Short-Term Impact of Dry Needling Treatment for Myofascial Pain on Equine Biomechanics Through Artificial Intelligence-Based Gait Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses and Treatment Allocation
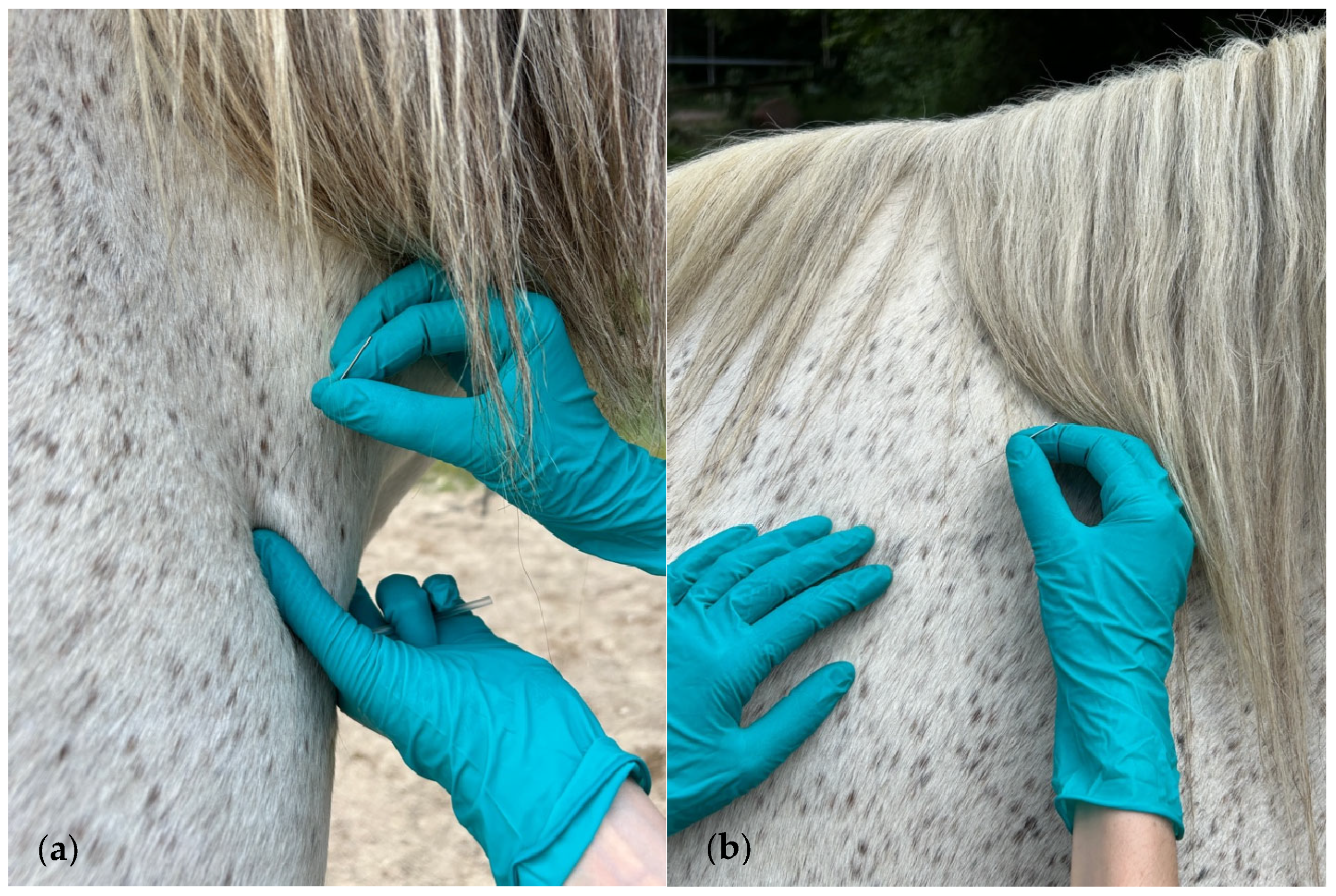
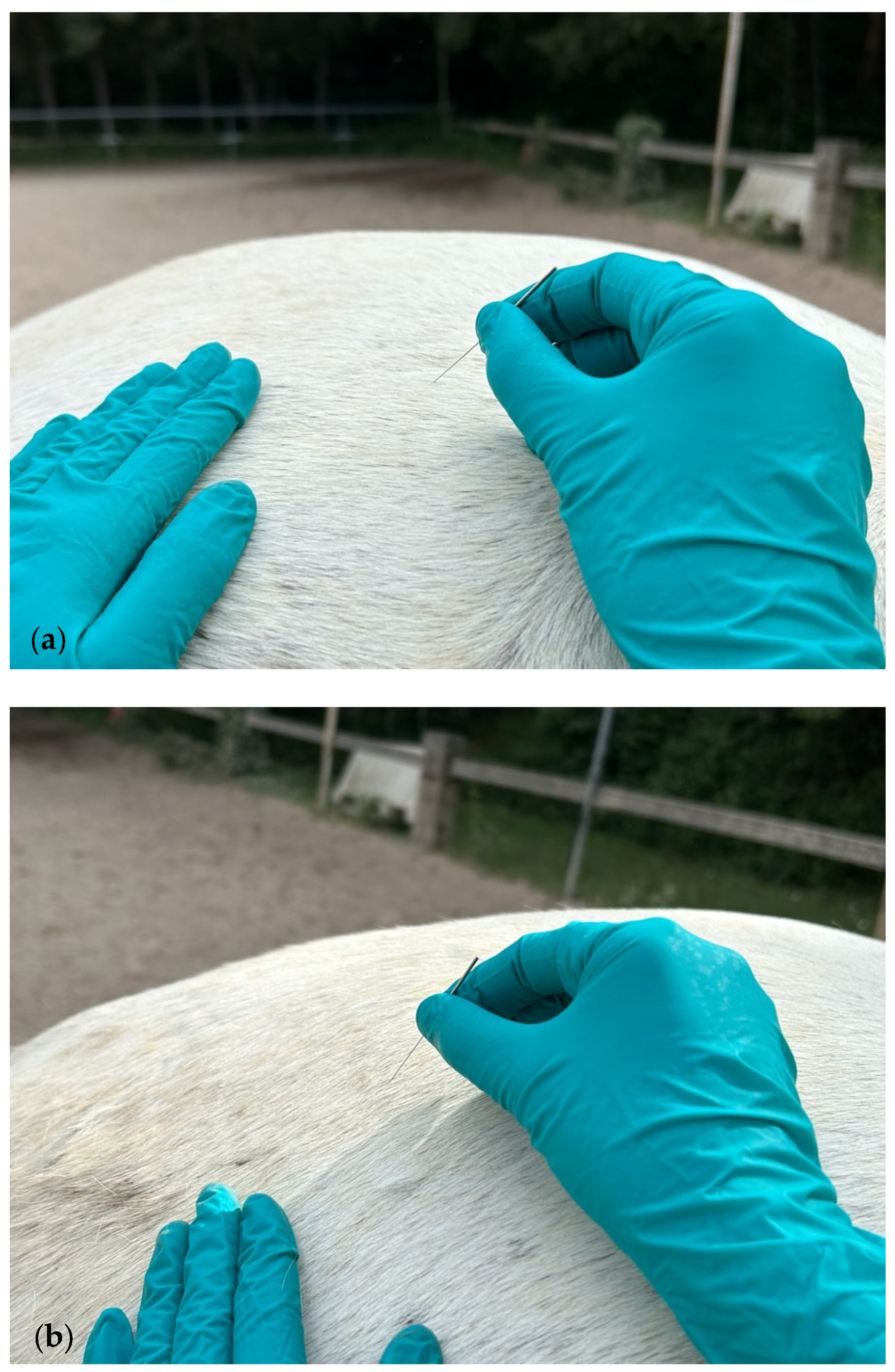
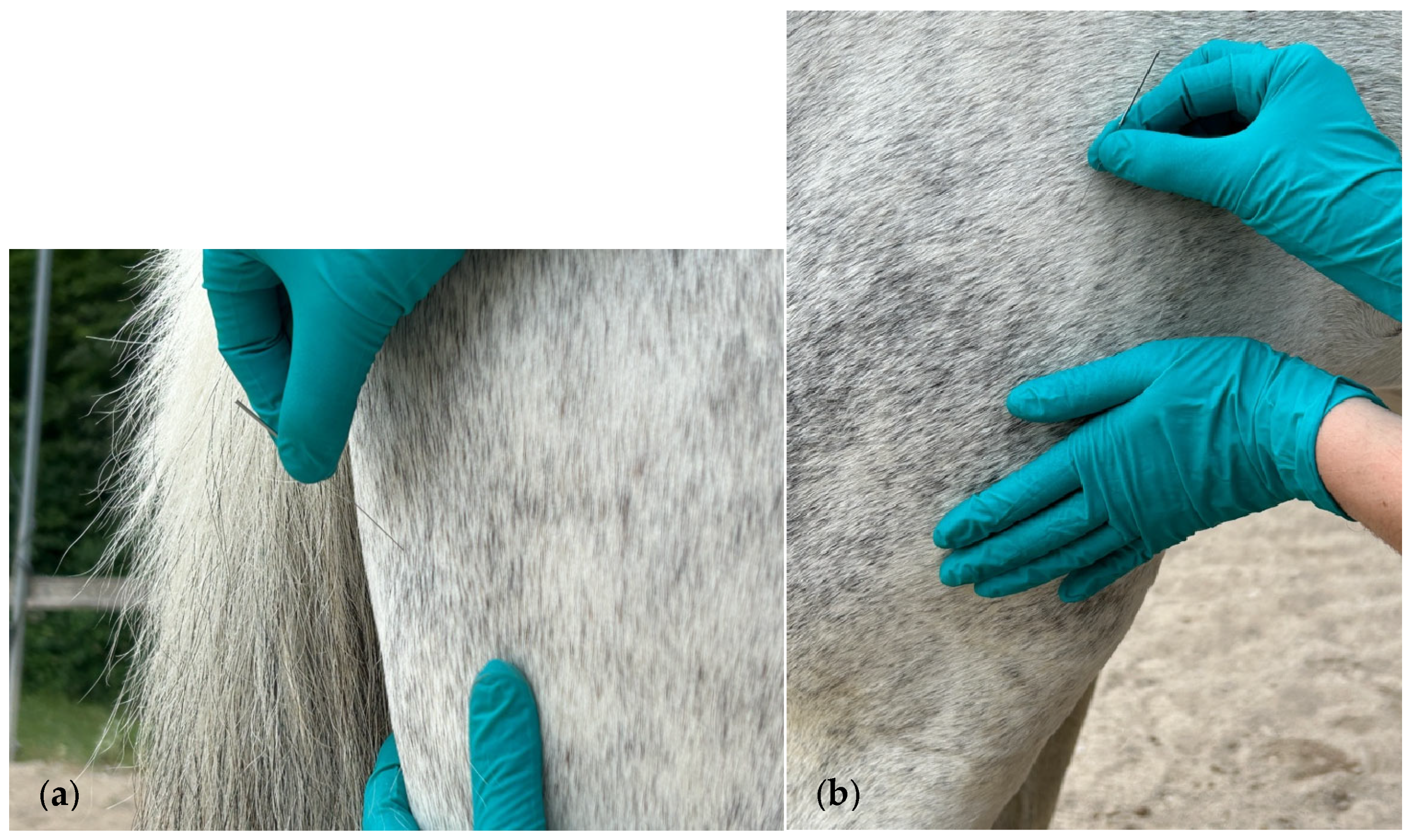
2.2. Muscle Assessment and DN Treatment Technique
2.3. Data Collection and Biomechanical Analysis
2.4. Procedure and Outcome Variable Measurements
2.5. Statistical Analysis
3. Results
3.1. Stride Frequency
3.2. Asymmetry Push-Off Phase
3.3. Asymmetry Impact Phase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPS | Myofascial pain syndrome |
| TrPs | Trigger points |
| DN | Dry needling |
| AI | Artificial intelligence |
| app | Smartphone application |
| TG | Treatment group |
| CG | Control group |
| SF | Stride frequency |
| FHROM | Forelimb head range of motion |
| LTRs | Local twitch responses |
| HPROM | Hindlimb pelvic range of motion |
| GLMMs | Generalized linear mixed models |
Appendix A
| Muscle | Anatomical References | Action | Needle Size (mm) |
|---|---|---|---|
| Brachiocephalicus | Origin: humeral crest. Insertion: mastoid process of the temporal bone. | Protraction of the forelimb. Bilateral: flexion of the neck. Unilateral: lateroflexion and rotation of the neck and poll. | 0.30 × 40 |
| Trapezius (cervical part) | Origin: nuchal and supraspinous ligament of C2–T10. Insertion: spine of the scapula. | Draws scapula cranially and upward. | 0.25 × 25 |
| Gluteus medius | Origin: longissimus dorsi, ilium, sacrum, and sacroiliac and sacrotuberous ligaments. Insertion: greater trochanter of the femur. | Extension, abduction, and internal rotation of the coxo-femoral joint. | 0.30 × 40 |
| Biceps femoris | Origin: spinous and transverse processes of Cd 3–5 and sacrotuberous ligament (vertebral part). Ischial tuberosity (pelvic part). Insertion: patella, intermediate and lateral patellar ligaments, tibial crest, crural fascia, and calcaneus. | Cranial part: extension of the coxo-femoral and stifle joint, as well as abduction. Caudal part: flexion of the knee, abduction, and extension of the tarsal joint. | 0.30 × 40 |
| Semitendinosus | Origin: last sacral vertebra, transverse processes of Cd 1–2, and sacrotuberous ligament (vertebral part). Ischial tuberosity (pelvic part). Insertion: cranial border of the tibia, crural fascia, and common calcanean tendon. | During weight-bearing: extension of the stifle and tarsal joint. During non-weight-bearing: flexion of the stifle joint and draws the limb caudally. | 0.30 × 40 |
| Quadriceps femoris (vastus lateralis) | Origin: lateral surface of the femur. Insertion: lateral part of the anterior surface and base of the patella. | Extension of the stifle joint. | 0.30 × 40 |
References
- Lawin, F.J.; Byström, A.; Roepstorff, C.; Rhodin, M.; Almlöf, M.; Silva, M.; Andersen, P.H.; Kjellström, H.; Hernlund, E. Is Markerless More or Less? Comparing a Smartphone Computer Vision Method for Equine Lameness Assessment to Multi-Camera Motion Capture. Animals 2023, 13, 390. [Google Scholar] [CrossRef]
- Ridgway, K.J. Diagnosis and Treatment of Equine Musculo-Skeletal Pain. The Role of the Complementary Modalities: Acupuncture and Chiropractic. AAEP Proc. 2005, 51, 403–408. [Google Scholar]
- Janssens, L.A. Trigger points in 48 dogs with myofascial pain syndromes. Vet. Surg. 1991, 20, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Janssens, L.A. Trigger point therapy. Probl. Vet. Med. 1992, 4, 117–124. [Google Scholar]
- Frank, E.M. Myofascial trigger point diagnostic criteria in the dog. J. Musculoskelet. Pain 1999, 7, 231–237. [Google Scholar] [CrossRef]
- Ridgway, K. Acupuncture as a treatment modality for back problems. Vet. Clin. N. Am. Equine Pract. 1999, 15, 211–221. [Google Scholar] [CrossRef]
- Rogers, P. Acupuncture for Equine Paraspinal Myofascial Pain. Am. J. Tradit. Chin. Vet. Med. 2012, 7, 69–75. [Google Scholar]
- Wall, R. Introduction to myofascial trigger points in dogs. Top. Companion Anim. Med. 2014, 29, 43–48. [Google Scholar] [CrossRef]
- Bowen, A.G.; Goff, L.M.; McGowan, C.M. Investigation of myofascial trigger points in equine pectoral muscles and girth-aversion behavior. J. Equine Vet. Sci. 2017, 48, 154–160.e1. [Google Scholar] [CrossRef]
- Sato, N.Y.S.; Bastos, B.B.B.; Pereira, M.A.A.; Campos, K.D.A.; Ambrósio, A.M.; Formenton, M.R.; Fantoni, D.T. Myofascial Pain Syndrome, myofascial trigger points and trigger points in veterinary medicine: A review. Braz. J. Vet. Res. Anim. Sci. 2020, 57, e164351. [Google Scholar] [CrossRef]
- Formenton, M.R.; Portier, K.; Gaspar, B.R.; Gauthier, L.; Yeng, L.T.; Fantoni, D.T. Location of Trigger Points in a Group of Police Working Dogs: A Preliminary Study. Animals 2023, 13, 2836. [Google Scholar] [CrossRef] [PubMed]
- Macgregor, J.; Graf von Schweinitz, D. Needle electromyographic activity of myofascial trigger points and control sites in equine cleidobrachialis muscle--an observational study. Acupunct. Med. 2006, 24, 61–70. [Google Scholar] [CrossRef]
- Simons, D. Myofascial Pain and Dysfunction: The Trigger Point Manual; Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- Dommerholt, J.; Gerwin, R.; Courtney, C. Pain sciences and myofascial pain. In Travell, Simons & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual; Wolters Kluwer: Baltimore, MD, USA, 2019; Volume 3, pp. 2–28. [Google Scholar]
- Galasso, A.; Urits, I.; An, D.; Nguyen, D.; Borchart, M.; Yazdi, C.; Manchikanti, L.; Kaye, R.J.; Kaye, A.D.; Mancuso, K.F. A comprehensive review of the treatment and management of myofascial pain syndrome. Curr. Pain Headache reports 2020, 24, 43. [Google Scholar] [CrossRef]
- Money, S. Pathophysiology of Trigger Points in Myofascial Pain Syndrome. J. Pain Palliat. Care Pharmacother. 2017, 31, 158–159. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Dommerholt, J. International Consensus on Diagnostic Criteria and Clinical Considerations of Myofascial Trigger Points: A Delphi Study. Pain Med. 2018, 19, 142–150. [Google Scholar] [CrossRef]
- Romero-Morales, C.; Bravo-Aguilar, M.; Abuín-Porras, V.; Almazán-Polo, J.; Calvo-Lobo, C.; Martínez-Jiménez, E.M.; López-López, D.; Navarro-Flores, E. Current advances and novel research on minimal invasive techniques for musculoskeletal disorders. Dis. Mon. 2021, 67, 101210. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Nijs, J. Trigger point dry needling for the treatment of myofascial pain syndrome: Current perspectives within a pain neuroscience paradigm. J. Pain Res. 2019, 12, 1899–1911. [Google Scholar] [CrossRef]
- Khan, I.; Ahmad, A.; Ahmed, A.; Sadiq, S.; Asim, H.M. Effects of dry needling in lower extremity myofascial trigger points. JPMA J. Pak. Med. Assoc. 2021, 71, 2596–2603. [Google Scholar] [CrossRef]
- Dach, F.; Ferreira, K.S. Tratamento da dor miofascial com agulhamento a seco: Uma revisão sistemática para as melhores práticas baseadas em evidências em lombalgia. Arq. Neuro-Psiquiatria 2024, 81, 1169–1178. [Google Scholar]
- Mouloodi, S.; Rahmanpanah, H.; Gohari, S.; Burvill, C.; Tse, K.M.; Davies, H.M.S. What can artificial intelligence and machine learning tell us? A review of applications to equine biomechanical research. J. Mech. Behav. Biomed. Mater. 2021, 123, 104728. [Google Scholar] [CrossRef]
- Biau, S.; Barrey, E. Relationship between stride characteristics and scores in dressage tests. Pferdeheilkunde 2004, 20, 140–144. [Google Scholar] [CrossRef]
- Hobbs, S.J.; Serra Braganca, F.M.; Rhodin, M.; Hernlund, E.; Peterson, M.; Clayton, H.M. Evaluating Overall Performance in High-Level Dressage Horse-Rider Combinations by Comparing Measurements from Inertial Sensors with General Impression Scores Awarded by Judges. Animals 2023, 13, 2496. [Google Scholar] [CrossRef]
- Stebbins, J.; Harrington, M.; Stewart, C. Clinical gait analysis 1973-2023: Evaluating progress to guide the future. J. Biomech. 2023, 160, 111827. [Google Scholar] [CrossRef]
- Bao, T.; Gao, J.; Wang, J.; Chen, Y.; Xu, F.; Qiao, G.; Li, F. A global bibliometric and visualized analysis of gait analysis and artificial intelligence research from 1992 to 2022. Front. Robot. AI 2023, 10, 1265543. [Google Scholar] [CrossRef]
- Molavian, R.; Fatahi, A.; Abbasi, H.; Khezri, D. Artificial Intelligence Approach in Biomechanics of Gait and Sport: A Systematic Literature Review. J. Biomed. Phys. Eng. 2023, 13, 383–402. [Google Scholar] [CrossRef]
- Ben Chaabane, N.; Conze, P.H.; Lempereur, M.; Quellec, G.; Rémy-Néris, O.; Brochard, S.; Cochener, B.; Lamard, M. Quantitative gait analysis and prediction using artificial intelligence for patients with gait disorders. Sci. Rep. 2023, 13, 23099. [Google Scholar] [CrossRef]
- Iseki, C.; Hayasaka, T.; Yanagawa, H.; Komoriya, Y.; Kondo, T.; Hoshi, M.; Fukami, T.; Kobayashi, Y.; Ueda, S.; Kawamae, K.; et al. Artificial Intelligence Distinguishes Pathological Gait: The Analysis of Markerless Motion Capture Gait Data Acquired by an iOS Application (TDPT-GT). Sensors 2023, 23, 6217. [Google Scholar] [CrossRef]
- Egenvall, A.; Byström, A.; Lindsten, A.; Clayton, H.M. A Scoping Review of Equine Biomechanics Revisited. J. Equine Vet. Sci. 2022, 113, 103920. [Google Scholar] [CrossRef]
- Egan, S.; Brama, P.; McGrath, D. Research trends in equine movement analysis, future opportunities and potential barriers in the digital age: A scoping review from 1978 to 2018. Equine Vet. J. 2019, 51, 813–824. [Google Scholar] [CrossRef]
- Feuser, A.K.; Gesell-May, S.; Müller, T.; May, A. Artificial Intelligence for Lameness Detection in Horses-A Preliminary Study. Animals 2022, 12, 2804. [Google Scholar] [CrossRef]
- Pfau, T.; Landsbergen, K.; Davis, B.L.; Kenny, O.; Kernot, N.; Rochard, N.; Porte-Proust, M.; Sparks, H.; Takahashi, Y.; Toth, K.; et al. Comparing Inertial Measurement Units to Markerless Video Analysis for Movement Symmetry in Quarter Horses. Sensors 2023, 23, 8414. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, A.; Ebrahimi Takamjani, I.; Sarrafzadeh, J.; Salehi, R. Could dry needling change the kinematics of gait in individuals with piriformis muscle syndromes? Secondary analysis of a randomized controlled trial. J. Bodyw. Mov. Ther. 2024, 37, 323–327. [Google Scholar] [CrossRef]
- Rajfur, J.; Rajfur, K.; Matusz, T.; Malarska, M.; Walewicz, K.; Ptaszkowski, K.; Dymarek, R.; Taradaj, J. Dry Needling with the Use of FRSc Technique in Addition to Standard Rehabilitation Program for Chronic Low Back Pain: A Randomized Controlled Trial Using Both PROMs and Measurement Tools. J. Pain Res. 2024, 17, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.Z. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am. J. Phys. Med. Rehabil. 1994, 73, 256–263. [Google Scholar] [CrossRef]
- Gerwin, R.D.; Dommerholt, J.; Shah, J.P. An expansion of Simons’ integrated hypothesis of trigger point formation. Curr. Pain Headache Rep. 2004, 8, 468–475. [Google Scholar] [CrossRef]
- Bordoni, B.; Sugumar, K.; Varacallo, M. Myofascial Pain; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Byrne, C.; Twist, C.; Eston, R. Neuromuscular function after exercise-induced muscle damage: Theoretical and applied implications. Sports Med. 2004, 34, 49–69. [Google Scholar] [CrossRef]
- Mendonça, T.; Bienboire-Frosini, C.; Menuge, F.; Leclercq, J.; Lafont-Lecuelle, C.; Arroub, S.; Pageat, P. The Impact of Equine-Assisted Therapy on Equine Behavioral and Physiological Responses. Animals 2019, 9, 409. [Google Scholar] [CrossRef]
- Cagnie, B.; Dewitte, V.; Barbe, T.; Timmermans, F.; Delrue, N.; Meeus, M. Physiologic effects of dry needling. Curr. Pain Headache Rep. 2013, 17, 348. [Google Scholar] [CrossRef]
- Dyson, S. Can lameness be graded reliably? Equine Vet. J. 2011, 43, 379–382. [Google Scholar] [CrossRef]
- Bowen, A.G.; Tabor, G.; Labens, R.; Randle, H. Visually Assessing Equine Quality of Movement: A Survey to Identify Key Movements and Patient-Specific Measures. Animals 2023, 13, 2822. [Google Scholar] [CrossRef]
- Buchnefp, H.; Savelberg, H.; Schamhardt, H.; Barneveld, A. Temporal stride patterns in horses with experimentally induced fore-or hindlimb lameness. Equine Vet. J. 1995, 27, 161–165. [Google Scholar] [CrossRef]
- Denoix, J.-M. Biomechanics and Physical Training of the Horse; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- van Loon, J.; Van Dierendonck, M.C. Objective pain assessment in horses (2014–2018). Vet. J. 2018, 242, 1–7. [Google Scholar] [CrossRef]
- Gleerup, K.; Lindegaard, C. Recognition and quantification of pain in horses: A tutorial review. Equine Vet. Educ. 2016, 28, 47–57. [Google Scholar] [CrossRef]



| Variable | Factor (Effect) | df | ChiSq | p |
|---|---|---|---|---|
| Number of front strides | Treatment | 1 | 1.501 | 0.221 |
| Time | 2 | 0.302 | 0.860 | |
| Treatment × time | 2 | 2.695 | 0.260 | |
| Variation of front strides | Treatment | 1 | 0.351 | 0.554 |
| Time | 2 | 0.192 | 0.909 | |
| Treatment × time | 2 | 0.191 | 0.909 | |
| Number of hind strides | Treatment | 1 | 3.277 | 0.070 |
| Time | 2 | 3.196 | 0.202 | |
| Treatment × time | 2 | 2.086 | 0.352 | |
| Variation of hind strides | Treatment | 1 | 0.103 | 0.748 |
| Time | 2 | 0.211 | 0.900 | |
| Treatment × time | 2 | 0.214 | 0.899 | |
| Stride frequency | Treatment | 1 | 1.389 | 0.239 |
| Time | 2 | 12.261 | 0.002 | |
| Treatment × time | 2 | 8.383 | 0.015 |
| Variable | Factor (Effect) | df | ChiSq | p |
|---|---|---|---|---|
| FHROM (%) | Treatment | 1 | 0.117 | 0.732 |
| Time | 2 | 1.209 | 0.546 | |
| Treatment × time | 2 | 4.898 | 0.086 | |
| FHROM severity | Treatment | 1 | 6.540 | 0.011 |
| Time | 2 | 5.467 | 0.065 | |
| Treatment × time | 2 | 5.451 | 0.066 | |
| HPROM (%) | Treatment | 1 | 0.649 | 0.420 |
| Time | 2 | 0.237 | 0.888 | |
| Treatment × time | 2 | 2.632 | 0.268 | |
| HPROM severity | Treatment | 1 | 0.426 | 0.514 |
| Time | 2 | 0.281 | 0.869 | |
| Treatment × time | 2 | 0.290 | 0.865 |
| Variable | Factor (Effect) | df | ChiSq | p |
|---|---|---|---|---|
| FHROM (%) | Treatment | 1 | 0.355 | 0.551 |
| Time | 2 | 1.798 | 0.407 | |
| Treatment × time | 2 | 0.393 | 0.822 | |
| FHROM severity | Treatment | 1 | 0.363 | 0.547 |
| Time | 2 | 0.718 | 0.698 | |
| Treatment × time | 2 | 0.825 | 0.662 | |
| HPROM (%) | Treatment | 1 | 0.341 | 0.559 |
| Time | 2 | 3.886 | 0.143 | |
| Treatment × time | 2 | 0.062 | 0.969 | |
| HPROM severity | Treatment | 1 | 0.111 | 0.739 |
| Time | 2 | 0.497 | 0.780 | |
| Treatment × time | 2 | 0.185 | 0.912 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resano-Zuazu, M.; Carmona, J.U.; Argüelles, D. Short-Term Impact of Dry Needling Treatment for Myofascial Pain on Equine Biomechanics Through Artificial Intelligence-Based Gait Analysis. Animals 2025, 15, 1517. https://doi.org/10.3390/ani15111517
Resano-Zuazu M, Carmona JU, Argüelles D. Short-Term Impact of Dry Needling Treatment for Myofascial Pain on Equine Biomechanics Through Artificial Intelligence-Based Gait Analysis. Animals. 2025; 15(11):1517. https://doi.org/10.3390/ani15111517
Chicago/Turabian StyleResano-Zuazu, María, Jorge U. Carmona, and David Argüelles. 2025. "Short-Term Impact of Dry Needling Treatment for Myofascial Pain on Equine Biomechanics Through Artificial Intelligence-Based Gait Analysis" Animals 15, no. 11: 1517. https://doi.org/10.3390/ani15111517
APA StyleResano-Zuazu, M., Carmona, J. U., & Argüelles, D. (2025). Short-Term Impact of Dry Needling Treatment for Myofascial Pain on Equine Biomechanics Through Artificial Intelligence-Based Gait Analysis. Animals, 15(11), 1517. https://doi.org/10.3390/ani15111517






