Dietary Galacto-Oligosaccharides Enhance Growth Performance and Modulate Gut Microbiota in Weaned Piglets: A Sustainable Alternative to Antibiotics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals and Experimental Design
2.3. Sample Collection
2.4. Growth Performance and Diarrhea Ratio
2.5. Morphological Examination
2.6. Intestinal Microbiome Structure
2.7. Bioinformatics Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth Performance and Diarrhea Rate
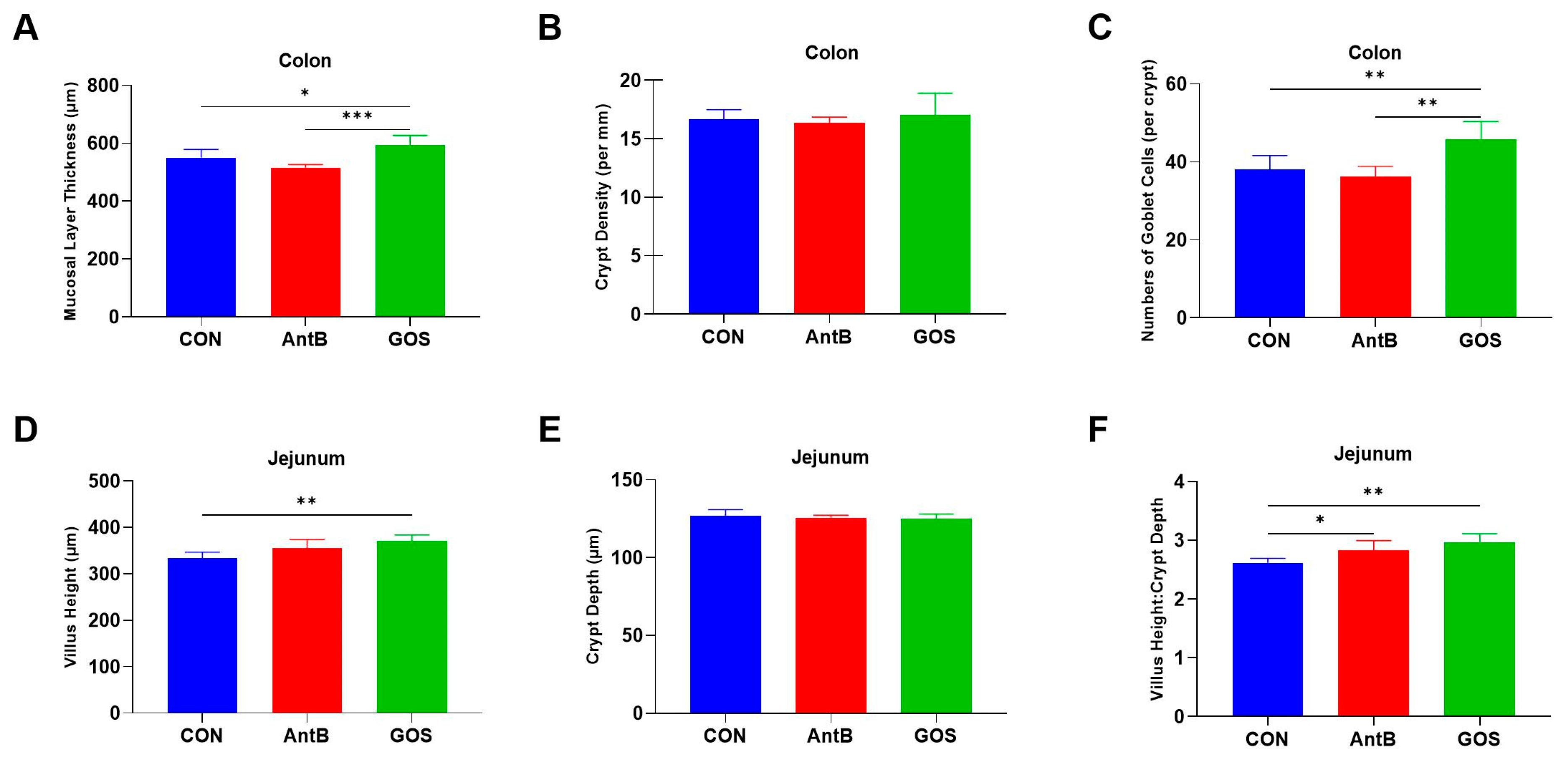
3.2. Effects of Dietary Treatments on Colonic and Jejunal Morphology
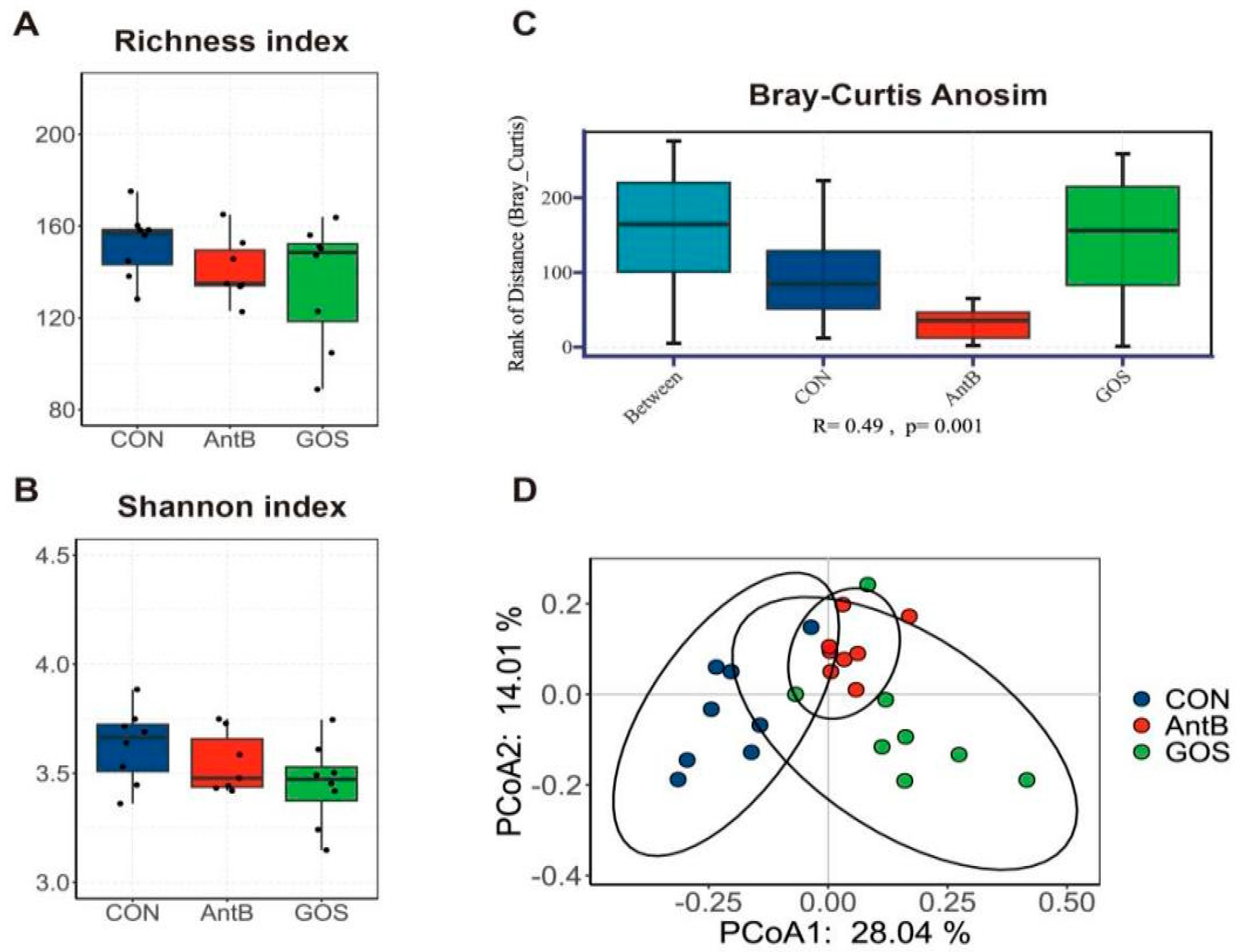
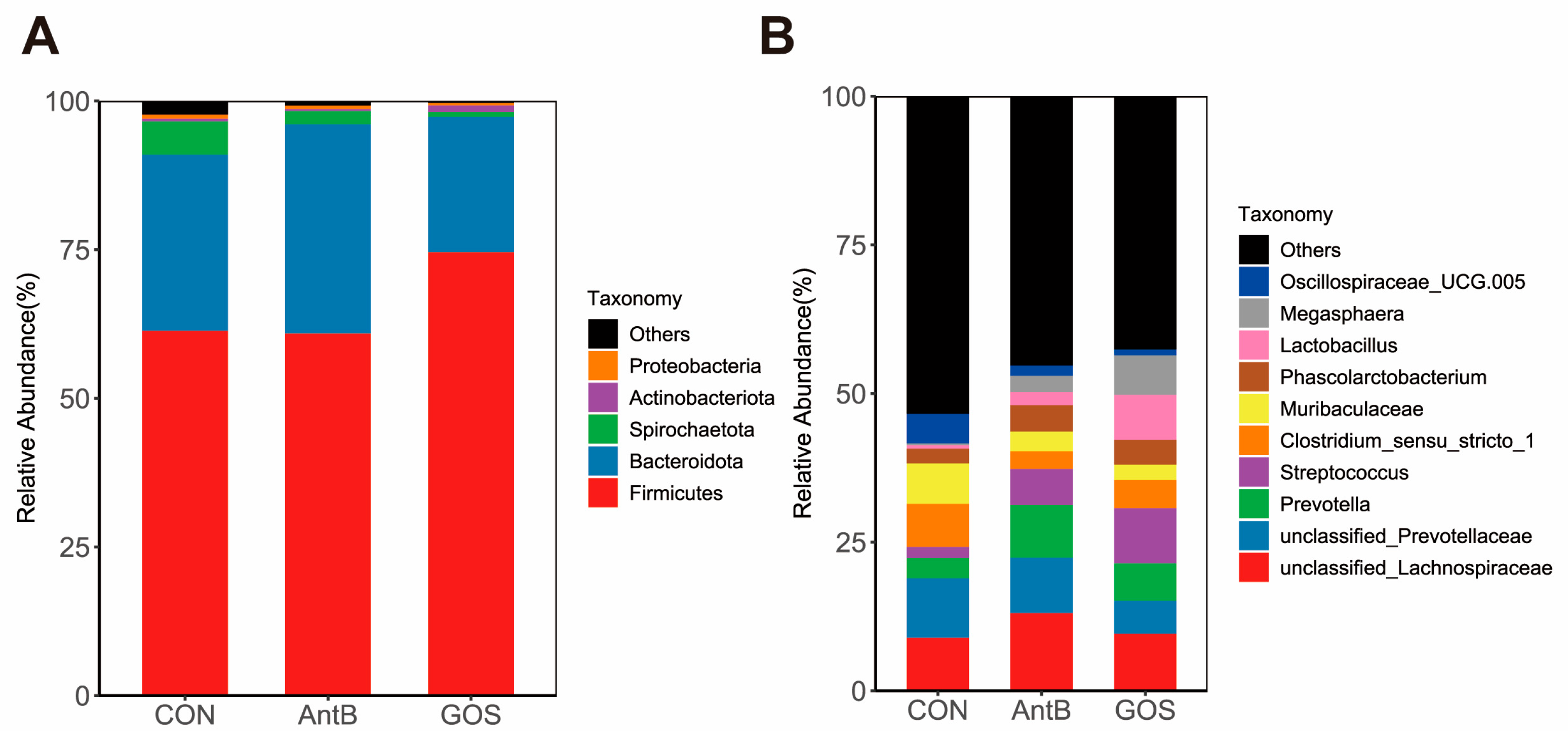
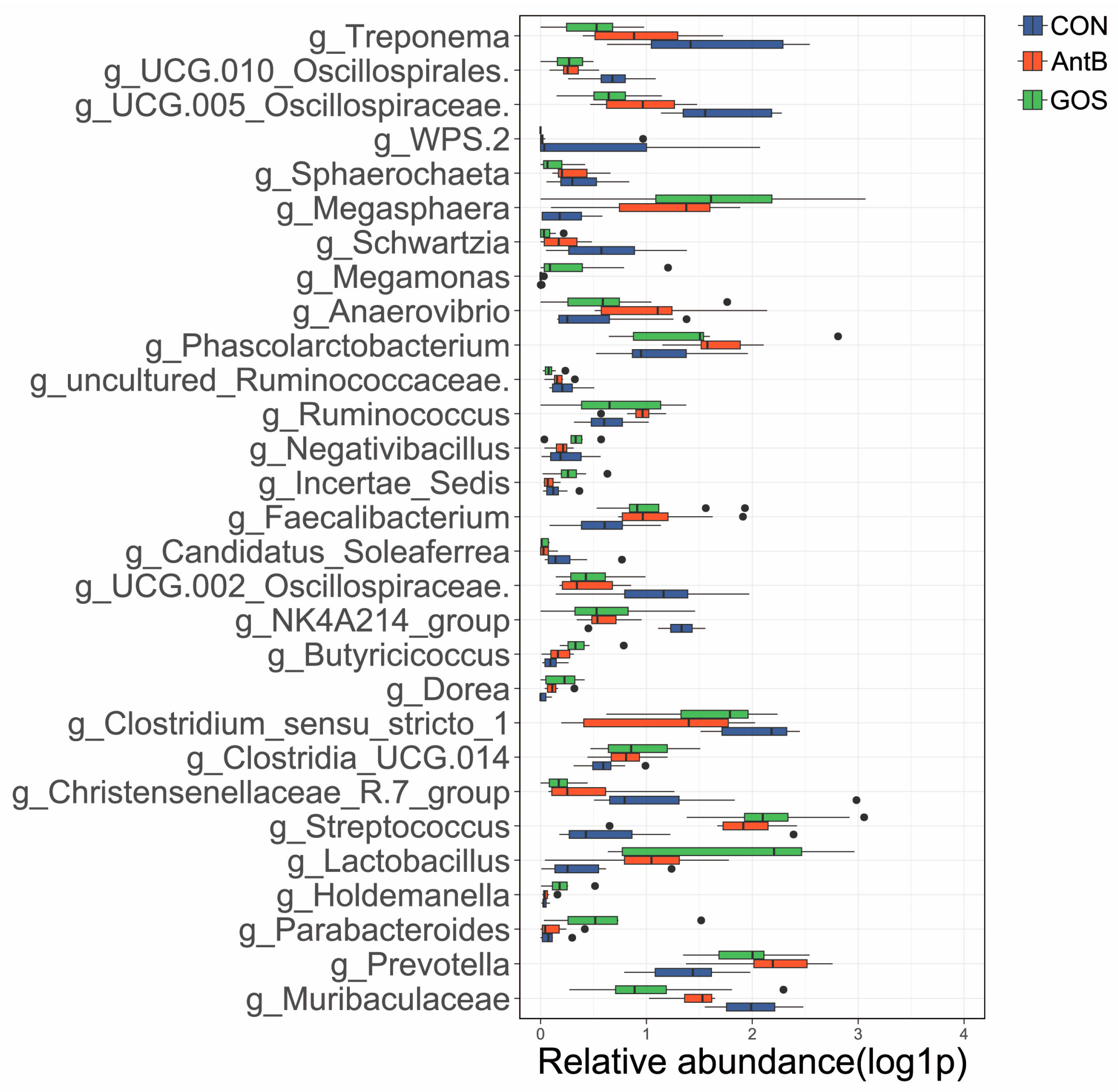
3.3. Effects of Dietary Treatments on the Colonic Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Wang, T.; Teng, K.; Liu, Y.; Shi, W.; Zhang, J.; Dong, E.; Zhang, X.; Tao, Y.; Zhong, J. Lactobacillus plantarum PFM 105 Promotes Intestinal Development Through Modulation of Gut Microbiota in Weaning Piglets. Front. Microbiol. 2019, 10, 90. [Google Scholar] [CrossRef]
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002, 13, 7–27. [Google Scholar] [CrossRef]
- Wijtten, P.J.; van der Meulen, J.; Verstegen, M.W. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, C.; Zhang, D.; Wang, R.; Gao, F. Effects of Low-Dose Antibiotics on Gut Immunity and Antibiotic Resistomes in Weaned Piglets. Front. Immunol. 2020, 11, 903. [Google Scholar] [CrossRef]
- Yang, H.; Paruch, L.; Chen, X.J.; Eerde, A.V.; Skomeda, H.; Wang, Y.L.; Liu, D.; Clarke, J.L. Antibiotic Application and Resistance in Swine Production in China: Current Situation and Future Perspectives. Front. Vet. Sci. 2019, 6, 136. [Google Scholar] [CrossRef]
- Neuman, H.; Forsythe, P.; Uzan, A.; Avni, O.; Koren, O. Antibiotics in early life: Dysbiosis and the damage done. FEMS Microbiol. Rev. 2018, 42, 489–499. [Google Scholar] [CrossRef]
- Toutain, P.L.; Ferran, A.A.; Bousquet-Melou, A.; Pelligand, L.; Lees, P. Veterinary Medicine Needs New Green Antimicrobial Drugs. Front. Microbiol. 2016, 7, 1196. [Google Scholar] [CrossRef]
- Herrero-Fresno, A.; Zachariasen, C.; Hansen, M.H.; Nielsen, A.; Hendriksen, R.S.; Nielsen, S.S.; Olsen, J.E. Apramycin treatment affects selection and spread ofa multidrug-resistant Escherichia coli strain able to colonize the human gut in the intestinal microbiota of pigs. Vet. Res. 2016, 47, 12. [Google Scholar] [CrossRef]
- Smith, M.G.; Jordan, D.; Chapman, T.A.; Chin, J.C.; Barton, M.D.; Do, T.N.; Fahy, V.; Fairbrother, J.; Trott, D. Antimicrobial resistance and virulence gene profiles in multi-drug resistant enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea. Vet. Microbiol. 2010, 145, 299–307. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Zhang, R.; Chen, Y.; Shen, Y.; Hu, F.; Liu, D.; Lu, J.; Guo, Y.; Xia, X.; et al. Changes in colistin resistance and mcr-1 abundance in Escherichia coli of animal and human origins following the ban of colistin-positive additives in China: An epidemiological comparative study. Lancet Infect. Dis. 2020, 20, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Reid, G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Edrington, T.S.; Anderson, R.C.; Harvey, R.B.; Genovese, K.J.; Kennedy, C.N.; Venn, D.W.; Nisbet, D.J. Probiotics, prebiotics and competitive exclusion for prophylaxis against bacterial disease. Anim. Health Res. Rev. 2008, 9, 217–225. [Google Scholar] [CrossRef]
- Mikkelsen, L.L.; Jakobsen, M.; Jensen, B.B. Effects of dietary oligosaccharides on microbial diversity and fructo-oligosaccharide degrading bacteria in faeces of piglets post-weaning. Anim. Feed Sci. Technol. 2003, 109, 133–150. [Google Scholar] [CrossRef]
- Mair, C.; Plitzner, C.; Domig, K.J.; Schedle, K.; Windisch, W. Impact of inulin and a multispecies probiotic formulation on performance, microbial ecology and concomitant fermentation patterns in newly weaned piglets. J. Anim. Physiol. Anim. Nutr. 2010, 94, e164–e177. [Google Scholar] [CrossRef]
- Alizadeh, A.; Akbari, P.; Difilippo, E.; Schols, H.A.; Ulfman, L.H.; Schoterman, M.H.C.; Braber, S. The piglet as a model for studying dietary components in infant diets: Effects of galacto-oligosaccharides on intestinal functions. Br. J. Nutr. 2016, 115, 605–618. [Google Scholar] [CrossRef]
- Samanta, A.K.; Jayapal, N.; Jayaram, C.; Roy, S.; Kolte, A.P.; Senani, S.; Sridhar, M. Xylooligosaccharides as prebiotics from agricultural by-products: Production and applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 62–71. [Google Scholar] [CrossRef]
- Hill, A.; Tian, F.; Karboune, S. Synthesis of Levan and Fructooligosaccharides by Levansucrase: Catalytic, Structural and Substrate-Specificity Properties. Curr. Org. Chem. 2016, 21, 149–161. [Google Scholar] [CrossRef][Green Version]
- Tzortzis, G. Functional properties of the second generation prebiotic Galacto-oligosaccharide (B-GOS). Agro Food Ind. Hi Tech. 2009, 20, 43–46. [Google Scholar]
- Vandenplas, Y.; Zakharova, I.; Dmitrieva, Y. Oligosaccharides in infant formula: More evidence to validate the role of prebiotics. Br. J. Nutr. 2015, 113, 1339–1344. [Google Scholar] [CrossRef]
- Boehm, G.; Jelinek, J.; Stahl, B.; Van Laere, K.; Knol, J.; Fanaro, S.; Vigi, V. Prebiotics in infant formulas. J. Clin. Gastroenterol. 2004, 38, S76–S79. [Google Scholar] [CrossRef]
- Martinez-Ferez, A.; Rudloff, S.; Guadix, A.; Henkel, C.A.; Pohlentz, G.; Boza, J.J.; Guadix, E.M.; Kunz, C. Goats’ milk as a natural source of lactose-derived oligosaccharides: Isolation by membrane technology. Int. Dairy J. 2006, 16, 173–181. [Google Scholar] [CrossRef]
- Cardellecobas, A.; Corzo, N.; Olano, A.; Peláez, C.; Requena, T.; Ávila, M. Galactooligosaccharides derived from lactose and lactulose: Influence of structure on lactobacillus, streptococcus and bifidobacterium growth. Int. J. Food Microbiol. 2011, 149, 81–87. [Google Scholar] [CrossRef]
- Gormley, A.; Garavito-Duarte, Y.; Kim, S.W. The Role of Milk Oligosaccharides in Enhancing Intestinal Microbiota, Intestinal Integrity, and Immune Function in Pigs: A Comparative Review. Biology 2024, 13, 663. [Google Scholar] [CrossRef]
- Farthing, M.J. Bugs and the gut: An unstable marriage. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 233–239. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Yu, H.; Wang, J.; Zhu, W. Effects of galacto-oligosaccharides on growth and gut function of newborn suckling piglets. J. Anim. Sci. Biotechnol. 2018, 9, 75. [Google Scholar] [CrossRef]
- Lee, A.; Mansbridge, S.C.; Liang, L.; Connerton, I.F.; Mellits, K.H. Galacto-Oligosaccharides Increase the Abundance of Beneficial Probiotic Bacteria and Improve Gut Architecture and Goblet Cell Expression in Poorly Performing Piglets, but Not Performance. Animals 2023, 13, 230. [Google Scholar] [CrossRef]
- Boston, T.E.; Wang, F.; Lin, X.; Kim, S.W.; Fellner, V.; Scott, M.F.; Odle, J. Prebiotic galactooligosaccharide improves piglet growth performance and intestinal health associated with alterations of the hindgut microbiota during the peri-weaning period. J. Anim. Sci. Biotechnol. 2024, 15, 88. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 7th ed.; National Academy Press: Washington, DC, USA, 2012.
- Song, Y.; Luo, Y.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Yu, J. Tannic acid extracted from gallnut prevents post-weaning diarrhea and improves intestinal health of weaned piglets. Anim. Nutr. 2021, 7, 1078–1086. [Google Scholar] [CrossRef]
- Shatos, M.A.; Ríos, J.D.; Horikawa, Y.; Hodges, R.R.; Chang, E.L.; Bernardino, C.R.; Dartt, D.A. Isolation and Characterization of Cultured Human Conjunctival Goblet Cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 550. [Google Scholar] [CrossRef]
- Richards, P.J.; Flaujac Lafontaine, G.M.; Connerton, P.L.; Liang, L.; Asiani, K.; Fish, N.M.; Connerton, I.F. Galacto-oligosaccharides modulate the juvenile gut microbiome and innate immunity to improve broiler chicken performance. Msystems 2020, 5, 10-1128. [Google Scholar] [CrossRef]
- Yu, X.; Ma, F.; Dai, H.; Liu, J.; Hashem, N.M.; Sun, P. Effects of Different Galacto-Oligosaccharide Supplementation on Growth Performance, Immune Function, Serum Nutrients, and Appetite-Related Hormones in Holstein Calves. Animals 2023, 13, 3366. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Wang, J.; Zhu, W. Differential Effects of Early-Life and Postweaning Galacto-oligosaccharide Intervention on Colonic Bacterial Composition and Function in Weaning Piglets. Appl. Environ. Microbiol. 2022, 88, e01318–e01321. [Google Scholar] [CrossRef]
- Chang, M.; Wang, F.; Ma, F.; Jin, Y.; Sun, P. Supplementation with galacto-oligosaccharides in early life persistently facilitates the microbial colonization of the rumen and promotes growth of preweaning Holstein dairy calves. Anim. Nutr. 2022, 10, 223–233. [Google Scholar] [CrossRef]
- Pan, L.; Mohammed, F.; Qin, G.; Yuan, Z.; Bao, N. The influences of soybean agglutinin and functional oligosaccharides on the intestinal tract of monogastric animals. Int. J. Mol. Sci. 2018, 19, 554. [Google Scholar] [CrossRef]
- Choe, D.W.; Loh, T.C.; Foo, H.L.; Hair-Bejo, M.; Awis, Q.S. Egg production, faecal pH and microbial population, small intestine morphology, and plasma and yolk cholesterol in laying hens given liquid metabolites produced by Lactobacillus plantarumstrains. Br. Poult. Sci. 2012, 53, 106–115. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Q.; Qi, R.; Wang, J.; Qiu, X.; Liu, Z.; Huang, J. Effects of Lactobacillus plantarum on the intestinal morphology, intestinal barrier function and microbiota composition of suckling piglets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1908–1918. [Google Scholar] [CrossRef]
- Zhu, C.; Yao, J.; Zhu, M.; Zhu, C.; Yuan, L.; Li, Z.; Liu, H.Y. A meta-analysis of Lactobacillus-based probiotics for growth performance and intestinal morphology in piglets. Front. Vet. Sci. 2022, 9, 1045965. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Y.; Wang, J.; Lv, Z.; Li, E.; Zhao, J.; Liu, L.; Wang, F.; Liu, H. Effects of reduced dietary protein at high temperature in summer on growth performance and carcass quality of finishing pigs. Animals 2022, 12, 599. [Google Scholar] [CrossRef]
- Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef]
- Jacobi, S.K.; Odle, J. Nutritional factors influencing intestinal health of the neonate. Adv. Nutr. 2012, 3, 687–696. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Q.; Li, Y.; Tang, Z.; Sun, W.; Zhang, X.; Sun, Z. Comparative effects of dietary supplementations with sodium butyrate, medium-chain fatty acids, and n-3 polyunsaturated fatty acids in late pregnancy and lactation on the reproductive performance of sows and growth performance of suckling piglets. J. Anim. Sci. 2019, 97, 4256–4267. [Google Scholar] [CrossRef]
- Hu, J.; Chen, L.; Zheng, W.; Shi, M.; Liu, L.; Xie, C.; Yan, X. Lactobacillus frumenti Facilitates Intestinal Epithelial Barrier Function Maintenance in Early Weaning Piglets. Front. Microbiol. 2018, 9, 897. [Google Scholar] [CrossRef]
- Nowland, T.L.; Plush, K.J.; Barton, M.; Kirkwood, R.N. Development and Function of the Intestinal Microbiome and Potential Implications for Pig Production. Animals 2019, 9, 76. [Google Scholar] [CrossRef]
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 109. [Google Scholar] [CrossRef]
- Gao, R.; Tian, S.; Wang, J.; Zhu, W. Galacto-oligosaccharides improve barrier function and relieve colonic inflammation via modulating mucosa-associated microbiota composition in lipopolysaccharides-challenged piglets. J. Anim. Sci. Biotechnol. 2021, 12, 92. [Google Scholar] [CrossRef]
- Ban-Tokuda, T.; Maekawa, S.; Miwa, T.; Ohkawara, S.; Matsui, H. Changes in faecal bacteria during fattening in finishing swine. Anaerobe 2017, 47, 188–193. [Google Scholar] [CrossRef]
- Spence, C.; Wells, W.G.; Smith, C.J. Characterization of the primary starch utilization operon in the obligate anaerobe Bacteroides fragilis: Regulation by carbon source and oxygen. J. Bacteriol. 2006, 188, 4663–4672. [Google Scholar] [CrossRef]
- Han, G.G.; Lee, J.Y.; Jin, G.D.; Park, J.; Choi, Y.H.; Chae, B.J.; Choi, Y.J. Evaluating the association between body weight and the intestinal microbiota of weaned piglets via 16S rRNA sequencing. Appl. Microbiol. Biotechnol. 2017, 101, 5903–5911. [Google Scholar] [CrossRef]
- Magnusson, K.R.; Hauck, L.; Jeffrey, B.M.; Elias, V.; Humphrey, A.; Nath, R.; Bermudez, L.E. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience 2015, 300, 128–140. [Google Scholar] [CrossRef]
- Guida, S.; Venema, K. Gut microbiota and obesity: Involvement of the adipose tissue. J. Funct. Foods 2015, 14, 407–423. [Google Scholar] [CrossRef]
- Ley, R.; Turnbaugh, P.; Klein, S.; Gordon, J.I. Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Lopezsiles, M.; Duncan, S.H.; Garciagil, L.J.; Martinezmedina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef]
- Kravtsov, E.G.; Yermolayev, A.V.; Anokhina, I.V.; Yashina, N.V.; Chesnokova, V.L.; Dalin, M.V. Adhesion characteristics of lactobacillus is a criterion of the probiotic choice. Bull. Exp. Biol. Med. 2008, 145, 232–234. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Follador, R. Metabolism of oligosaccharides and starch in lactobacilli: A review. Front. Microbiol. 2012, 3, 340. [Google Scholar] [CrossRef]
- Xing, Y.-Y.; Li, K.-N.; Xu, Y.-Q.; Wu, Y.-Z.; Shi, L.-L.; Guo, S.-W.; Yan, S.-M.; Jin, X.; Shi, B.-L. Effects of galacto-oligosaccharide on growth performance, feacal microbiota, immune response and antioxidant capability in weaned piglets. J. Appl. Anim. Res. 2020, 48, 63–69. [Google Scholar] [CrossRef]
- Li, Y.; Hou, S.; Chen, J.; Peng, W.; Wen, W.; Chen, F.; Huang, X. Oral administration of Lactobacillus delbrueckii during the suckling period improves intestinal integrity after weaning in piglets. J. Funct. Foods 2019, 63, 103591. [Google Scholar] [CrossRef]
- Dumonceaux, T.J.; Hill, J.E.; Hemmingsen, S.M.; Van Kessel, A.G. Characterization ofintestinal microbiota and response to dietary virginiamycin supplementation in the broiler chicken. Appl. Environ. Microbiol. 2006, 72, 2815–2823. [Google Scholar] [CrossRef]
- Lin, J. Effect ofantibiotic growth promoters on intestinal microbiota in food animals: A novel model for studying the relationship between gut microbiota and human obesity? Front. Microbiol. 2011, 2, 53. [Google Scholar] [CrossRef]
- Ushida, K.; Kishimoto, A.; Piao, S.J.; Itoh, M.; Shiga, A.; Nakanishi, N.; Tsukahara, T. An epidemiological survey on pigs showing symptoms of infectious enteric diseases and dyspepsia in Japan. Anim. Sci. J. 2009, 80, 556–561. [Google Scholar] [CrossRef]
- Ariyoshi, T.; Hagihara, M.; Takahashi, M.; Mikamo, H. Effect of Clostridium butyricum on gastrointestinal infections. Biomedicines 2022, 10, 483. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Liu, J.; Zhu, W.; Mao, S. A high grain diet dynamically shifted the composition ofmucosa-associated microbiota and induced mucosal injuries in the colon of sheep. Front. Microbiol. 2017, 8, 2080. [Google Scholar] [CrossRef]
- Ho, E.L.; Lukehart, S.A. Syphilis: Using modern approaches to understand an old disease. J. Clin. Investig. 2011, 121, 4584–4592. [Google Scholar] [CrossRef]
- Zhao, J.B.; Liu, P.; Huang, C.F.; Liu, L.; Li, E.K.; Zhang, G.; Zhang, S. Effect of wheat bran on apparent total tract digestibility, growth performance, fecal microbiota and their metabolites in growing pigs. Anim. Feed. Sci. Technol. 2018, 239, 14–26. [Google Scholar] [CrossRef]
- Oladele, P.; Li, E.; Lu, H.; Cozannet, P.; Nakatsu, C.; Johnson, T.; Adeola, O.; Ajuwon, K.M. Effect of a carbohydrase admixture in growing pigs fed wheat-based diets in thermoneutral and heat stress conditions. J. Anim. Sci. 2021, 99, skab254. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, H.; Qiu, Y.; Bai, Z.; Yang, Z.; Li, E.; Ma, X.; Xiao, D. Alternative Uses of Fermented Wheat Bran: A Mini Review. Fermentation 2024, 10, 611. [Google Scholar] [CrossRef]
| Items | Content (%) |
|---|---|
| Ingredients | |
| Corn | 52.53 |
| Soybean meal | 19.00 |
| Full-fat soybean powder | 9.00 |
| Fish meal | 4.00 |
| Whey powder | 8.00 |
| Soybean oil | 3.60 |
| Dicalcium phosphate | 0.90 |
| L-Lysine-HCl, 78% | 0.46 |
| L-Threonine | 0.15 |
| DL-Methionine | 0.10 |
| L-Tryptophan | 0.03 |
| L-Valine | 0.12 |
| Salt | 0.30 |
| Limestone | 0.81 |
| Premix a | 1.00 |
| Total | 100.00 |
| Calculated nutrients | |
| Digestible energy (MJ/kg) | 14.53 |
| Crude protein | 19.38 |
| SID Lys | 1.35 |
| SID Met | 0.39 |
| SID Thr | 0.72 |
| SID Try | 0.22 |
| Items | CON | AntB | GOS | SEM | p Value |
|---|---|---|---|---|---|
| d 0 BW, kg | 7.75 | 7.76 | 7.79 | 0.06 | 0.89 |
| d 7 BW, kg | 9.00 b | 9.26 a | 9.27 a | 0.06 | 0.01 |
| d 14 BW, kg | 10.92 b | 11.50 a | 11.53 a | 0.07 | <0.01 |
| Diarrhea rate, % | 19.64 a | 8.04 b | 9.82 b | 3.20 | 0.047 |
| d 1~7 | |||||
| ADG, g/d | 179 b | 214 a | 212 a | 2.33 | <0.01 |
| ADFI, g/d | 336 | 358 | 359 | 17.74 | 0.59 |
| G:F | 0.55 | 0.61 | 0.60 | 0.04 | 0.48 |
| d 7~14 | |||||
| ADG, g/d | 274 b | 319 a | 323 a | 5.94 | <0.01 |
| ADFI, g/d | 639 | 615 | 619 | 15.18 | 0.53 |
| G:F | 0.43 b | 0.52 a | 0.52 a | 0.01 | <0.01 |
| d 1~14 | |||||
| ADG, g/d | 227 b | 267 a | 268 a | 2.56 | <0.01 |
| ADFI, g/d | 487 | 486 | 489 | 9.27 | 0.98 |
| G:F | 0.47 b | 0.55 a | 0.55 a | 0.01 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, Z.; Chen, G.; Xing, Y.; Wang, J.; Zhao, Y.; Kang, M.; Huang, K.; Li, E.; Ma, X. Dietary Galacto-Oligosaccharides Enhance Growth Performance and Modulate Gut Microbiota in Weaned Piglets: A Sustainable Alternative to Antibiotics. Animals 2025, 15, 1508. https://doi.org/10.3390/ani15111508
Wang Y, Li Z, Chen G, Xing Y, Wang J, Zhao Y, Kang M, Huang K, Li E, Ma X. Dietary Galacto-Oligosaccharides Enhance Growth Performance and Modulate Gut Microbiota in Weaned Piglets: A Sustainable Alternative to Antibiotics. Animals. 2025; 15(11):1508. https://doi.org/10.3390/ani15111508
Chicago/Turabian StyleWang, Yongchao, Zhong Li, Guowu Chen, Yiyuan Xing, Jingjing Wang, Yujie Zhao, Meng Kang, Ke Huang, Enkai Li, and Xiaokang Ma. 2025. "Dietary Galacto-Oligosaccharides Enhance Growth Performance and Modulate Gut Microbiota in Weaned Piglets: A Sustainable Alternative to Antibiotics" Animals 15, no. 11: 1508. https://doi.org/10.3390/ani15111508
APA StyleWang, Y., Li, Z., Chen, G., Xing, Y., Wang, J., Zhao, Y., Kang, M., Huang, K., Li, E., & Ma, X. (2025). Dietary Galacto-Oligosaccharides Enhance Growth Performance and Modulate Gut Microbiota in Weaned Piglets: A Sustainable Alternative to Antibiotics. Animals, 15(11), 1508. https://doi.org/10.3390/ani15111508







