergus microspilotus (Caudata: Salamandridae). Acta Zool. 2018, 99, 9–19. [Google Scholar] [CrossRef]
- Bueno-Villafañe, D.; Caballero-Gini, A.; Ferreira, M.; Netto, F.; Ríos, D.F.; Brusquetti, F. Ontogenetic changes in the ventral colouration of post metamorphic Elachistocleis haroi Pereyra, Akmentins, Laufer, Vaira, 2013 (Anura: Microhylidae). Amphib.-Reptil. 2020, 41, 191–200. [Google Scholar] [CrossRef]
- Casas-Cardona, S.; Márquez, R.; Vargas-Salinas, F. Different colour morphs of the poison frog Andinobates bombetes (Dendrobatidae) are similarly effective visual predator deterrents. Ethology 2018, 124, 245–255. [Google Scholar] [CrossRef]
- Sztatecsny, M.; Preininger, D.; Freudmann, A.; Loretto, M.-C.; Maier, F.; Hödl, W. Don’t get the blues: Conspicuous nuptial colouration of male moor frogs (Rana arvalis) supports visual mate recognition during scramble competition in large breeding aggregations. Behav. Ecol. Sociobiol. 2012, 66, 1587–1593. [Google Scholar] [CrossRef]
- Gardner, K.M.; Mennill, D.J.; Newman, A.E.M.; Doucet, S.M. Social and physiological drivers of rapid colour change in a tropical toad. General. Comp. Endocrinol. 2020, 285, 113292. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, E.; Beltran, J.; Márquez, R. Melanophore metachrosis response in amphibian tadpoles: Effect of background colour, light and temperature. Amphib.-Reptil. 2020, 42, 133–140. [Google Scholar] [CrossRef]
- Laumeier, R.; Brändle, M.; Rödel, M.-O.; Brunzel, S.; Brandl, R.; Pinkert, S. The global importance and interplay of colour-based protective and thermoregulatory functions in frogs. Nat. Commun. 2023, 14, 8117. [Google Scholar] [CrossRef]
- Camargo, C.R.; Visconti, M.A.; Castrucci, A.M.d.L. Physiological color change in the bullfrog, Rana catesbeiana. J. Exp. Zool. 1999, 283, 160–169. [Google Scholar] [CrossRef]
- Cadena, V.; Rankin, K.; Smith, K.R.; Endler, J.A.; Stuart-Fox, D. Temperature-induced colour change varies seasonally in bearded dragon lizards. Biol. J. Linn. Soc. 2017, 123, 422–430. [Google Scholar] [CrossRef]
- Wendy, H.W.; John, B.P. Seasonal Color Change in a Population of Pacific Treefrogs (Pseudacris regilla). J. Herpetol. 2005, 39, 161–165. [Google Scholar] [CrossRef]
- Schwartz, W.J.; Daan, S. Origins: A Brief Account of the Ancestry of Circadian Biology. In Biological Timekeeping: Clocks, Rhythms and Behaviour; Kumar, V., Ed.; Springer: New Delhi, India, 2017; pp. 3–22. [Google Scholar] [CrossRef]
- Oishi, T.; Kiyoko, N.; Yumiko, H.; Mayumi, N.; Masumi, O.; Emi, K.; Tamotsu, S. Circadian Rhythms in Amphibians and Reptiles: Ecological Implications. Biol. Rhythm Res. 2004, 35, 105–120. [Google Scholar] [CrossRef]
- Miwa, T. Diel activity patterns during autumn migration to hibernation and breeding sites in a Japanese explosive breeding frog Rana sakuraii. Herpetol. J. 2017, 27, 173–180. [Google Scholar]
- Krishna, M.P.; Sreepada, K.S.; Sridhar, K.R.; Hemachandra, A. Diurnal Periodicity of the Pond Frogs in the Western Ghats of India. Proc. Zool. Soc. 2019, 72, 290–300. [Google Scholar] [CrossRef]
- Prakash, A.; Gower, D.J.; Vengot, R.; Kotharambath, R. Circadian rhythm and surface activity in soil-dwelling caecilians (Amphibia: Gymnophiona). Sci. Rep. 2024, 14, 9950. [Google Scholar] [CrossRef]
- Granato, F.C.; Tironi, T.S.; Maciel, F.E.; Rosa, C.E.; Vargas, M.A.; Nery, L.E.M. Circadian rhythm of pigment migration induced by chromatrophorotropins in melanophores of the crab Chasmagnathus granulata. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 138, 313–319. [Google Scholar] [CrossRef]
- Stevens, M.; Rong, C.P.; Todd, P.A. Colour change and camouflage in the horned ghost crab cypode ceratophthalmus. Biol. J. Linn. Soc. 2013, 109, 257–270. [Google Scholar] [CrossRef]
- Andreazzoli, M.; Angeloni, D. The Amphibian Clock System. In Biological Timekeeping: Clocks, Rhythms and Behaviour; Kumar, V., Ed.; Springer: New Delhi, India, 2017; pp. 211–222. [Google Scholar] [CrossRef]
- Filadelfi, A.M.C.; Vieira, A.; Louzada, F.M. Circadian rhythm of physiological color change in the amphibian Bufo ictericus under different photoperiods. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 142, 370–375. [Google Scholar] [CrossRef]
- Alfakih, A.; Watt, P.J.; Nadeau, N.J. The physiological cost of colour change: Evidence, implications and mitigations. J. Exp. Biol. 2022, 225, jeb210401. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.K.; Li, H.; Wang, Z.; Hime, P.M.; McMillan, A.; Wu, M.; Diaz, R.; Hongxing, Z.; McGinnity, D.; Briggler, J.T. The giant salamanders (Cryptobranchidae): Part A. palaeontology, phylogeny, genetics, and morphology. Amphib. Reptile Conserv. 2012, 5, 17–29. [Google Scholar]
- Yan, F.; Lü, J.; Zhang, B.; Yuan, Z.; Zhao, H.; Huang, S.; Wei, G.; Mi, X.; Zou, D.; Xu, W.; et al. The Chinese giant salamander exemplifies the hidden extinction of cryptic species. Curr. Biol. 2018, 28, R590–R592. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.-Q.; Chen, W.-T.; Wang, D.-Q.; Zhang, S.-H.; Wang, C.-R.; He, S.-P.; Wu, Y.-A.; He, P.; Xie, J.; Li, C.-W.; et al. Phylogeographic patterns and conservation implications of the endangered Chinese giant salamander. Ecol. Evol. 2019, 9, 3879–3890. [Google Scholar] [CrossRef]
- Turvey, S.T.; Marr, M.M.; Barnes, I.; Brace, S.; Tapley, B.; Murphy, R.W.; Zhao, E.; Cunningham, A.A. Historical museum collections clarify the evolutionary history of cryptic species radiation in the world’s largest amphibians. Ecol. Evol. 2019, 9, 10070–10084. [Google Scholar] [CrossRef]
- Blanchard, E. On a new gigantic salamander (Sieboldia Davidiana, Blanch.) from Western China. Ann. Mag. Nat. Hist. 1871, 8, 212–214. [Google Scholar] [CrossRef]
- Bòulenger, E.G. On a new Giant Salamander, living in the Society’s Gardens. Proc. Zool. Soc. Lond. 1924, 94, 173–174. [Google Scholar] [CrossRef]
- Chai, J.; Lu, C.-Q.; Yi, M.-R.; Dai, N.-H.; Weng, X.-D.; Di, M.-X.; Peng, Y.; Tang, Y.; Shan, Q.-H.; Wang, K.; et al. Discovery of a wild, genetically pure Chinese giant salamander creates new conservation opportunities. Zool. Res. 2022, 43, 469–480. [Google Scholar] [CrossRef]
- Gong, Y.A.; Xu, J.C.; Huang, S.; Huang, R.Y.; Li, J.Q.; Jiang, Y.Q.; Yang, D.C.; Yu, J.; Zhang, Y.; Li, W.J. A New Species of the Giant Salamander of the Genus Andrias from Qimeng, Anhui, China (Amphibia:Cryptorchiidae). Chin. J. Zool. 2023, 58, 651–657. [Google Scholar] [CrossRef]
- Zhang, K.J.; Wang, X.M.; Wu, W.; Wang, Z.H.; Huang, S. Advances in conservation biology of Chinese giant salamander. Biodivers. Sci. 2002, 10, 291–297. [Google Scholar]
- National Forestry and Grassland Administration of China. Official Release of the Updated List of Wild Animals under Special State Protection in China. Available online: https://www.forestry.gov.cn/main/5461/20210205/122418860831352.html (accessed on 8 February 2021).
- Liang, G.; Geng, B.; Zhao, E. Andrias davidianus. In The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2004. [Google Scholar]
- Fei, L.; Ye, C.Y.; Jiang, J.P. Amphibians of China and Their Distributions: A Colored Atlas; Sichuan Science and Technology Press: Chengdu, China, 2012; p. 77. [Google Scholar]
- Liang, Z.Q. Conservation Biology Research of the Chinese Giant Salamander (Andrias davidianus). Ph.D. Thesis, Southwest University, Chongqing, China, 2015. [Google Scholar]
- Endler, J.A. On the measurement and classification of colour in studies of animal colour patterns. Biol. J. Linn. Soc. 1990, 41, 315–352. [Google Scholar] [CrossRef]
- Maia, R.; Gruson, H.; Endler, J.A.; White, T.E. 2: New tools for the spectral and spatial analysis of colour in r. Methods Ecol. Evol. 2019, 10, 1097–1107. [Google Scholar] [CrossRef]
- Fan, M.; Stuart-Fox, D.; Cadena, V. Cyclic Colour Change in the Bearded Dragon Pogona vitticeps under Different Photoperiods. PLoS ONE 2014, 9, e111504. [Google Scholar] [CrossRef]
- Parsons, R.; Parsons, R.; Garner, N.; Oster, H.; Rawashdeh, O. CircaCompare: A method to estimate and statistically support differences in mesor, amplitude and phase, between circadian rhythms. Bioinformatics 2019, 36, 1208–1212. [Google Scholar] [CrossRef]
- Mistlberger, R.E.; Rusak, B. Biological Rhythms and Behavior. In The Behavior of Animals, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 78–110. [Google Scholar] [CrossRef]
- Vaze, K.M.; Sharma, V.K. On the Adaptive Significance of Circadian Clocks for Their Owners. Chronobiol. Int. 2013, 30, 413–433. [Google Scholar] [CrossRef]
- Helm, B.; Visser, M.E.; Schwartz, W.; Kronfeld-Schor, N.; Gerkema, M.; Piersma, T.; Bloch, G. Two sides of a coin: Ecological and chronobiological perspectives of timing in the wild. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160246. [Google Scholar] [CrossRef]
- Krittika, S.; Yadav, P. Circadian clocks: An overview on its adaptive significance. Biol. Rhythm Res. 2020, 51, 1109–1132. [Google Scholar] [CrossRef]
- Figon, F.; Casas, J. Morphological and Physiological Colour Changes in the Animal Kingdom. In eLS; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 1–11. [Google Scholar] [CrossRef]
- Nilsson Sköld, H.; Aspengren, S.; Wallin, M. Rapid color change in fish and amphibians—Function, regulation, and emerging applications. Pigment Cell Melanoma Res. 2013, 26, 29–38. [Google Scholar] [CrossRef]
- Ligon, R.A.; McCartney, K.L. Biochemical regulation of pigment motility in vertebrate chromatophores: A review of physiological color change mechanisms. Curr. Zool. 2016, 62, 237–252. [Google Scholar] [CrossRef]
- Johnsen, S.; Johnsen, S. Absorption. In The Optics of Life: A Biologist’s Guide to Light in Nature; Princeton University Press: Princeton, NJ, USA, 2011; pp. 75–115. [Google Scholar] [CrossRef]
- Endler, J.A. The Color of Light in Forests and Its Implications. Ecol. Monogr. 1993, 63, 1–27. [Google Scholar] [CrossRef]
- Pauers, M.J.; Kuchenbecker, J.A.; Neitz, M.; Neitz, J. Changes in the colour of light cue circadian activity. Anim. Behav. 2012, 83, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M. Color Change, Phenotypic Plasticity, and Camouflage. Front. Ecol. Evol. 2016, 4, 51. [Google Scholar] [CrossRef]
- Duarte, R.C.; Flores, A.A.V.; Stevens, M. Camouflage through colour change: Mechanisms, adaptive value and ecological significance. Philos. Trans. R. Soc. B: Biol. Sci. 2017, 372, 20160342. [Google Scholar] [CrossRef]
- Croteau, M.C.; Davidson, M.A.; Lean, D.R.S.; Trudeau, V.L. Global Increases in Ultraviolet B Radiation: Potential Impacts on Amphibian Development and Metamorphosis. Physiol. Biochem. Zool. 2008, 81, 743–761. [Google Scholar] [CrossRef]
- Garcia, T.S.; Stacy, J.; Sih, A. Larval salamander response to UV radiation and predation risk: Color change and microhabitat use. Ecol. Appl. 2004, 14, 1055–1064. [Google Scholar] [CrossRef]
- Garcia, T.S.; Paoletti, D.J.; Blaustein, A.R. Correlated trait responses to multiple selection pressures in larval amphibians reveal conflict avoidance strategies. Freshw. Biol. 2009, 54, 1066–1077. [Google Scholar] [CrossRef]
- Garcia, T.S.; Paoletti, D.J.; Blaustein, A.R. Correlated trait response: Comparing amphibian defense strategies across a stress gradient. Can. J. Zool. 2009, 87, 41–49. [Google Scholar] [CrossRef]
- Aspengren, S.; Hedberg, D.; Sköld, H.N.; Wallin, M. Chapter 6 New Insights into Melanosome Transport in Vertebrate Pigment Cells. In International Review of Cell and Molecular Biology; Academic Press: San Diego, CA, USA, 2008; Volume 272, pp. 245–302. [Google Scholar]
- Polo-Cavia, N.; Gomez-Mestre, I. Pigmentation plasticity enhances crypsis in larval newts: Associated metabolic cost and background choice behaviour. Sci. Rep. 2017, 7, 39739. [Google Scholar] [CrossRef]
- Ikeda, Y. Color Change in Cephalopods. In Pigments, Pigment Cells and Pigment Patterns; Hashimoto, H., Goda, M., Futahashi, R., Kelsh, R., Akiyama, T., Eds.; Springer: Singapore, 2021; pp. 425–449. [Google Scholar] [CrossRef]
- Rodgers, G.M.; Gladman, N.W.; Corless, H.F.; Morrell, L.J. Costs of colour change in fish: Food intake and behavioural decisions. J. Exp. Biol. 2013, 216, 2760–2767. [Google Scholar] [CrossRef]
- Green, S.D.; Duarte, R.C.; Kellett, E.; Alagaratnam, N.; Stevens, M. Colour change and behavioural choice facilitate chameleon prawn camouflage against different seaweed backgrounds. Commun. Biol. 2019, 2, 230. [Google Scholar] [CrossRef]
- Gamble, F.W.; Keeble, F.W. Hippolyte varians: A Study in Colour-change. J. Cell Sci. 1900, s2-43, 589–698. [Google Scholar] [CrossRef]
- Clusella Trullas, S.; van Wyk, J.H.; Spotila, J.R. Thermal melanism in ectotherms. J. Therm. Biol. 2007, 32, 235–245. [Google Scholar] [CrossRef]


| Photoperiodic | Rhythmic p | Mesor | Amplitude | Acrophase (h) | Peak Time |
|---|---|---|---|---|---|
| LD12:12 (n = 10) | 0.000 *** | 20.395 ± 1.167 | 7.600 ± 0.989 | 15.905 ± 0.355 | 00:54 |
| DD (n = 10) | 0.000 *** | 20.758 ± 1.023 | 8.459 ± 1.165 | 15.908 ± 0.462 | 00:54 |
| LD16:8 (n = 10) | 0.000 *** | 18.980 ± 0.622 | 6.612 ± 0.594 | 17.416 ± 0.443 | 02:25 |
| LD8:16 (n = 10) | 0.000 *** | 22.536 ± 0.873 | 10.520 ± 1.182 | 15.778 ± 0.211 | 00:47 |
| Group Comparison | Mesor diff. | P (Mesor diff.) | Amplitude diff. | P (Amplitude diff.) | Acrophase diff. | P (Acrophase diff.) |
|---|---|---|---|---|---|---|
| DDvsLD12:12 | 0.363 | 0.283 | 0.859 | 0.081 | 0.002 | 0.980 |
| LD16:8vsLD12:12 | −1.415 | 0.000 *** | −0.988 | 0.020 * | 1.510 | 0.000 *** |
| LD8:16vsLD12:12 | 2.141 | 0.000 *** | 2.919 | 0.000 *** | −0.128 | 0.428 |
| LD16:8vsDD | −1.777 | 0.000 *** | −1.847 | 0.000 *** | 1.508 | 0.000 *** |
| LD8:16vsDD | 1.778 | 0.000 *** | 2.061 | 0.000 *** | −0.130 | 0.508 |
| LD16:8vsLD8:16 | −3.556 | 0.000 *** | −3.908 | 0.000 *** | 1.638 | 0.000 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Z.; Luo, Q.; Li, H.; Wang, P.; Wang, J.; Li, D.; Wang, W.; Yuan, K.; Zhou, Y.; et al. Circadian Rhythm of Body Color Change in the Juvenile Chinese Giant Salamander (Andrias davidianus) Under Different Photoperiods. Animals 2025, 15, 1526. https://doi.org/10.3390/ani15111526
Zhang Y, Wang Z, Luo Q, Li H, Wang P, Wang J, Li D, Wang W, Yuan K, Zhou Y, et al. Circadian Rhythm of Body Color Change in the Juvenile Chinese Giant Salamander (Andrias davidianus) Under Different Photoperiods. Animals. 2025; 15(11):1526. https://doi.org/10.3390/ani15111526
Chicago/Turabian StyleZhang, Yifang, Ziteng Wang, Qinghua Luo, Honghui Li, Pei Wang, Jiuxiang Wang, Dafeng Li, Wentao Wang, Kangle Yuan, Yan Zhou, and et al. 2025. "Circadian Rhythm of Body Color Change in the Juvenile Chinese Giant Salamander (Andrias davidianus) Under Different Photoperiods" Animals 15, no. 11: 1526. https://doi.org/10.3390/ani15111526
APA StyleZhang, Y., Wang, Z., Luo, Q., Li, H., Wang, P., Wang, J., Li, D., Wang, W., Yuan, K., Zhou, Y., Luo, S., & Tian, H. (2025). Circadian Rhythm of Body Color Change in the Juvenile Chinese Giant Salamander (Andrias davidianus) Under Different Photoperiods. Animals, 15(11), 1526. https://doi.org/10.3390/ani15111526





