Effects of Quinoa Secondary Metabolites on In Vitro Fermentation and Gas Production
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Test Site
2.2. Plant Material
2.3. In Vitro Fermentation
2.4. Chemical Analyses
2.5. Statistical Analyses
3. Results
3.1. Fermentation Quality
3.2. Secondary Metabolites
3.3. In Vitro Fermentation Production
3.4. Gas Composition
3.5. Digestibility and Energy Value
3.6. Correlation
3.7. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Misiukiewicz, A.; Gao, M.; Filipiak, W.; Cieslak, A.; Patra, A.K.; Szumacher-Strabel, M. Review: Methanogens and methane production in the digestive systems of nonruminant farm animals. Animal 2021, 15, 100060. [Google Scholar] [CrossRef]
- Khanna, N.; Lin, J.; Liu, X.; Wang, W. An assessment of China’s methane mitigation potential and costs and uncertainties through 2060. Nat. Commun. 2024, 15, 9694. [Google Scholar] [CrossRef] [PubMed]
- Cardador, M.J.; Reyes-Palomo, C.; Díaz-Gaona, C.; Arce, L.; Rodríguez-Estévez, V. Review of the Methodologies for Measurement of Greenhouse Gas Emissions in Livestock Farming: Pig Farms as a Case of Study. Crit. Rev. Anal. Chem. 2022, 52, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, Y.; Guo, T.; Liu, W.; Tong, X.; Zhang, Z.; Sun, J.; Yang, Y.; Yang, S.; Li, D.; et al. Sargassum mcclurei Mitigating Methane Emissions and Affecting Rumen Microbial Community in In Vitro Rumen Fermentation. Animals 2024, 14, 2057. [Google Scholar] [CrossRef]
- Eugène, M.; Klumpp, K.; Sauvant, D. Methane mitigating options with forages fed to ruminants. Grass Forage Sci. 2021, 76, 196–204. [Google Scholar] [CrossRef]
- Ibrahim, T.A.; Hassen, A.; Apostolides, Z. The Antimethanogenic Potentials of Plant Extracts: Their Yields and Phytochemical Compositions as Affected by Extractive Solvents. Plants 2022, 11, 3296. [Google Scholar] [CrossRef] [PubMed]
- Lileikis, T.; Nainiene, R.; Bliznikas, S.; Uchockis, V. Dietary Ruminant Enteric Methane Mitigation Strategies: Current Findings, Potential Risks and Applicability. Animals 2023, 13, 2586. [Google Scholar] [CrossRef]
- Cobellis, G.; Trabalza-Marinucci, M.; Yu, Z. Critical evaluation of essential oils as rumen modifiers in ruminant nutrition: A review. Sci. Total Environ. 2016, 545–546, 556–568. [Google Scholar] [CrossRef]
- Ku-Vera, J.C.; Jimenez-Ocampo, R.; Valencia-Salazar, S.S.; Montoya-Flores, M.D.; Molina-Botero, I.C.; Arango, J.; Gomez-Bravo, C.A.; Aguilar-Perez, C.F.; Solorio-Sanchez, F.J. Role of Secondary Plant Metabolites on Enteric Methane Mitigation in Ruminants. Front. Vet. Sci. 2020, 7, 584. [Google Scholar] [CrossRef]
- Voronov, S.; Pleskachiov, Y.; Shitikova, A.; Zargar, M.; Abdelkader, M. Diversity of the Biological and Proteinogenic Characteristics of Quinoa Genotypes as a Multi-Purpose Crop. Agronomy 2023, 13, 279. [Google Scholar] [CrossRef]
- Yilmaz, Ş.; Ertekin, İ.; Atiş, İ. Forage yield and quality of quinoa (Chenopodium quinoa Willd.) genotypes harvested at different cutting stages under Mediterranean conditions. Turk. J. Field Crops 2021, 26, 202–209. [Google Scholar] [CrossRef]
- Villacres, E.; Quelal, M.; Galarza, S.; Iza, D.; Silva, E. Nutritional Value and Bioactive Compounds of Leaves and Grains from Quinoa (Chenopodium quinoa Willd.). Plants 2022, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Kolba, N.; Khen, N.; Even, C.; Turjeman, S.; Koren, O.; Tako, E. Quinoa Soluble Fiber and Quercetin Alter the Composition of the Gut Microbiome and Improve Brush Border Membrane Morphology In Vivo (Gallus gallus). Nutrients 2022, 14, 448. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Teng, C.; Fan, X.; Guo, S.; Zhao, G.; Zhang, L.; Liang, Z.; Qin, P. Nutrient composition, functional activity and industrial applications of quinoa (Chenopodium quinoa Willd.). Food Chem. 2023, 410, 135290. [Google Scholar] [CrossRef]
- Kuljanabhagavad, T.; Wink, M. Biological activities and chemistry of saponins from Chenopodium quinoa Willd. Phytochem. Rev. 2009, 8, 473–490. [Google Scholar] [CrossRef]
- Alvar-Beltrán, J.; Dalla Marta, A.; Vivoli, R.; Verdi, L.; Orlandini, S. Greenhouse Gas Emissions and Yield Production from an Organic and Conventional Fertilization on Quinoa. Agronomy 2022, 12, 1015. [Google Scholar] [CrossRef]
- Zulkadir, G.; İdikut, L. The impact of various sowing applications on the nutritional value of Quinoa Dry Herb. J. Food Process. Preserv. 2021, 45, e15730. [Google Scholar] [CrossRef]
- Ebeid, H.M.; Kholif, A.E.; El-Bordeny, N.; Chrenkova, M.; Mlynekova, Z.; Hansen, H.H. Nutritive value of quinoa (Chenopodium quinoa) as a feed for ruminants: In sacco degradability and in vitro gas production. Environ. Sci. Pollut. Res. Int. 2022, 29, 35241–35252. [Google Scholar] [CrossRef]
- Mustafa, S.; Riaz, M.A.; Masoud, M.S.; Qasim, M.; Riaz, A. Impact of dietary inclusion of Chenopodium quinoa on growth performance and survival of Hubbard chicken. PLoS ONE 2022, 17, e0276524. [Google Scholar] [CrossRef]
- Marino, R.; Caroprese, M.; Annicchiarico, G.; Ciampi, F.; Ciliberti, M.G.; della Malva, A.; Santillo, A.; Sevi, A.; Albenzio, M. Effect of Diet Supplementation with Quinoa Seed and/or Linseed on Immune Response, Productivity and Meat Quality in Merinos Derived Lambs. Animals 2018, 8, 204. [Google Scholar] [CrossRef]
- Jafari, S.; Ebrahimi, M.; Goh, Y.M.; Rajion, M.A.; Jahromi, M.F.; Al-Jumaili, W.S. Manipulation of Rumen Fermentation and Methane Gas Production by Plant Secondary Metabolites (Saponin, Tannin and Essential Oil)—A Review of Ten-Year Studies. Ann. Anim. Sci. 2019, 19, 3–29. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Vallejo, L.H.; Salem, A.Z.M.; Mellado, M.; Camacho, L.M.; Cipriano, M.; Olafadehan, O.A.; Olivares, J.; Rojas, S. Moringa oleifera leaf meal as an environmental friendly protein source for ruminants: Biomethane and carbon dioxide production, and fermentation characteristics. J. Clean. Prod. 2017, 165, 1229–1238. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas productionusing rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Matra, M.; Suriyapha, C.; Dagaew, G.; Prachumchai, R.; Phupaboon, S.; Sommai, S.; Wanapat, M. Advantageous effects of rumen-protected phytonutrients from tropical plant extracts on rumen fermentation efficiency and methane mitigation using in vitro fermentation technique. Anim. Biosci. 2024, 38, 489–500. [Google Scholar] [CrossRef]
- HJ 38-2017; Stationary Source Emission—Determination of Total Hydrocarbons, Methane and Nonmethane Hydrocarbons—Gas Chromatography. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2017.
- D’Souza, G.M.; Norris, A.B.; Tedeschi, L.O. Evaluation of methane concentration sampling methods of gas produced from in vitro fermentation. J. Anim. Sci. 2020, 98, 54–55. [Google Scholar] [CrossRef]
- Tonamo, A.; Tamir, B.; Goshu, G. Assessment of Cattle Feed Resources; Chemical Composition and Digestibility of Major Feeds in Essera District, Southern Ethiopia. Sci. Technol. Arts Res. J. 2016, 4, 89. [Google Scholar] [CrossRef]
- Gebremariam, T.; Belay, S. Chemical Composition and Digestibility of Major Feed Resources in Tanqua-Abergelle District of Central Tigray, Northern Ethiopia. Sci. World J. 2021, 2021, 5234831. [Google Scholar] [CrossRef] [PubMed]
- Hare, K.S.; Lambert, K.; Chagas, A.C.; Watanabe, D.H.M.; Penner, G.B. PSXII-30 Changes in Ruminal Digesta and Ruminal Ammonia-N Concentration with Differing Durations Feed Restriction for Sheep. J. Anim. Sci. 2023, 101, 637–638. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, C.; Gong, W.; Xiong, Y.; Zhou, Y.; Guo, W.; Li, B.; Wang, Y. Trade-off between shade tolerance and chemical resistance of invasive Phytolacca americana under different light levels compared with its native and exotic non-invasive congeners. Environ. Exp. Bot. 2022, 196, 104809. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, C.; Xiong, Y.; Xiao, F.; Wang, Y. Heavy metal induced resistance to herbivore of invasive plant: Implications from inter- and intraspecific comparisons. Front. Plant Sci. 2023, 14, 1222867. [Google Scholar] [CrossRef]
- Sun, J.; Shi, W.; Wu, Y.; Ji, J.; Feng, J.; Zhao, J.; Shi, X.; Du, C.; Chen, W.; Liu, J.; et al. Variations in Acorn Traits in Two Oak Species: Quercus mongolica Fisch. ex Ledeb. and Quercus variabilis Blume. Forests 2021, 12, 1755. [Google Scholar] [CrossRef]
- Jiang, Z.; He, J.; Fang, Y.; Lin, J.; Liu, S.; Wu, Y.; Huang, X. Effects of herbivore on seagrass, epiphyte and sediment carbon sequestration in tropical seagrass bed. Mar. Environ. Res. 2023, 190, 106122. [Google Scholar] [CrossRef]
- Ni, B.B.; Liu, H.; Wang, Z.S.; Zhang, G.Y.; Sang, Z.Y.; Liu, J.J.; He, C.Y.; Zhang, J.G. A chromosome-scale genome of Rhus chinensis Mill. provides new insights into plant-insect interaction and gallotannins biosynthesis. Plant J. 2024, 118, 766–786. [Google Scholar] [CrossRef]
- Olfaz, M.; Kilic, U.; Boga, M.; Abdi, A.M. Determination of the In Vitro Gas Production and Potential Feed Value of Olive, Mulberry and Sour Orange Tree Leaves. Open Life Sci. 2018, 13, 269–278. [Google Scholar] [CrossRef]
- He, C.; Li, Q.; Xiao, H.; Sun, X.; Gao, Z.; Cai, Y.; Zhao, S. Effects of Mixing Ratio and Lactic Acid Bacteria Preparation on the Quality of Whole-Plant Quinoa and Whole-Plant Corn or Stevia Powder Mixed Silage. Microorganisms 2025, 13, 78. [Google Scholar] [CrossRef]
- Asher, A.; Galili, S.; Whitney, T.; Rubinovich, L. The potential of quinoa (Chenopodium quinoa) cultivation in Israel as a dual-purpose crop for grain production and livestock feed. Sci. Hortic. 2020, 272, 109534. [Google Scholar] [CrossRef]
- Pazla, R.; Jamarun, N.; Agustin, F.; Arief, A.; Elihasridas, E.; Ramaiyulis, R.; Yanti, G.; Ardani, L.R.; Sucitra, L.S.; Ikhlas, Z. Nutrition profile and rumen fermentation of Tithonia diversifolia fermented with Lactobacillus bulgaricus at different times and doses. J. Adv. Vet. Anim. Res. 2024, 11, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Li, X.; Fang, D.; Wang, S.; Li, J.; Dong, D.; Wang, Y.; Shao, T. Effects of additives on the fermentation quality and bacterial community of silage prepared from fresh-cut whole-plant quinoa (Chenopodium quinoa willd.). Ital. J. Anim. Sci. 2022, 21, 1558–1568. [Google Scholar] [CrossRef]
- Ertekin, I.; Atis, I.; Yilmaz, S. The Effect of Cultivar and Stage of Growth on the Fermentation, Aerobic Stability and Nutritive Value of Ensiled Quinoa. J. Agric. Sci.-Tarim Bilim. Derg. 2023, 29, 478–490. [Google Scholar] [CrossRef]
- Fang, D.; Dong, Z.H.; Wang, D.L.; Li, B.; Shi, P.B.A.; Yan, J.; Zhuang, D.Y.; Shao, T.; Wang, W.Y.; Gu, M.F. Evaluating the fermentation quality and bacterial community of high-moisture whole-plant quinoa silage ensiled with different additives. J. Appl. Microbiol. 2022, 132, 3578–3589. [Google Scholar] [CrossRef]
- Limon-Hernandez, D.; Rayas-Amor, A.A.; Garcia-Martinez, A.; Estrada-Flores, J.G.; Lopez, M.N.; Cruz Monterrosa, R.G.; Morales-Almaraz, E. Chemical composition, in vitro gas production, methane production and fatty acid profile of canola silage (Brassica napus) with four levels of molasses. Trop. Anim. Health Prod. 2019, 51, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Suárez Mvz, M.N.J.P.; Escobar Mvz, E.M.M.I.; Molano Zoot, E.M.C.E.R. Valor nutricional del ensilaje de forraje de quinua (Chenopodium quinoa willd) con adición de microorganismos eficientes. CES Med. Vet. Y Zootec. 2019, 14, 16–28. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Dietary phytochemicals as rumen modifiers: A review of the effects on microbial populations. Antonie Leeuwenhoek 2009, 96, 363–375. [Google Scholar] [CrossRef]
- Zhou, X.; Yue, T.; Wei, Z.; Yang, L.; Zhang, L.; Wu, B. Evaluation of nutritional value, bioactivity and mineral content of quinoa bran in China and its potential use in the food industry. Curr. Res. Food Sci. 2023, 7, 100562. [Google Scholar] [CrossRef] [PubMed]
- Totakul, P.; Matra, M.; Sommai, S.; Viennasay, B.; Wanapat, M. Combination effects of phytonutrient pellet and lemongrass (Cymbopogon citratus) powder on rumen fermentation efficiency and nutrient degradability using in vitro technique. Trop. Anim. Health Prod. 2024, 56, 97. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P.S.; Becker, K. Changes in microbial community structure, methanogenesis and rumen fermentation in response to saponin-rich fractions from different plant materials. J. Appl. Microbiol. 2008, 105, 770–777. [Google Scholar] [CrossRef]
- Aderao, G.N.; Sahoo, A.; Bhatt, R.S.; Kumawat, P.K.; Soni, L. In vitro rumen fermentation kinetics, metabolite production, methane and substrate degradability of polyphenol rich plant leaves and their component complete feed blocks. J. Anim. Sci. Technol. 2018, 60, 26. [Google Scholar] [CrossRef]
- Zeru, A.E.; Hassen, A.; Apostolides, Z.; Tjelele, J. Relationships Between Agronomic Traits of Moringa Accessions and In Vitro Gas Production Characteristics of a Test Feed Incubated with or Without Moringa Plant Leaf Extracts. Plants 2022, 11, 2901. [Google Scholar] [CrossRef]
- Wang, L.; Gao, H.; Sun, C.; Huang, L. Protective Application of Morus and Its Extracts in Animal Production. Animals 2022, 12, 3541. [Google Scholar] [CrossRef]
- Hassan, F.U.; Arshad, M.A.; Li, M.; Rehman, M.S.; Loor, J.J.; Huang, J. Potential of Mulberry Leaf Biomass and Its Flavonoids to Improve Production and Health in Ruminants: Mechanistic Insights and Prospects. Animals 2020, 10, 2076. [Google Scholar] [CrossRef]
- Fiorentino, S.; Bellani, L.; Santin, M.; Castagna, A.; Echeverria, M.C.; Giorgetti, L. Effects of Microalgae as Biostimulants on Plant Growth, Content of Antioxidant Molecules and Total Antioxidant Capacity in Chenopodium quinoa Exposed to Salt Stress. Plants 2025, 14, 781. [Google Scholar] [CrossRef] [PubMed]
- Ku-Vera, J.C.; Castelan-Ortega, O.A.; Galindo-Maldonado, F.A.; Arango, J.; Chirinda, N.; Jimenez-Ocampo, R.; Valencia-Salazar, S.S.; Flores-Santiago, E.J.; Montoya-Flores, M.D.; Molina-Botero, I.C.; et al. Review: Strategies for enteric methane mitigation in cattle fed tropical forages. Animal 2020, 14, s453–s463. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, M.; Cieslak, A.; Józwik, A.; El-Sherbiny, M.; Stochmal, A.; Oleszek, W.; Kowalczyk, M.; Filipiak, W.; Szumacher-Strabel, M. The effect of total and individual alfalfa saponins on rumen methane production. J. Sci. Food Agric. 2020, 100, 1922–1930. [Google Scholar] [CrossRef]
- Mahmoud, A.E.M.; Abbas, M.S.; Cieslak, A.; Szumacher-Strabel, M. Evaluation of chemical composition and in vitro dry and organic matter digestibility of some forage plant species derived from Egyptian rangelands. J. Anim. Plant Sci. 2017, 27, 1573–1581. [Google Scholar]
- Lwin, D.S.; Williams, A.; Barber, D.E.; Benvenutti, M.A.; Williams, B.; Poppi, D.P.; Harper, K.J.; Watt, L. Comparison of equations to predict the metabolisable energy content as applied to the vertical strata and plant parts of forage sorghum. Anim. Prod. Sci. 2022, 62, 1006–1013. [Google Scholar] [CrossRef]
- Quintero-Anzueta, S.; Molina-Botero, I.C.; Ramirez-Navas, J.S.; Rao, I.; Chirinda, N.; Barahona-Rosales, R.; Moorby, J.; Arango, J. Nutritional Evaluation of Tropical Forage Grass Alone and Grass-Legume Diets to Reduce In Vitro Methane Production. Front. Sustain. Food Syst. 2021, 5, 663003. [Google Scholar] [CrossRef]
- Pal, K.; Patra, A.K.; Sahoo, A. Evaluation of feeds from tropical origin for in vitro methane production potential and rumen fermentation in vitro. Span. J. Agric. Res. 2015, 13, e0608. [Google Scholar] [CrossRef]
- Hariadi, B.T.; Santoso, B. Evaluation of tropical plants containing tannin on in vitro methanogenesis and fermentation parameters using rumen fluid. J. Sci. Food Agric. 2010, 90, 456–461. [Google Scholar] [CrossRef]
- Angeles-Mayorga, Y.; Cen-Cen, E.R.; Crosby-Galvan, M.M.; Ramirez-Bribiesca, J.E.; Candelaria-Martinez, B.; Sanchez-Villarreal, A.; Ramirez-Mella, M. Foliage of Tropical Trees and Shrubs and Their Secondary Metabolites Modify In Vitro Ruminal Fermentation, Methane and Gas Production without a Tight Correlation with the Microbiota. Animals 2022, 12, 2628. [Google Scholar] [CrossRef]
- Trotta, R.J.; Kreikemeier, K.K.; Foote, S.; McLeod, K.R.; Harmon, D.L. Influence of Anti-Coccidial Compounds and Phytogenic Saponin Extracts on In Vitro and In Vivo Ruminal Fermentation and Methane Production of Cattle. Animals 2023, 13, 2308. [Google Scholar] [CrossRef]
- Jensen, R.H.; Ronn, M.; Thorsteinsson, M.; Olijhoek, D.W.; Nielsen, M.O.; Norskov, N.P. Untargeted Metabolomics Combined with Solid Phase Fractionation for Systematic Characterization of Bioactive Compounds in Hemp with Methane Mitigation Potential. Metabolites 2022, 12, 77. [Google Scholar] [CrossRef]
- Jayanegara, A.; Makkar, H.; Becker, K. The use of principal component analysis in identifying and integrating variables related to forage quality and methane production. J. Indones. Trop. Anim. Agric. 2009, 34, 241–247. [Google Scholar] [CrossRef]

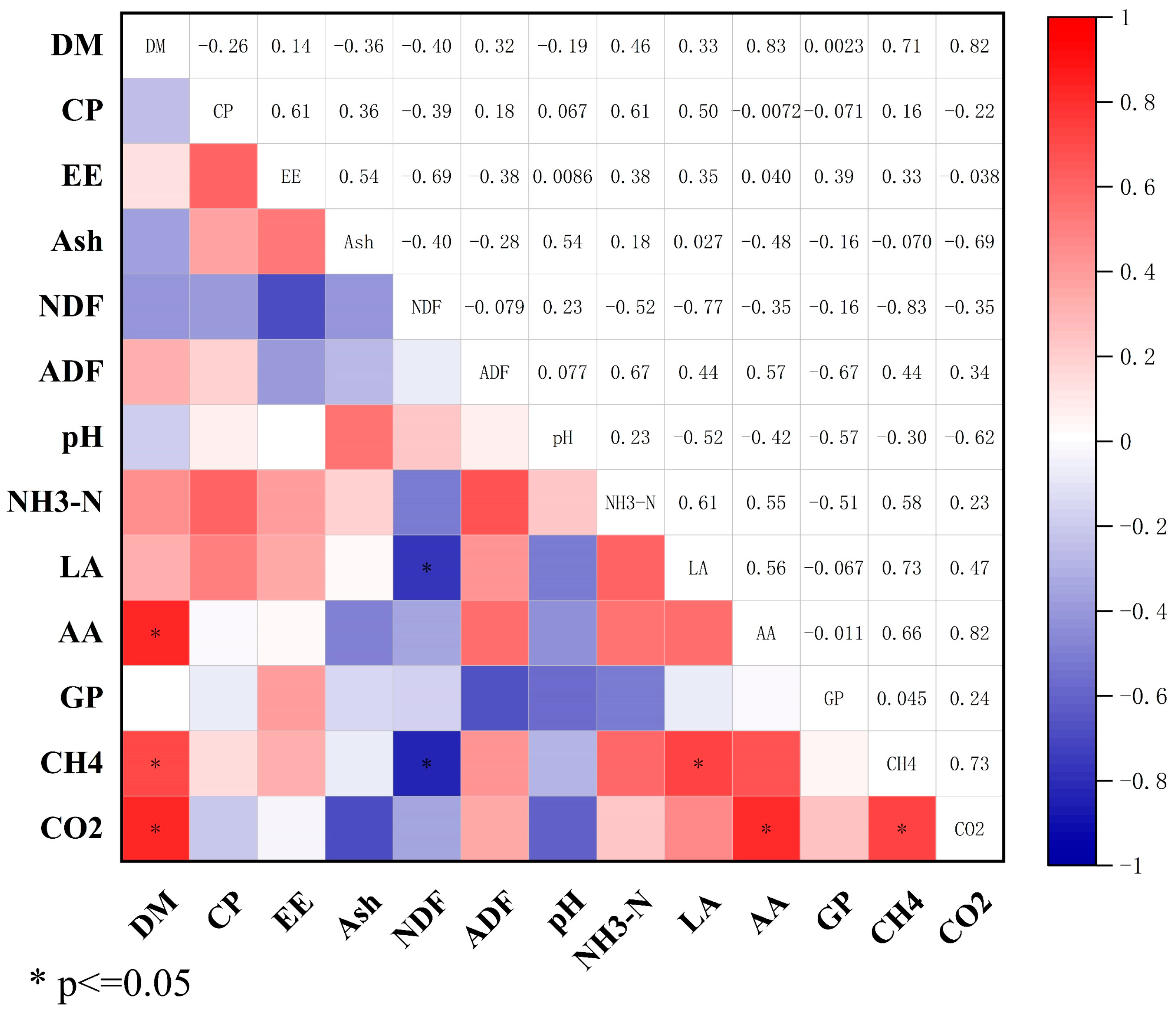
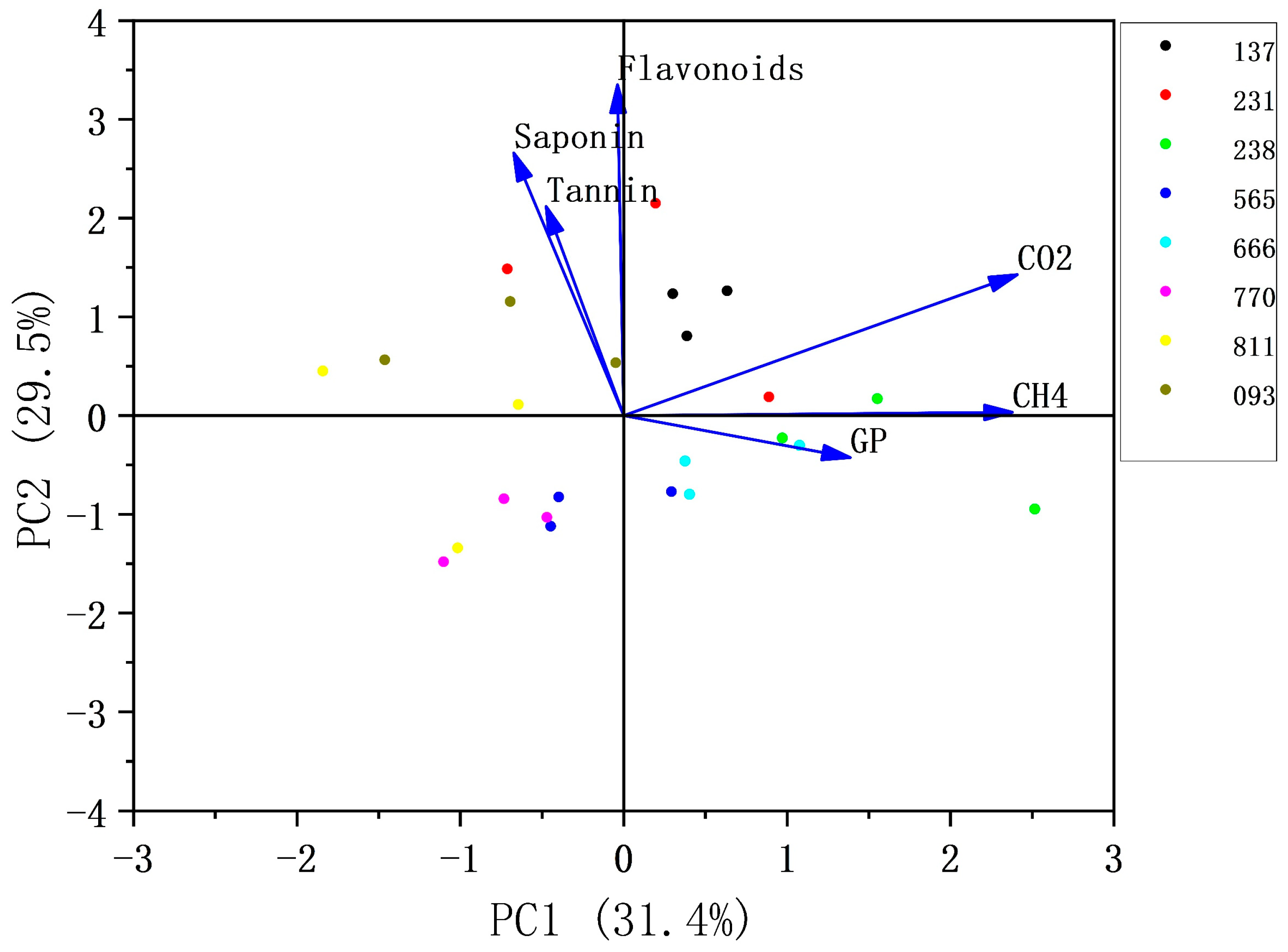
| Code | CP | EE | Ash | NDF | ADF | Source |
|---|---|---|---|---|---|---|
| 093 | 10.11 | 2.15 | 19.43 | 59.47 | 34.97 | Chile |
| 137 | 9.50 | 1.98 | 17.00 | 61.91 | 36.06 | Chile |
| 231 | 10.17 | 2.33 | 19.74 | 57.73 | 35.24 | Chile |
| 238 | 10.18 | 2.25 | 20.58 | 49.31 | 37.31 | Chile |
| 565 | 10.69 | 2.14 | 18.90 | 62.53 | 36.66 | Argentina |
| 666 | 8.84 | 2.38 | 20.13 | 51.74 | 33.96 | United States |
| 770 | 10.32 | 2.10 | 21.20 | 59.42 | 34.84 | Argentina |
| 811 | 9.93 | 2.30 | 23.14 | 58.02 | 33.29 | United States |
| Stock Solution | Volume (mL) |
|---|---|
| Buffer (B solution) | 208.1 |
| Macroelement solution (C liquid) | 208.1 |
| Trace element solution (solution A) | 0.1 |
| 0.1% resazurin solution (D solution) | 1 |
| Deionized water | 520.2 |
| Reductant solution (E liquid) | 62.4 |
| Varieties | pH | NH3-N (g/kg FM) | LA (g/kg FM) | AA (g/kg FM) | PA | BA |
|---|---|---|---|---|---|---|
| 093 | 4.63 ± 0.03 b | 32.46 ± 7.6 ab | 11.46 ± 4.73 ab | 230.84 ± 78.38 ab | ND | ND |
| 137 | 4.59 ± 0.02 bc | 23.19 ± 8.25 ab | 4.15 ± 1.13 b | 364.54 ± 92.69 ab | ND | ND |
| 231 | 4.5 ± 0.01 de | 32.32 ± 9.68 ab | 12.75 ± 5.47 ab | 473.02 ± 190.68 a | ND | ND |
| 238 | 4.53 ± 0.01 cd | 40.14 ± 5.73 a | 19.65 ± 2.14 a | 463.68 ± 78.85 a | ND | ND |
| 565 | 4.55 ± 0.02 cd | 30.29 ± 7.56 ab | 11 ± 3.85 ab | 275.96 ± 58.06 ab | ND | ND |
| 666 | 4.44 ± 0.01 e | 19.28 ± 1.26 b | 9.43 ± 2.4 b | 163.36 ± 30.11 b | ND | ND |
| 770 | 4.57 ± 0.01 bc | 21.16 ± 2.13 ab | 9.87 ± 0.48 b | 161.99 ± 11.79 b | ND | ND |
| 811 | 4.89 ± 0.02 a | 31.88 ± 5.12 ab | 6.17 ± 1.52 b | 133.53 ± 36.66 b | ND | ND |
| SEM | 0.03 | 2.37 | 1.36 | 37.3 | - | - |
| p value | <0.01 | 0.38 | 0.09 | 0.08 | - | - |
| Varieties | Total Saponins (g/kg DM) | Tannin (g/kg FM) | Flavonoid (g/kg DM) |
|---|---|---|---|
| 093 | 5.6 ± 0.24 a | 5.65 ± 0.74 a | 8.47 ± 0.2 b |
| 137 | 5.05 ± 0.22 ab | 5.56 ± 0.58 a | 9.65 ± 0.32 a |
| 231 | 5.15 ± 0.26 ab | 5.59 ± 0.99 a | 10.43 ± 0.55 a |
| 238 | 4.5 ± 0.64 abc | 4.55 ± 0.17 a | 6.94 ± 0.34 cd |
| 565 | 2.71 ± 0.03 d | 5.79 ± 0.32 a | 7.24 ± 0.07 cd |
| 666 | 3.68 ± 0.19 cd | 5.33 ± 0.16 a | 7.74 ± 0.3 bc |
| 770 | 4.26 ± 0.14 bc | 4.53 ± 0.5 a | 6.36 ± 0.25 d |
| 811 | 5.09 ± 0.45 ab | 5.15 ± 0.75 a | 7.6 ± 0.43 bc |
| SEM | 0.21 | 0.20 | 0.29 |
| p value | <0.01 | 0.69 | <0.01 |
| Varieties | 1 h | 2 h | 4 h | 8 h | 16 h | 32 h | 48 h |
|---|---|---|---|---|---|---|---|
| 093 | 10.46 ± 0.21 a | 16.35 ± 0.16 b | 25.48 ± 0.1 c | 45.59 ± 0.36 c | 85.22 ± 0.65 c | 109.6 ± 0.61 c | 116.99 ± 0.96 c |
| 137 | 12.77 ± 1.51 a | 20.45 ± 1.39 a | 32.7 ± 1.77 ab | 62.63 ± 2.69 ab | 94.98 ± 0.4 ab | 116.3 ± 1.38 b | 123.7 ± 1.47 b |
| 231 | 12.42 ± 0.64 a | 19.47 ± 0.84 ab | 31.78 ± 1.12 ab | 62.69 ± 1.99 ab | 95.68 ± 1.95 ab | 116.76 ± 1.85 b | 124.85 ± 2.37 b |
| 238 | 14.04 ± 0.66 a | 20.86 ± 0.75 a | 33.11 ± 1.06 ab | 63.03 ± 2.02 ab | 93.42 ± 1.39 b | 116.07 ± 3.26 b | 121.85 ± 2.66 bc |
| 565 | 13.38 ± 0.8 a | 19.9 ± 0.87 ab | 29.54 ± 1.15 abc | 54.01 ± 1.92 bc | 90.48 ± 1.59 bc | 115.37 ± 1.73 bc | 123.41 ± 1.86 b |
| 666 | 14.01 ± 1.43 a | 21.21 ± 1.87 a | 33.83 ± 3.37 a | 66.42 ± 6.64 a | 100.81 ± 4.9 ab | 125.07 ± 2.46 a | 132.97 ± 2.17 a |
| 770 | 13.52 ± 0.72 a | 19.83 ± 0.66 ab | 30.16 ± 0.44 abc | 56.44 ± 1.01 ab | 91.03 ± 0.36 bc | 113.63 ± 0.85 bc | 120.77 ± 1.21 bc |
| 811 | 12.48 ± 0.62 a | 18.09 ± 0.75 ab | 27.73 ± 0.78 bc | 54.84 ± 1.99 bc | 91.79 ± 1.29 bc | 112.59 ± 1.23 bc | 120.56 ± 1.4 bc |
| SEM | 0.71 | 0.87 | 1.45 | 3.14 | 2.15 | 2.06 | 2.12 |
| p value | 0.201 | 0.069 | 0.017 | 0.03 | 0.005 | 0.002 | 0.001 |
| Varieties | GP (mL/g) | CH4 (mL/g) | CO2 (mL/g) | O2, N2 (mL/g) |
|---|---|---|---|---|
| 093 | 116.99 ± 0.95 c | 8.59 ± 0.54 b | 27.78 ± 1.81 cd | 77.19 ± 1.99 d |
| 137 | 123.7 ± 1.47 b | 9.43 ± 0.18 b | 32.19 ± 0.33 ab | 78.38 ± 1.03 cd |
| 231 | 124.85 ± 2.36 b | 9.2 ± 0.5 b | 29.8 ± 1.55 abc | 82.06 ± 1.85 bcd |
| 238 | 121.85 ± 2.65 bc | 14.03 ± 0.83 a | 32.93 ± 1.09 a | 70.82 ± 2.79 e |
| 565 | 123.41 ± 1.86 b | 8.36 ± 0.23 b | 27.03 ± 0.83 cd | 84.55 ± 0.87 b |
| 666 | 132.97 ± 2.17 a | 9.19 ± 0.43 b | 28.59 ± 0.41 bcd | 90.76 ± 1.57 a |
| 770 | 120.77 ± 1.2b c | 7.99 ± 0.16 b | 24.76 ± 0.97 de | 83.74 ± 1.22 bc |
| 811 | 120.56 ± 1.4 bc | 8.17 ± 0.64 b | 22.72 ± 1.22 e | 85.17 ± 1.86 ab |
| SEM | 1.06 | 10.6 | 0.8 | 1.3 |
| p value | <0.01 | <0.01 | <0.01 | <0.01 |
| Varieties | OMD (%) | ME (MJ/kg DM) | NEL (MJ/kg DM) |
|---|---|---|---|
| 093 | 52.86 ± 0.21 cd | 5.97 ± 0.03 c | 3.12 ± 0.02 c |
| 137 | 52.2 ± 0.34 d | 6.12 ± 0.06 bc | 3.2 ± 0.04 bc |
| 231 | 54.49 ± 0.51 ab | 6.19 ± 0.07 ab | 3.3 ± 0.05 ab |
| 238 | 54.51 ± 0.56 ab | 6.11 ± 0.07 bc | 3.23 ± 0.05 bc |
| 565 | 53.92 ± 0.4 bc | 6.18 ± 0.05 ab | 3.27 ± 0.04 ab |
| 666 | 55.59 ± 0.44 a | 6.34 ± 0.06 a | 3.4 ± 0.04 a |
| 770 | 54.78 ± 0.17 ab | 6.09 ± 0.03 bc | 3.2 ± 0.02 bc |
| 811 | 55.84 ± 0.32 a | 6.06 ± 0.04 bc | 3.2 ± 0.03 bc |
| SEM | 0.27 | 0.03 | 0.02 |
| p value | <0.01 | <0.01 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, J.; Yang, Y.; Lu, H.; Wang, B.; Yang, H.; Guo, S. Effects of Quinoa Secondary Metabolites on In Vitro Fermentation and Gas Production. Animals 2025, 15, 1522. https://doi.org/10.3390/ani15111522
Ge J, Yang Y, Lu H, Wang B, Yang H, Guo S. Effects of Quinoa Secondary Metabolites on In Vitro Fermentation and Gas Production. Animals. 2025; 15(11):1522. https://doi.org/10.3390/ani15111522
Chicago/Turabian StyleGe, Junfeng, Yindi Yang, Hao Lu, Bo Wang, Hongjin Yang, and Shanli Guo. 2025. "Effects of Quinoa Secondary Metabolites on In Vitro Fermentation and Gas Production" Animals 15, no. 11: 1522. https://doi.org/10.3390/ani15111522
APA StyleGe, J., Yang, Y., Lu, H., Wang, B., Yang, H., & Guo, S. (2025). Effects of Quinoa Secondary Metabolites on In Vitro Fermentation and Gas Production. Animals, 15(11), 1522. https://doi.org/10.3390/ani15111522





