Effects of Grape Seed Proanthocyanidins on Growth Performance, Jejunal Antioxidant Capacity, Gut Microbial Diversity, and Metabolites in Kangle Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Experimental Animals and Treatments
2.2. The Sample Collection and Processing
2.3. Measurement of the Indicators and Methods
2.3.1. Growth Performance
2.3.2. Serum and Jejunal Tissue Indicators
2.3.3. Jejunal Histology
2.4. Microbiota 16s rRNA Sequencing of the Jejunal Contents
2.5. The Metabolomic Analysis of the Jejunal Contents
2.6. The Statistical Analysis
3. Results
3.1. The Effects of Dietary GSPs on the Growth Performance of Kangle Chickens
3.2. The Effects of Dietary GSPs on the Visceral Organ Indices of the Kangle Chickens
3.3. The Effects of the Dietary GSPs on the Serum Biochemical Parameters of the Kangle Chickens
3.4. The Effects of the Dietary GSPs on the Jejunal Morphology of Kangle Chickens
3.5. The Effects of the Dietary GSPs on the Jejunal Antioxidant Capacity of the Kangle Chickens
3.6. The Effects of the GSPs on the Jejunal Microbiota Composition in the CON and HGSP Groups
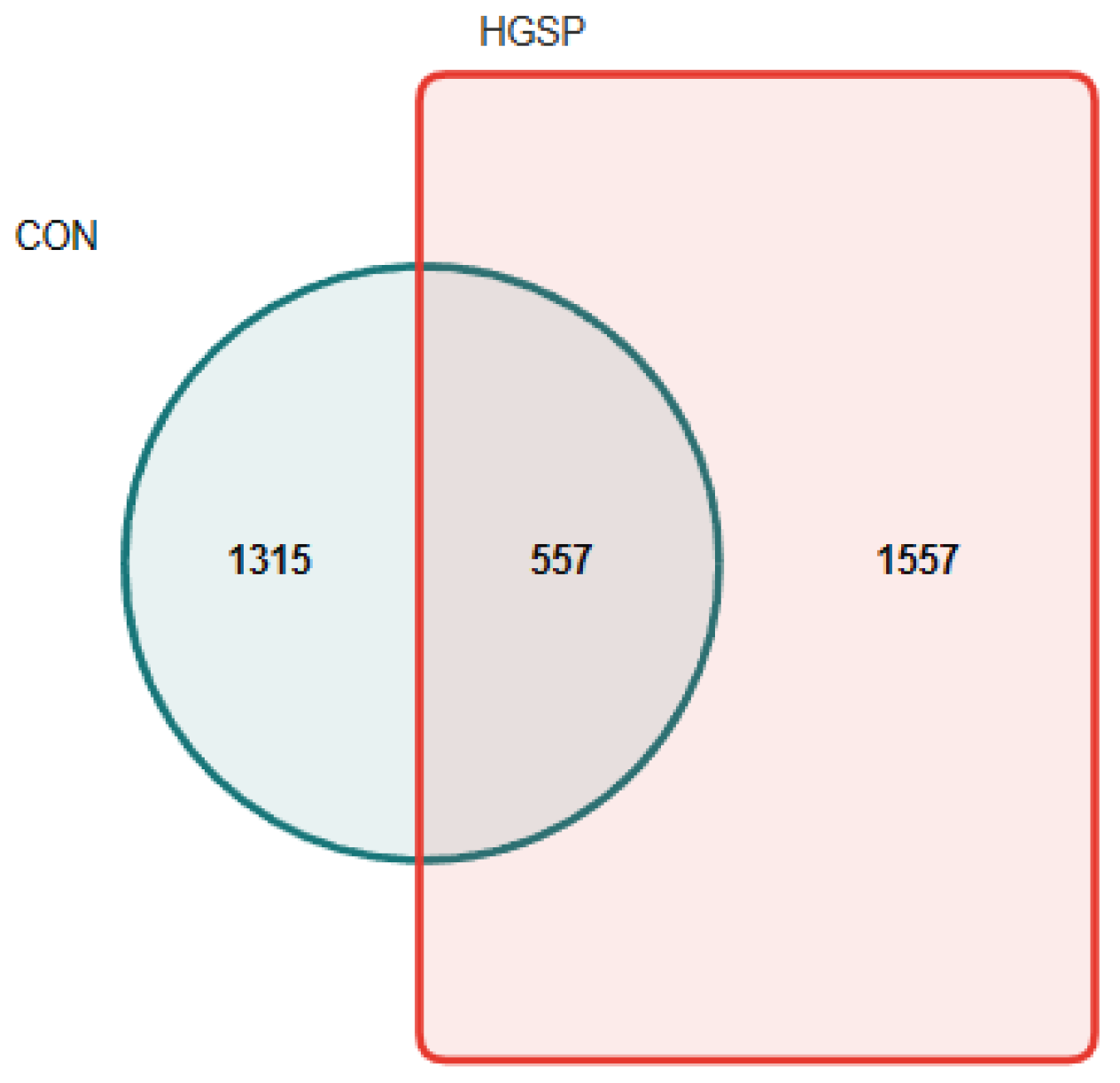
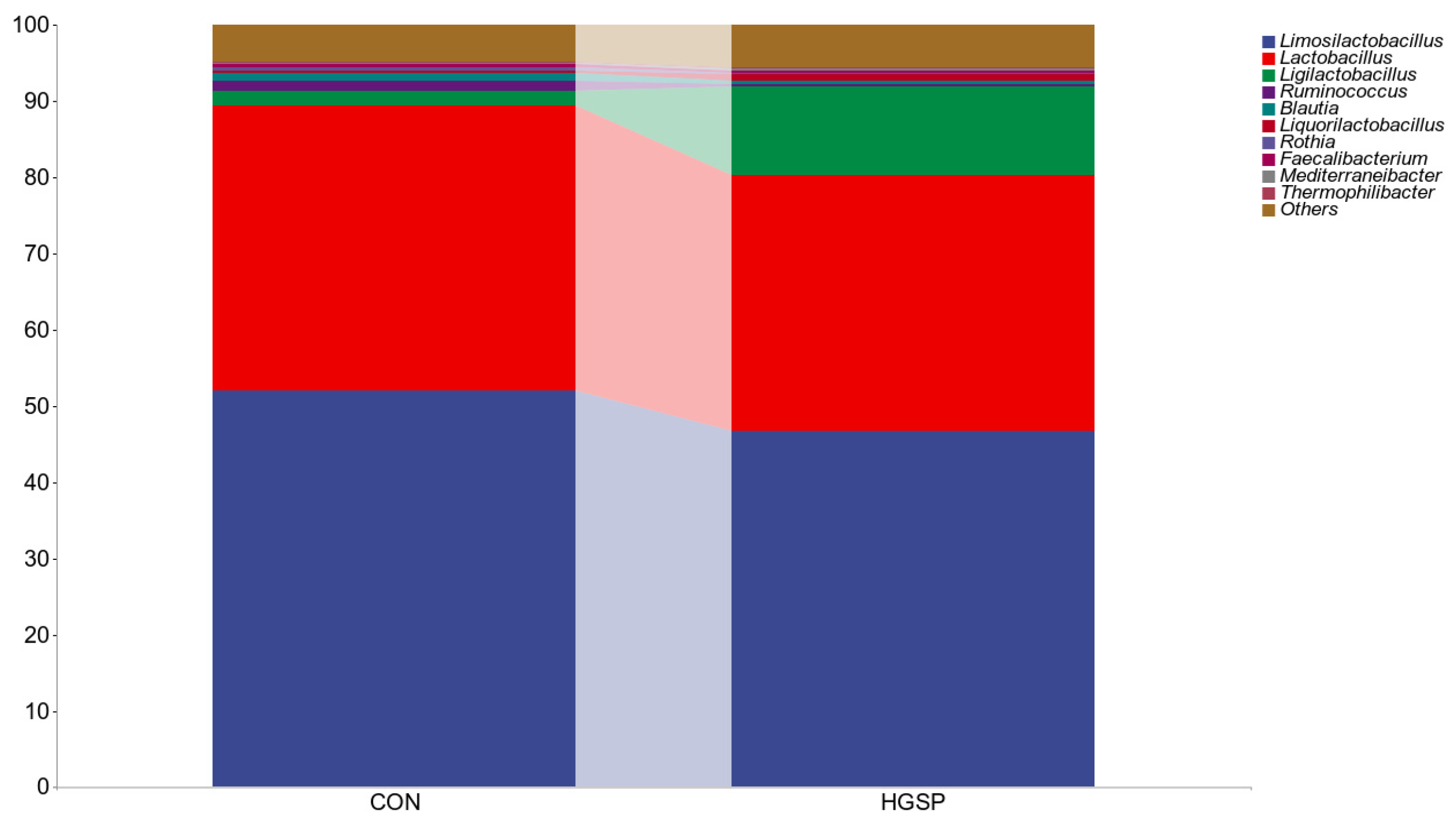
3.6.1. Analysis of the Jejunal Microbiota Composition
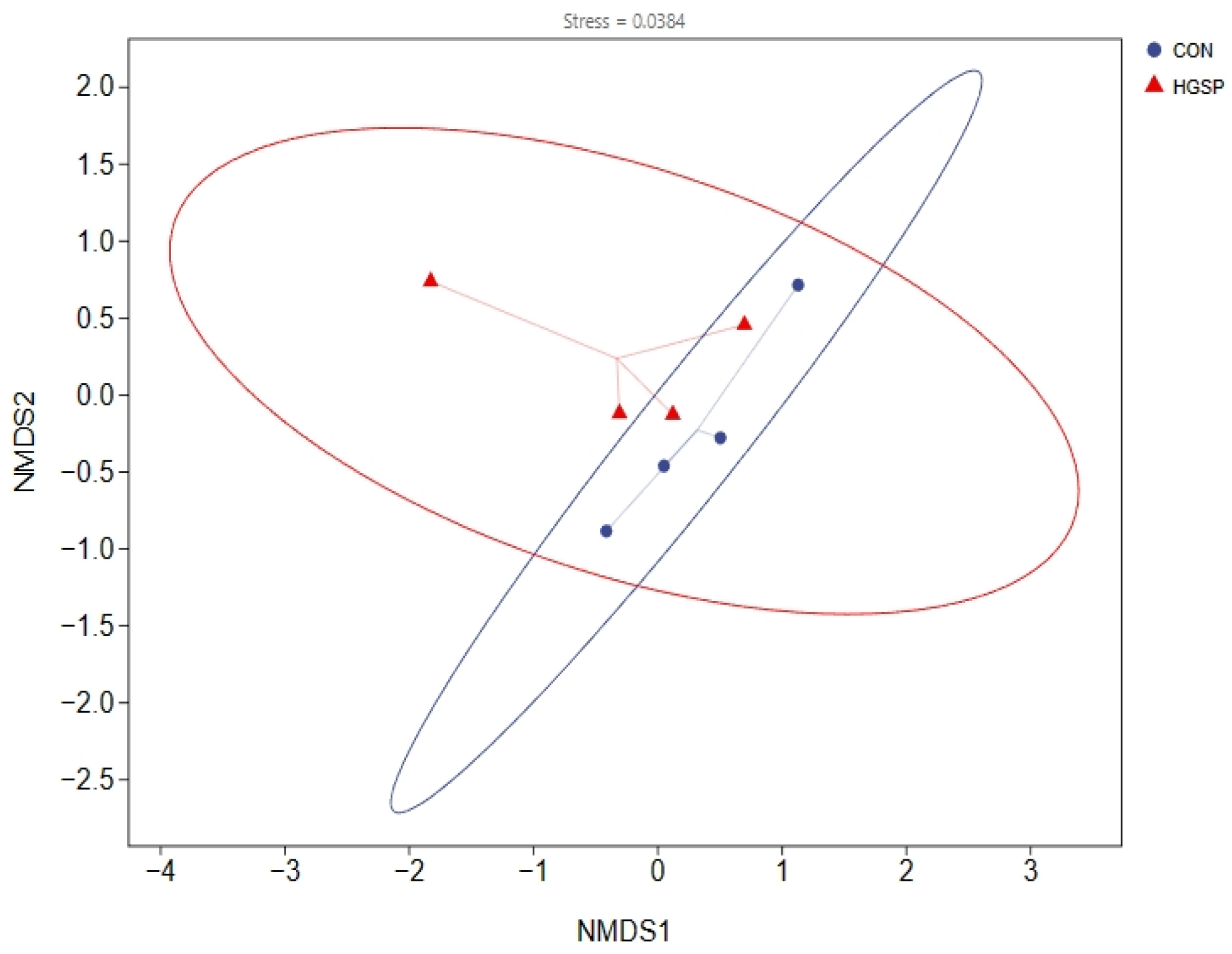
3.6.2. The Analysis of the Jejunal Microbiota Diversity Between the CON and HGSP Groups
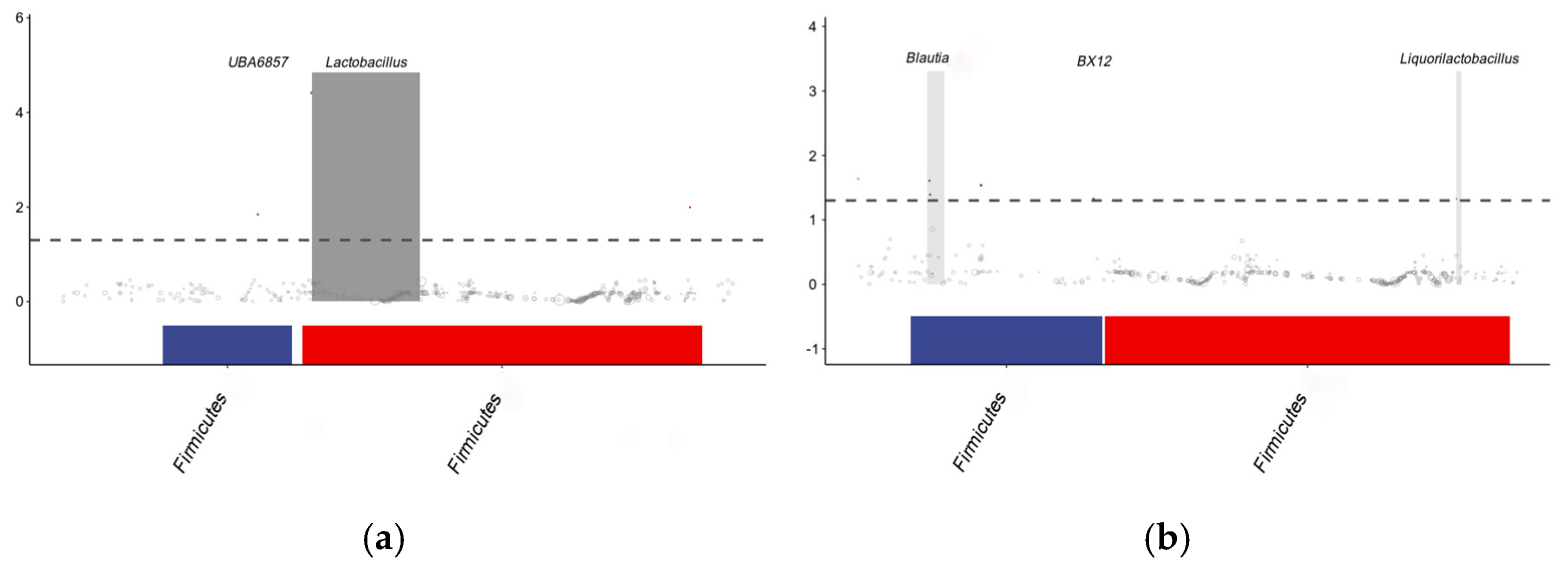
3.6.3. Analysis of the Differential Microbial Taxa Between the CON and HGSP Groups
3.7. The Metabolomic Analysis of the Jejunal Contents Between the CON and HGSP Groups
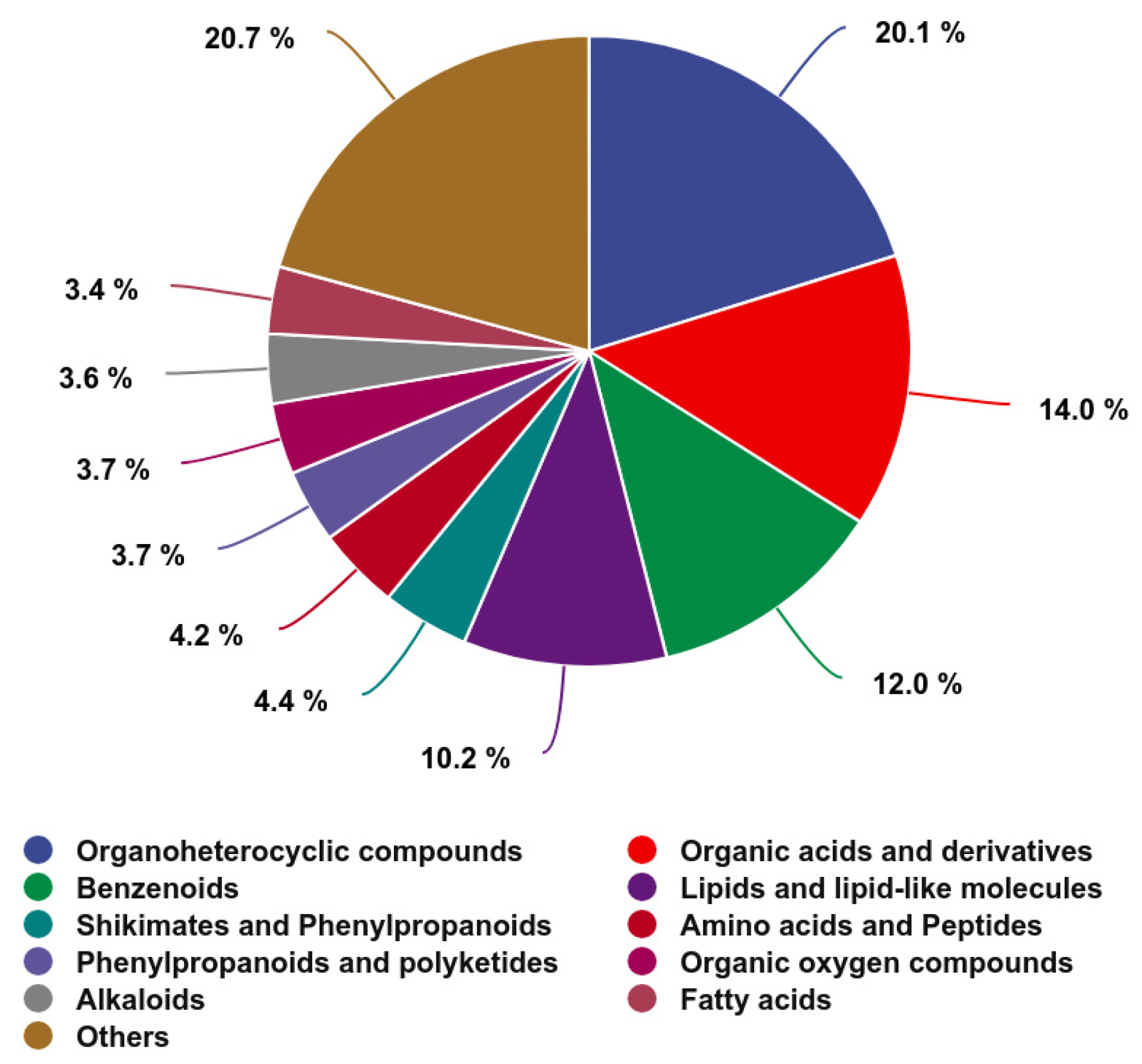
3.7.1. The Chemical Composition of the Jejunal Metabolites
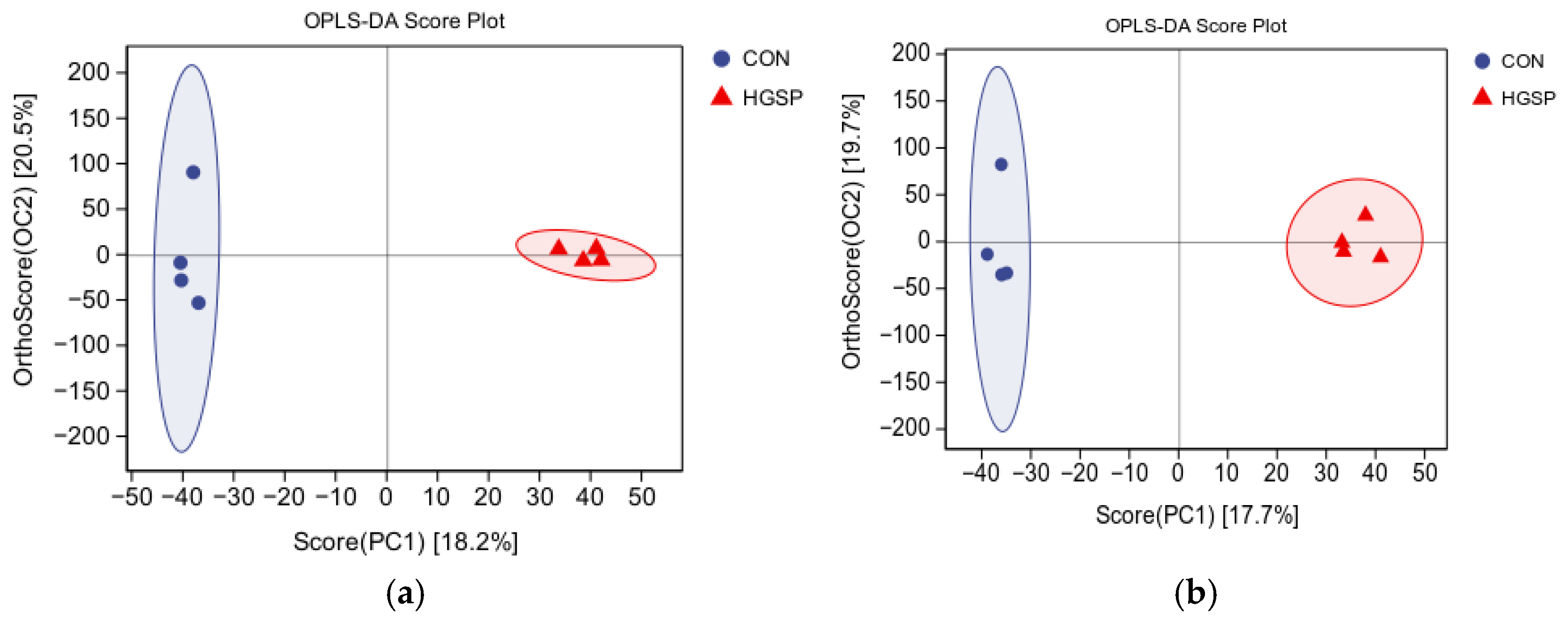
3.7.2. The Statistical Analysis of the Differentially Abundant Metabolites Between the CON and HGSP Groups
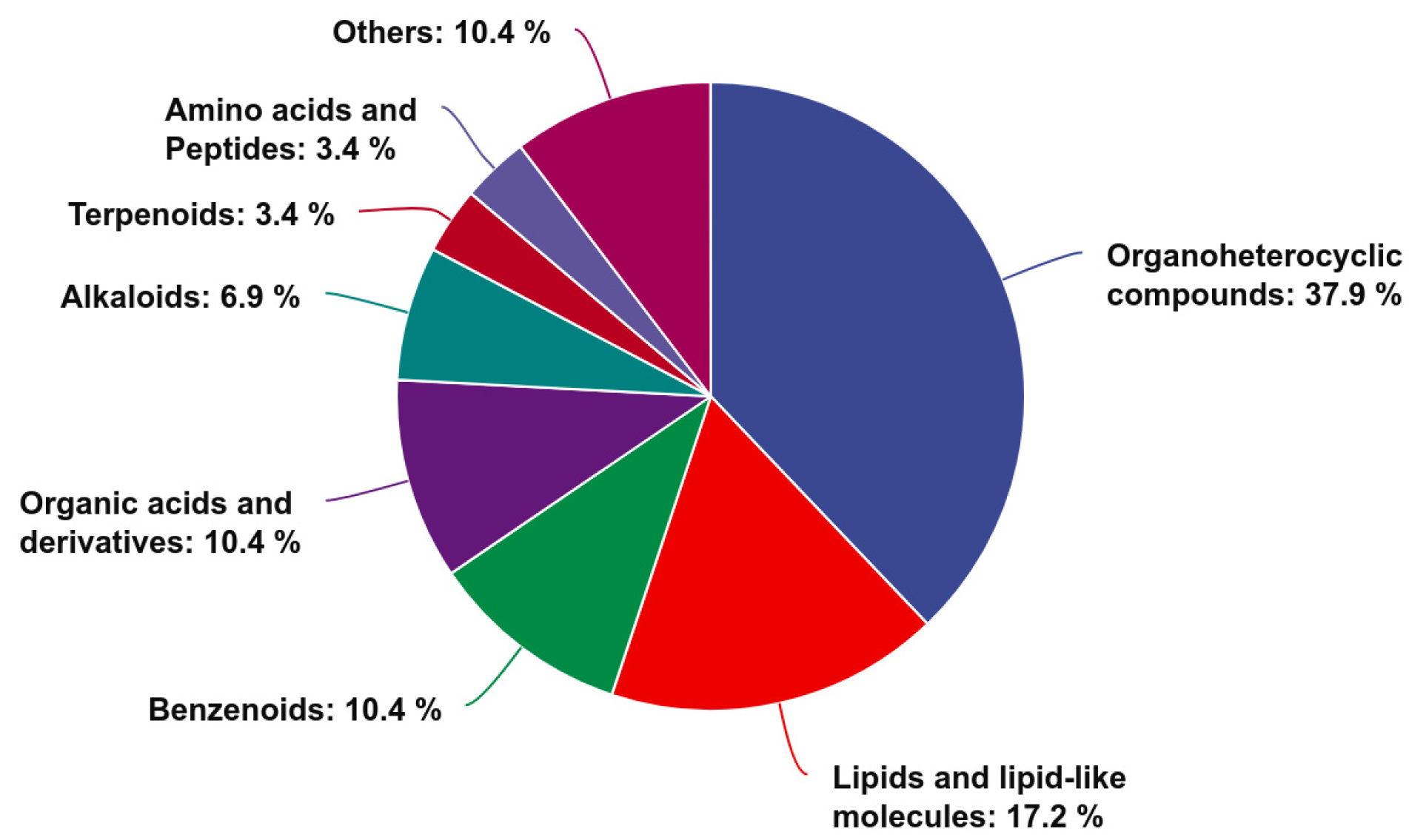
3.7.3. Identification and Classification of Differentially Abundant Metabolites
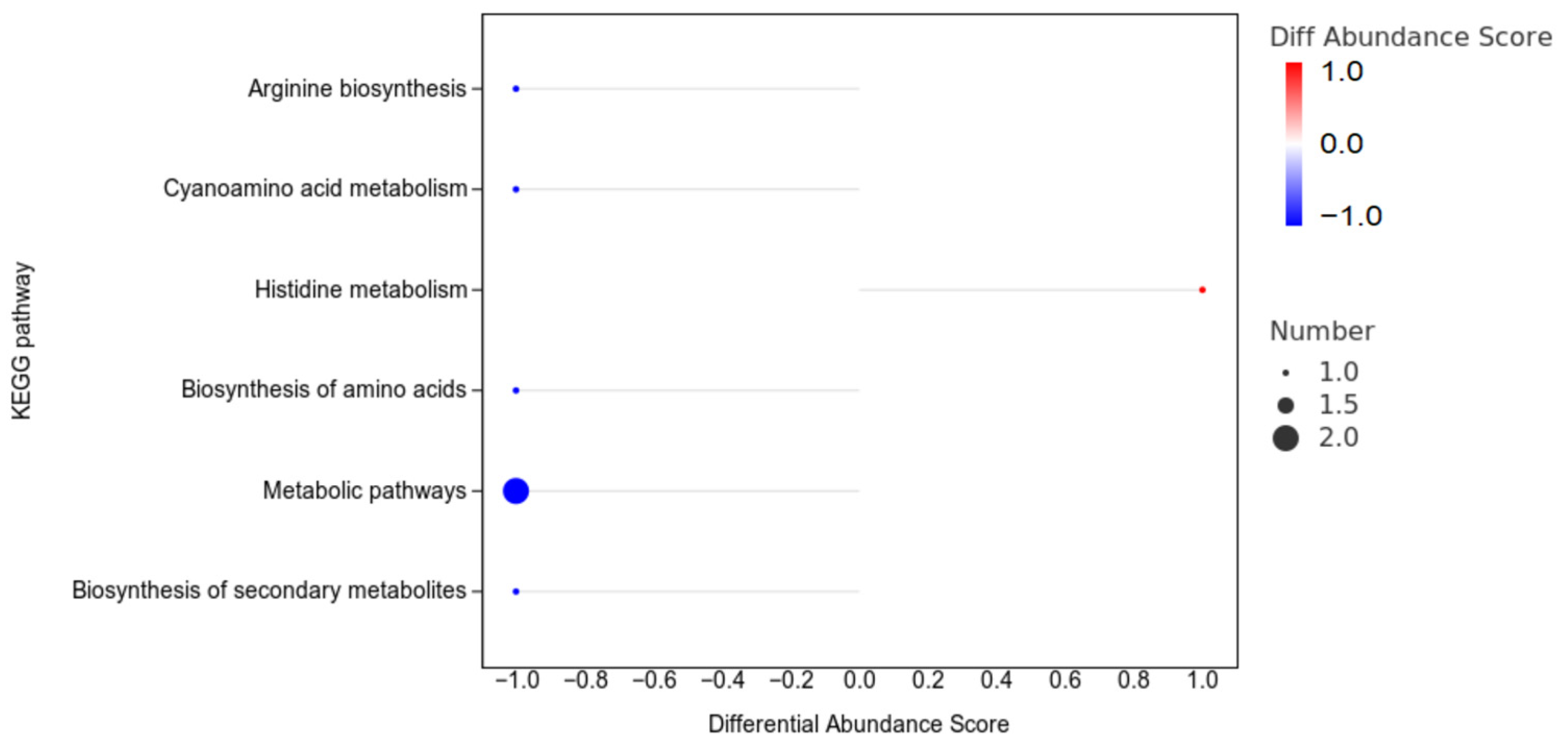
3.7.4. The Enrichment Analysis of the Differentially Abundant Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Cao, G.; Zeng, X.; Liu, J.; Li, X.; Zhang, Y. Change of serum metabolome and cecal microflora in broiler chickens supplemented with grape seed extracts. Front. Immunol. 2020, 11, 610934. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.A.; Sun, L.; Zhang, N.Y.; Khalil, M.M.; Ling, Z.; Chong, L.; Wang, S.; Rajput, I.R.; Bloch, D.M.; Khan, F.A.; et al. Grape Seed Proanthocyanidin Extract Alleviates AflatoxinB1-Induced Immunotoxicity and Oxidative Stress via Modulation of NF-κB and Nrf2 Signaling Pathways in Broilers. Toxins 2019, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, X.; Mi, Y.; Li, J.; Zhang, C. Grape Seed Proanthocyanidin Extract Prevents Ovarian Aging by Inhibiting Oxidative Stress in the Hens. Oxid. Med. Cell. Longev. 2018, 2018, 9390810. [Google Scholar] [CrossRef]
- Rodrigues, I.; Choct, M. The foregut and its manipulation via feeding practices in the chicken. Poult. Sci. 2018, 97, 3188–3206. [Google Scholar] [CrossRef]
- Gong, M.; Liu, L.; Li, F.; Chen, J. Grape Seed Proanthocyanidin Extract Improves Growth Performance and Protects Against Hydrogen Peroxide-Induced Oxidative Stress to the Liver and Intestine in Weaned Hyla Rabbits. Animals 2025, 15, 327. [Google Scholar] [CrossRef]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef]
- Deng, C.; Zhai, Y.; Yang, X.; Chen, Z.; Li, Q.; Hao, R. Effects of grape seed procyanidins on antioxidant function, barrier function, microbial community, and metabolites of cecum in geese. Poult. Sci. 2023, 102, 102878. [Google Scholar] [CrossRef]
- NY/T 33-2004; Feeding Standards for Chickens. Ministry of Agriculture and Rural Affairs: Beijing, China, 2004.
- Cozannet, P.; Jlali, M.; Moore, D.; Archibeque, M.; Preynat, A. Evaluation of phytase dose effect on performance, bone mineralization, and prececal phosphorus digestibility in broilers fed diets with varying metabolizable energy, digestible amino acids, and available phosphorus concentration. Poult. Sci. 2023, 102, 102755. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Swaroop, A.; Preuss, H.G.; Bagchi, M. Free radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: An overview. Mutat. Res. 2014, 768, 69–73. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef]
- Farahat, M.H.; Abdallah, F.M.; Ali, H.A.; Hernandez-Santana, A. Effect of dietary supplementation of grape seed extract on the growth performance, lipid profile, antioxidant status and immune response of broiler chickens. Animal 2017, 11, 771–777. [Google Scholar] [CrossRef]
- Chen, S.; Yan, C.; Xiao, J.; Liu, W.; Li, Z.; Liu, H.; Liu, J.; Zhang, X.; Ou, M.; Chen, Z.; et al. Domestication and Feed Restriction Programming Organ Index, Dopamine, and Hippocampal Transcriptome Profile in Chickens. Front. Vet. Sci. 2021, 8, 701850. [Google Scholar] [CrossRef] [PubMed]
- Qaisrani, S.N.; Moquet, P.C.A.; Krimpen, M.M.; Kwakkel, R.P. Protein source and dietary structure influence growth performance, gut morphology, and hindgut fermentation characteristics in broilers. Poult. Sci. 2014, 93, 3053–3064. [Google Scholar] [CrossRef]
- Erinle, T.J.; Oladokun, S.; MacIsaac, J.; Rathgeber, B. Dietary grape pomace—Effects on growth performance, intestinal health, blood parameters, and breast muscle myopathies of broiler chickens. Poult. Sci. 2022, 101, 101519. [Google Scholar] [CrossRef] [PubMed]
- Geng, A.L.; Zhang, Y.; Zhang, J.; Zeng, L.C.; Chang, C.; Wang, H.H.; Yan, Z.X.; Chu, Q.; Liu, H.G. Effects of light regime on the hatching performance, body development and serum biochemical indexes in Beijing You Chicken. Poult. Sci. 2021, 100, 101270. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, H.; Zhang, H.; Liu, G.; Yin, X.; Zhang, C.; Jiang, R.; Wang, Z.; Ding, X. Effect of Lactobacillus paracasei LK01 on Growth Performance, Antioxidant Capacity, Immunity, Intestinal Health, and Serum Biochemical Indices in Broilers. Animals 2024, 14, 3474. [Google Scholar] [CrossRef]
- Wang, W.; Xu, M.; Diao, H.; Long, Q.; Gan, F.; Mao, Y. Effects of grape seed proanthocyanidin extract on cholesterol metabolism and antioxidant dtatus in finishing pigs. Sci. Rep. 2024, 14, 21117. [Google Scholar] [CrossRef]
- Shamkhi Noor, A.; Essa Al-Mashhdani, H.; Hasan Kadhim, A. Effects of grape seed powder on productive performance, lipid profile and total bacteria in duodenum and ceca of broiler chickens. Arch. Razi Inst. 2022, 77, 2159–2164. [Google Scholar] [CrossRef]
- Nan, S.; Yao, M.; Zhang, X.; Li, X.; Zhang, Y. Fermented grape seed meal promotes broiler growth and reduces abdominal fat deposition through intestinal microorganisms. Front. Microbiol. 2022, 13, 994033. [Google Scholar] [CrossRef]
- Abu Hafsa, S.H.; Ibrahim, S.A. Effect of dietary polyphenol-rich grape seed on growth performance, antioxidant capacity and ileal microflora in broiler chicks. J. Anim. Physiol. Anim. Nutr. 2018, 102, 268–275. [Google Scholar] [CrossRef]
- Mancini, M.; Cerny, M.E.V.; Cardoso, N.S.; Ferreira, C.F. Grape seed components as protectors of inflammation, DNA damage, and cancer. Curr. Nutr. Rep. 2023, 12, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, K.; Li, S.; Yang, G.; Lin, Z.; Miao, Z. Grape seed-derived procyanidin inhibits glyphosate-induced hepatocyte ferroptosis via enhancing crosstalk between Nrf2 and FGF12. Phytomedicine 2024, 123, 155278. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; He, Z.; Xiong, Z.; Li, X.; Zhang, Y. Effects of dietary supplementation of grape seed extract in comparison with excessive level of vitamin E on growth performance and antioxidant function of broilers. Anim. Biotechnol. 2024, 35, 2331640. [Google Scholar] [CrossRef] [PubMed]
- Bindari, Y.R.; Gerber, P.F. Centennial Review: Factors affecting the chicken gastrointestinal microbial composition and their association with gut health and productive performance. Poult. Sci. 2022, 101, 101612. [Google Scholar] [CrossRef]
- Liu, Q.; Akhtar, M.; Kong, N.; Zhang, R.; Liang, Y.; Gu, Y.; Yang, D.; Nafady, A.A.; Shi, D.; Ansari, A.R.; et al. Early fecal microbiota transplantation continuously improves chicken growth performance by inhibiting age-related Lactobacillus decline in jejunum. Microbiome 2025, 13, 49. [Google Scholar] [CrossRef]
- Iqbal, Y.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Gut Microbiota-Polyphenol Interactions in Chicken: A Review. Animals 2020, 10, 1391. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Bauermeister, A.; Mannochio-Russo, H.; Costa-Lotufo, L.V.; Jarmusch, A.K.; Dorrestein, P.C. Mass spectrometry-based metabolomics in microbiome investigations. Nat. Rev. Microbiol. 2022, 20, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for investigating physiological and pathophysiological processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H. Molecular and integrative physiology of intestinal peptide transport. Annu. Rev. Physiol. 2004, 66, 361–384. [Google Scholar] [CrossRef]
- Miner-Williams, W.M.; Stevens, B.R.; Moughan, P.J. Are intact peptides absorbed from the healthy gut in the adult human? Nutr. Res. Rev. 2014, 27, 308–329. [Google Scholar] [CrossRef]
- Zheng, K.Y.; Gao, B.; Wang, H.J.; He, J.G.; Chen, H.S.; Hu, Z.L.; Long, L.H.; Chen, J.G.; Wang, F. Melatonin Ameliorates Depressive-Like Behaviors in Ovariectomized Mice by Improving Tryptophan Metabolism via Inhibition of Gut Microbe Alistipes Inops. Adv. Sci. 2024, 11, e2309473. [Google Scholar] [CrossRef] [PubMed]
- Gruß, H.; Sewald, N. Late-stage diversification of tryptophan-derived biomolecules. Chemistry 2020, 26, 5328–5340. [Google Scholar] [CrossRef]
- Xu, K.; Liu, G.; Fu, C. The Tryptophan Pathway Targeting Antioxidant Capacity in the Placenta. Oxid. Med. Cell. Longev. 2018, 2018, 1054797. [Google Scholar] [CrossRef]
- Ozawa, H.; Miyazawa, T.; Burdeos, G.C.; Miyazawa, T. Biological Functions of Antioxidant Dipeptides. J. Nutr. Sci. Vitaminol. 2022, 68, 162–171. [Google Scholar] [CrossRef]
- de Oliveira Silva, E.; Batista, R. Ferulic Acid and Naturally Occurring Compounds Bearing a Feruloyl Moiety: A Review on Their Structures, Occurrence, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2017, 16, 580–616. [Google Scholar] [CrossRef]
- Liu, Z.; You, C. The bile acid profile. Clin. Chim. Acta 2025, 565, 120004. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Gao, Y.; Yang, R. Gut microbiota derived bile acid metabolites maintain the homeostasis of gut and systemic immunity. Front. Immunol. 2023, 14, 1127743. [Google Scholar] [CrossRef]
- Vico-Oton, E.; Volet, C.; Jacquemin, N.; Dong, Y.; Hapfelmeier, S.; Meibom, K.L.; Bernier-Latmani, R. Strain-dependent induction of primary bile acid 7-dehydroxylation by cholic acid. BMC Microbiol. 2024, 24, 286. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef]
- Holecek, M. Histidine in health and disease: Metabolism, physiological importance, and use as a supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M.; Kerti, K.B.; Farmani, J.; Alizadeh, M. Antioxidant peptides: Overview of production, properties, and applications in food systems. Compr. Rev. Food Sci. Food Saf. 2023, 22, 46–106. [Google Scholar] [CrossRef]
- Hart, P.H.; Norval, M. The multiple roles of urocanic acid in health and disease. J. Investig. Dermatol. 2021, 141, 496–502. [Google Scholar] [CrossRef]
- Yang, H.; Hou, X.; Xing, L.; Tian, F. Carnosine and bone (Review). Mol. Med. Rep. 2023, 27, 12. [Google Scholar] [CrossRef]

| Ingredients | Content (%) | Nutrient Levels | Content (%) |
|---|---|---|---|
| Maize | 66.70 | Metabolizable energy (MJ/kg) 2 | 12.59 |
| Soybean meal | 27.35 | Crude protein | 17.68 |
| Soybean oil | 2.41 | Calcium | 0.75 |
| Limestone | 1.21 | Phosphorus | 0.56 |
| Salt | 0.21 | Lysine | 0.92 |
| Calcium hydrogen phosphate | 0.92 | Methionine | 0.42 |
| L-lysine hydrochloride | 0.11 | Non-phytic acid phosphor | 0.34 |
| DL-Methionine | 0.09 | ||
| Premix 1 | 1.00 | ||
| Total | 100.00 |
| Items 1 | Treatments 2 | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | LGSP | HGSP | |||
| IBW (g) | 146.20 | 145.39 | 147.72 | 3.02 | 0.642 |
| FBW (g) | 471.74 | 489.64 | 483.14 | 8.15 | 0.488 |
| ADG (g/d) | 10.85 | 11.48 | 11.18 | 0.31 | 0.538 |
| ADFI (g/d) | 36.83 | 37.64 | 36.47 | 0.92 | 0.846 |
| FCR | 3.39 | 3.28 | 3.26 | 0.14 | 0.491 |
| Items | Treatments 1 | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | LGSP | HGSP | |||
| Liver index (%) | 2.99 b | 2.87 b | 3.39 a | 0.08 | 0.004 |
| Spleen index (%) | 0.24 | 0.24 | 0.23 | 0.01 | 0.871 |
| Jejunum index (%) | 3.06 b | 3.02 b | 3.76 a | 0.17 | 0.036 |
| Items 1 | Treatments 2 | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | LGSP | HGSP | |||
| TP (g/L) | 33.28 | 33.42 | 33.32 | 0.59 | 0.728 |
| ALB (g/L) | 14.28 | 13.72 | 14.52 | 0.25 | 0.490 |
| AST (U/L) | 238.60 | 233.50 | 220.50 | 7.72 | 0.759 |
| ALT (U/L) | 3.20 | 3.67 | 4.17 | 0.26 | 0.296 |
| GLU (mmol/L) | 11.65 a | 10.43 b | 10.82 ab | 0.20 | 0.026 |
| TG (mmol/L) | 0.84 | 0.91 | 1.49 | 0.13 | 0.074 |
| TC (mmol/L) | 2.32 b | 2.53 ab | 2.87 a | 0.09 | 0.028 |
| Items 1 | Treatments 2 | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | LGSP | HGSP | |||
| VH (μm) | 1043.66 | 1143.96 | 1063.26 | 27.98 | 0.342 |
| CD (μm) | 212.72 a | 179.77 ab | 139.99 b | 14.05 | 0.046 |
| V/C | 5.20 b | 6.57 ab | 7.59 a | 0.44 | 0.022 |
| Items 1 | Treatments 2 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | LGSP | HGSP | ||||
| Jejunum | T-AOC (μmol/mg prot) | 64.04 | 67.17 | 68.44 | 3.70 | 0.300 |
| SOD (U/mg prot) | 148.45 | 153.09 | 149.88 | 5.42 | 0.640 | |
| CAT (U/mg prot) | 9.13 a | 6.30 b | 6.56 b | 0.49 | 0.021 | |
| GSH-Px (U/mg prot) | 33.13 | 37.23 | 44.20 | 1.99 | 0.073 | |
| MDA (nmol/mg prot) | 0.24 a | 0.22 a | 0.13 b | 0.02 | 0.002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Fan, Q.; Yang, X.; Hu, W.; Zheng, L.; Zhou, L.; Shi, J.; Zhao, X.; Zhang, Y. Effects of Grape Seed Proanthocyanidins on Growth Performance, Jejunal Antioxidant Capacity, Gut Microbial Diversity, and Metabolites in Kangle Chickens. Animals 2025, 15, 1481. https://doi.org/10.3390/ani15101481
Wang Q, Fan Q, Yang X, Hu W, Zheng L, Zhou L, Shi J, Zhao X, Zhang Y. Effects of Grape Seed Proanthocyanidins on Growth Performance, Jejunal Antioxidant Capacity, Gut Microbial Diversity, and Metabolites in Kangle Chickens. Animals. 2025; 15(10):1481. https://doi.org/10.3390/ani15101481
Chicago/Turabian StyleWang, Qianqian, Qingcan Fan, Xue Yang, Wei Hu, Lucheng Zheng, Lijun Zhou, Jinmeng Shi, Xingxu Zhao, and Yong Zhang. 2025. "Effects of Grape Seed Proanthocyanidins on Growth Performance, Jejunal Antioxidant Capacity, Gut Microbial Diversity, and Metabolites in Kangle Chickens" Animals 15, no. 10: 1481. https://doi.org/10.3390/ani15101481
APA StyleWang, Q., Fan, Q., Yang, X., Hu, W., Zheng, L., Zhou, L., Shi, J., Zhao, X., & Zhang, Y. (2025). Effects of Grape Seed Proanthocyanidins on Growth Performance, Jejunal Antioxidant Capacity, Gut Microbial Diversity, and Metabolites in Kangle Chickens. Animals, 15(10), 1481. https://doi.org/10.3390/ani15101481






