Genome-Wide Association Study That Identifies Molecular Markers with Freezing Resistance in Duroc Boar Sperm
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Semen Collection
2.2. Semen Cryopreservation and Evaluation
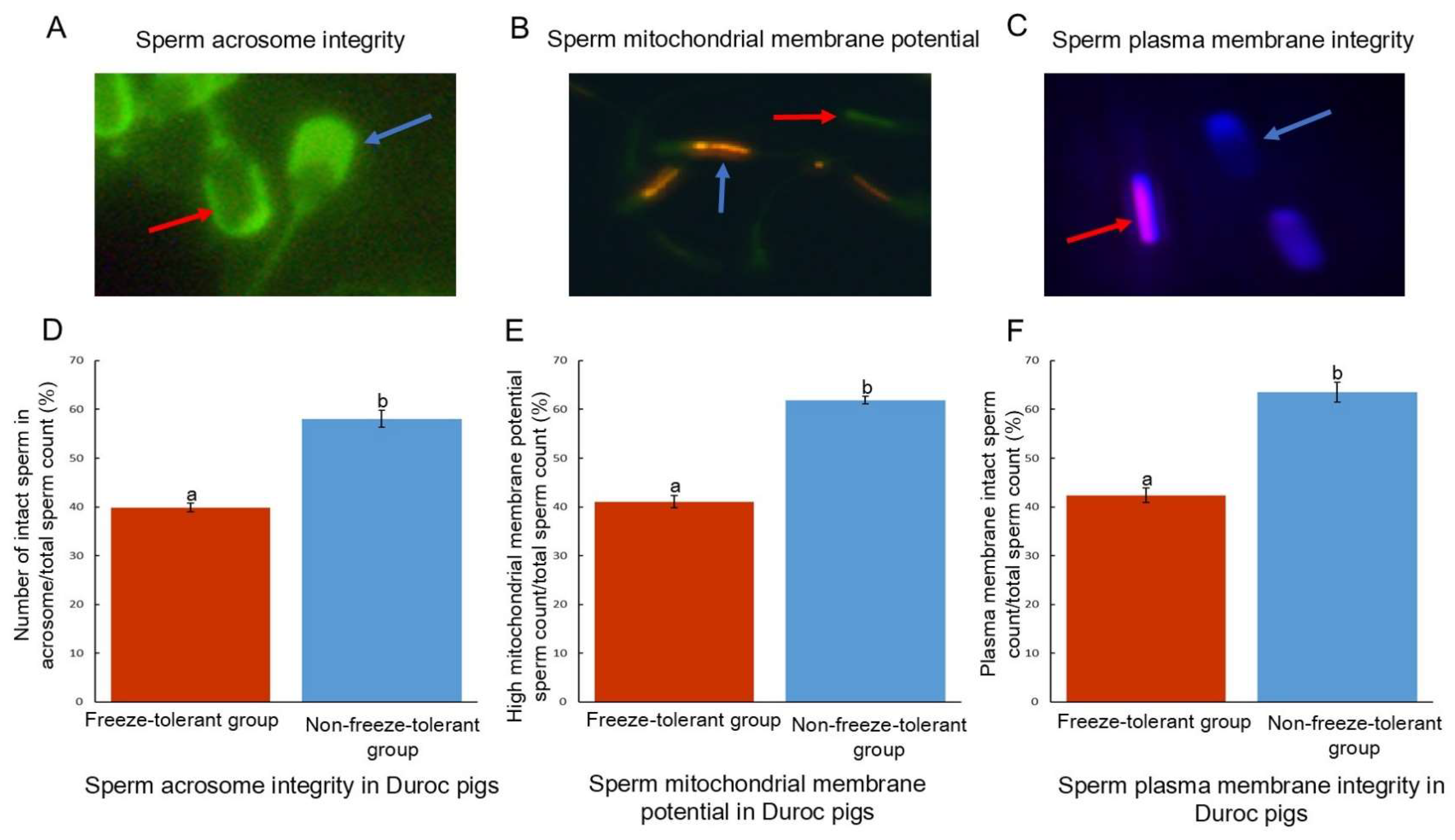
2.3. Structural Testing of Sperm
2.4. Genotype Data Acquisition and Quality Control
2.5. Estimation of Heritability and Population Affinity Coefficients for SRRs
2.6. Genome-Wide Association Analysis of Semen Freezing Tolerance
2.7. Statistical Analyses
3. Results
3.1. Sperm Motility and Structure Differ Before and After Freezing
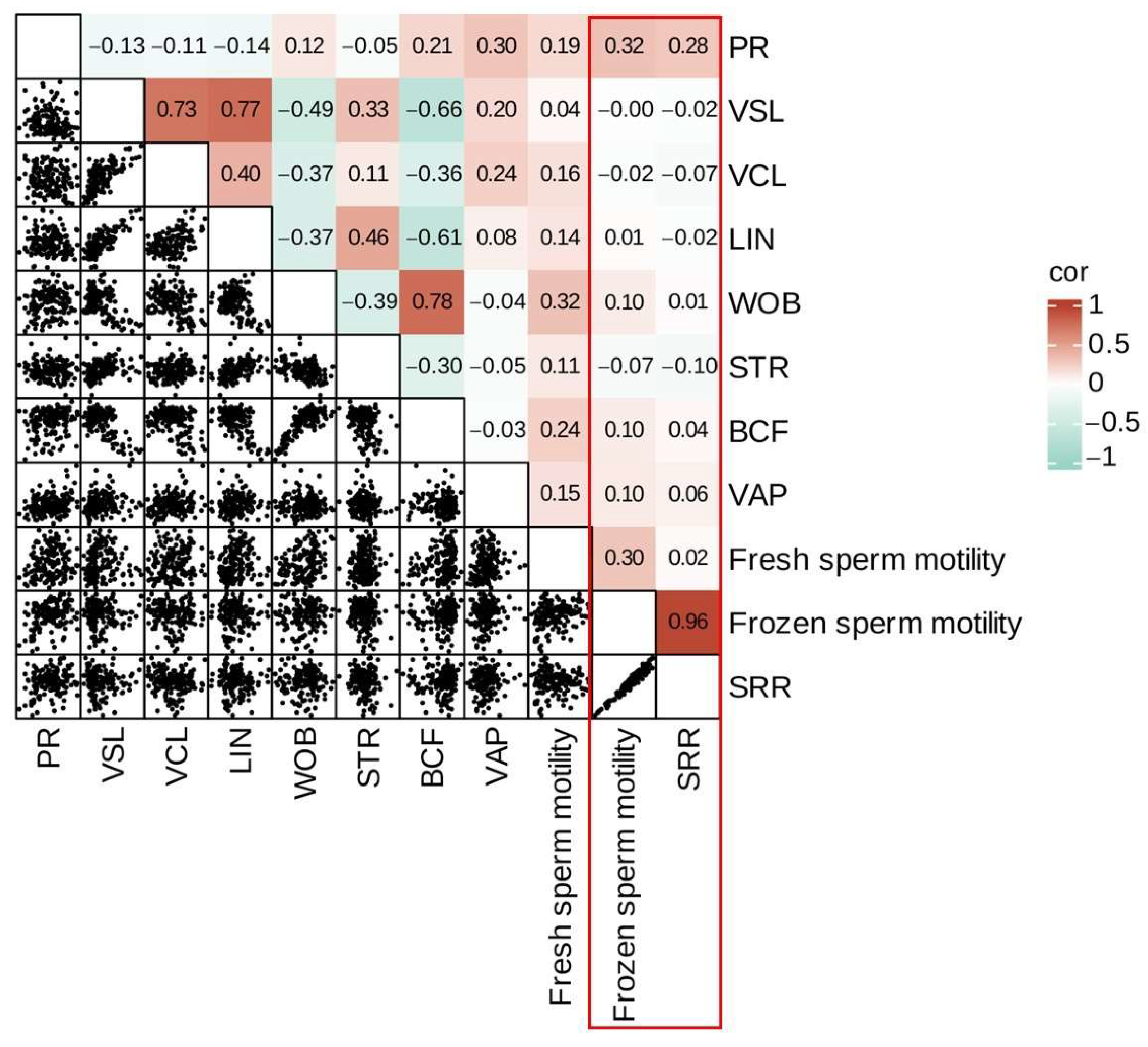
3.2. Correlation Analysis of Sperm Motility Parameters
3.3. Population Genetic Structure Analysis and Heritability Estimation of the SRR
3.4. Genome-Wide Association Analysis and Candidate Gene Identification
3.5. Determination-Dominant Genotypes Associated with SRR Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GWAS | Genome-wide association study |
| SNP | Single-nucleotide polymorphism |
| SRR | Sperm recovery rate |
| VSL | Straight line velocity |
| VCL | Curvilinear velocity |
| LIN | Linearity |
| VAP | Average path velocity |
| WOB | Wobble |
| STR | Straightness |
| BCF | Beat cross frequency |
| PR | Progressive motility |
References
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm Cryo-preservation: A Review on Current Molecular Cryobiology and Advanced Approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Yánez-Ortiz, I.; Catalán, J.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Advances in Sperm Cryopreservation in Farm Animals: Cattle, Horse, Pig and Sheep. Anim. Reprod. Sci. 2022, 246, 106904. [Google Scholar] [CrossRef]
- Yeste, M. Recent Advances in Boar Sperm Cryopreservation: State of the Art and Current Perspectives. Reprod. Domest. Anim. 2015, 50, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Lopez Rodriguez, A.; Van Soom, A.; Arsenakis, I.; Maes, D. Boar Management and Semen Handling Factors Affect the Quality of Boar Extended Semen. Porc. Health Manag. 2017, 3, 15. [Google Scholar] [CrossRef]
- Waterhouse, K.E.; Hofmo, P.O.; Tverdal, A.; Miller, R.R. Within and between Breed Differences in Freezing Tolerance and Plasma Membrane Fatty Acid Composition of Boar Sperm. Reproduction 2006, 131, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Mańkowska, A.; Brym, P.; Sobiech, P.; Fraser, L. Promoter Polymorphisms in STK35 and IFT27 Genes and Their Associations with Boar Sperm Freezability. Theriogenology 2022, 189, 199–208. [Google Scholar] [CrossRef]
- Wang, W.; Bai, Y.; Wang, X.; Zhang, J.; Li, B.; Zhang, H.; Zhou, X.; Wang, H.; Liu, B. Identification of Key Genes and Variants Associated with Boar Sperm Freezability Using Whole Genome Resequencing. Int. J. Bio-Log. Macromol. 2025, 294, 139268. [Google Scholar] [CrossRef]
- Fraser, L.; Brym, P.; Pareek, C.S.; Mogielnicka-Brzozowska, M.; Paukszto, Ł.; Jastrzębski, J.P.; Wasilewska-Sakowska, K.; Mańkowska, A.; Sobiech, P.; Żukowski, K. Transcriptome Analysis of Boar Spermatozoa with Different Freezability Using RNA-Seq. Theriogenology 2020, 142, 400–413. [Google Scholar] [CrossRef]
- Gao, N.; Chen, Y.; Liu, X.; Zhao, Y.; Zhu, L.; Liu, A.; Jiang, W.; Peng, X.; Zhang, C.; Tang, Z.; et al. Weighted Single-Step GWAS Identified Candidate Genes Associated with Semen Traits in a Duroc Boar Population. BMC Genom. 2019, 20, 797. [Google Scholar] [CrossRef]
- Mei, Q.; Fu, C.; Sahana, G.; Chen, Y.; Yin, L.; Miao, Y.; Zhao, S.; Xiang, T. Identification of new semen trait-related candidate genes in Duroc boars through genome-wide association and weighted gene co-expression network analyses. J. Anim. Sci. 2021, 99, skab188. [Google Scholar] [CrossRef]
- Zhuang, Z.; Li, K.; Yang, K.; Gao, G.; Li, Z.; Zhu, X.; Zhao, Y. Genome-Wide Association Study Reveals Novel Candidate Genes Influencing Semen Traits in Landrace Pigs. Animals 2024, 14, 1839. [Google Scholar] [CrossRef]
- Alonso, V.; Muela, E.; Gutiérrez, B.; Calanche, J.B.; Roncalés, P.; Beltrán, J.A. The Inclusion of Duroc Breed in Maternal Line Affects Pork Quality and Fatty Acid Profile. Meat Sci. 2015, 107, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bai, Y.; Zhang, J.; Wang, H.; Li, J.; Wang, W. Sperm Metabolomics Identifies Freezability Markers in Duroc, Landrace, and Large White Boars. Theriogenology 2025, 240, 117395. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zeng, Y.; Tian, Y.; Cheng, L.; Li, W.; Cheng, S.; Wang, J.; Li, L. Genome-Wide Association Study Reveals Novel Loci and Candidate Genes for Birth Weight in Pigs. Animals 2025, 15, 825. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhong, Z.; Xu, Z.; Teng, J.; Wei, C.; Chen, Z.; Zhang, W.; Ding, X.; Li, J.; Zhang, Z. Meta-analysis of ge-nome-wide association studies uncovers shared candidate genes across breeds for pig fatness trait. BMC Genom. 2022, 23, 786. [Google Scholar] [CrossRef]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial Dysfunction and Oxidative Stress Caused by Cryopreservation in Reproductive Cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef]
- Hai, E.; Li, B.; Zhang, J.; Zhang, J. Sperm Freezing Damage: The Role of Regulated Cell Death. Cell Death Discov. 2024, 10, 239. [Google Scholar] [CrossRef]
- McNeil, P.L.; Steinhardt, R.A. Plasma Membrane Disruption: Repair, Prevention, Adaptation. Annu. Rev. Cell Dev. Biol. 2003, 19, 697–731. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R. Effects of Reactive Oxygen Species on Sperm Function. Theriogenology 2012, 78, 1700–1708. [Google Scholar] [CrossRef]
- Sun, W.; Jiang, S.; Su, J.; Zhang, J.; Bao, X.; Ding, R.; Shi, P.; Li, S.; Wu, C.; Zhao, G.; et al. The Effects of Cryopreservation on the Acrosome Structure, Enzyme Activity, Motility, and Fertility of Bovine, Ovine, and Goat Sperm. Anim. Reprod. 2021, 17, e20200219. [Google Scholar] [CrossRef]
- Afrin, S.; Lee, Y.M.; Haque, M.A.; Iqbal, A.; Choo, H.; Kim, J.J. Estimation of Genetic Parameters and Breeding Value Accuracy for Growth and Egg Production Traits in Korean Native Chicken Pure Lines. Livest. Sci. 2024, 282, 105436. [Google Scholar] [CrossRef]
- Visscher, P.M.; Hill, W.G.; Wray, N.R. Heritability in the Genomics Era—Concepts and Misconceptions. Nat. Rev. Genet. 2008, 9, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.P.; Eivers, B.; Dunne, G.; McParland, S. Genetics of Bull Semen Characteristics in a Multi-Breed Cattle Popu-lation. Theriogenology 2019, 123, 202–208. [Google Scholar] [CrossRef]
- Smital, J.; De Sousa, L.L.; Mohsen, A. Differences among Breeds and Manifestation of Heterosis in AI Boar Sperm Output. Anim. Reprod. Sci. 2004, 80, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Burren, A.; Joerg, H.; Erbe, M.; Gilmour, A.R.; Witschi, U.; Schmitz-Hsu, F. Genetic Parameters for Semen Production Traits in Swiss Dairy Bulls. Reprod. Domest. Anim. 2019, 54, 1177–1181. [Google Scholar] [CrossRef]
- Fietz, D.; Bakhaus, K.; Wapelhorst, B.; Grosser, G.; Günther, S.; Alber, J.; Döring, B.; Kliesch, S.; Weidner, W.; Galuska, C.E.; et al. Membrane Transporters for Sulfated Steroids in the Human Testis—Cellular Localization, Expression Pattern and Functional Analysis. PLoS ONE 2013, 8, e62638. [Google Scholar] [CrossRef]
- Karoui, S.; Díaz, C.; Serrano, M.; Cue, R.; Celorrio, I.; Carabaño, M.J. Time Trends, Environmental Factors and Genetic Basis of Semen Traits Collected in Holstein Bulls under Commercial Conditions. Anim. Reprod. Sci. 2011, 124, 28–38. [Google Scholar] [CrossRef]
- Toe, F.; Rege, J.E.; Mukasa-Mugerwa, E.; Tembely, S.; Anindo, D.; Baker, R.L.; Lahlou-Kassi, A. Reproductive Characteristics of Ethiopian Highland Sheep. Small Rumin. Res. 2000, 37, 173–187. [Google Scholar] [CrossRef]
- Druet, T.; Fritz, S.; Sellem, E.; Basso, B.; Gérard, O.; Salas-Cortes, L.; Humblot, P.; Druart, X.; Eggen, A. Estimation of Genetic Parameters and Genome Scan for 15 Semen Characteristics Traits of Holstein Bulls. J. Anim. Breed. Genet. 2009, 126, 269–277. [Google Scholar] [CrossRef]
- Wannowius, M.; Karakus, E.; Aktürk, Z.; Breuer, J.; Geyer, J. Role of the Sodium-Dependent Organic Anion Transporter (SOAT/SLC10A6) in Physiology and Pathophysiology. Int. J. Mol. Sci. 2023, 24, 9926. [Google Scholar] [CrossRef]
- Bakhaus, K.; Bennien, J.; Fietz, D.; Sánchez-Guijo, A.; Hartmann, M.; Serafini, R.; Love, C.C.; Golovko, A.; Wudy, S.A.; Bergmann, M.; et al. Sodium-Dependent Organic Anion Transporter (Slc10a6−/−) Knockout Mice Show Normal Spermatogenesis and Reproduction, but Elevated Serum Levels for Cholesterol Sulfate. J. Steroid Biochem. Mol. Biol. 2018, 179, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Geyer, J.; Döring, B.; Meerkamp, K.; Ugele, B.; Bakhiya, N.; Fernandes, C.F.; Godoy, J.R.; Glatt, H.; Petzinger, E. Cloning and Functional Characterization of Human Sodium-Dependent Organic Anion Transporter (SLC10A6). J. Bio-Log. Chem 2007, 282, 19728–19741. [Google Scholar] [CrossRef]
- Grosser, G.; Fietz, D.; Günther, S.; Bakhaus, K.; Schweigmann, H.; Ugele, B.; Brehm, R.; Petzinger, E.; Bergmann, M.; Geyer, J. Cloning and Functional Characterization of the Mouse Sodium-Dependent Organic Anion Transporter Soat (Slc10a6). J. Steroid Biochem. Mol. Biol. 2013, 138, 90–99. [Google Scholar] [CrossRef]
- Bujalka, H.; Koenning, M.; Jackson, S.; Perreau, V.M.; Pope, B.; Hay, C.M.; Mitew, S.; Hill, A.F.; Lu, Q.R.; Wegner, M.; et al. MYRF Is a Membrane-Associated Transcription Factor That Autoproteolytically Cleaves to Directly Activate Myelin Genes. PLoS Biol. 2013, 11, e1001625. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, K.; Takata, A.; Uchiyama, Y.; Miyatake, S.; Miyake, N.; Mitsuhashi, S.; Iwama, K.; Fujita, A.; Imagawa, E.; Alkanaq, A.N.; et al. MYRF haploinsufficiency causes 46,XY and 46,XX disorders of sex development: Bioinformatics consideration. Hum. Mol. Genet. 2019, 28, 2319–2329. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Chen, Y.; Han, T.; Pan, Q.; Gao, F.; Li, G. GGA1 Participates in Spermatogenesis in Mice under Stress. PeerJ 2023, 11, e15673. [Google Scholar] [CrossRef]
- Hirst, J.; Carmichael, J. A Potential Role for the Clathrin Adaptor GGA in Drosophila Spermatogenesis. BMC Cell Biol. 2011, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Khawar, M.B.; Gao, H.; Li, W. Mechanism of Acrosome Biogenesis in Mammals. Front. Cell Dev. Biol 2019, 7, 195. [Google Scholar] [CrossRef]
- Venditti, M.; Minucci, S. Differential Expression and Localization of EHBP1L1 during the First Wave of Rat Spermatogenesis Suggest Its Involvement in Acrosome Biogenesis. Biomedicines 2022, 10, 181. [Google Scholar] [CrossRef]
- ’t Hoen, P.A.C.; de Meijer, E.J.; Boer, J.M.; Vossen, R.H.A.M.; Turk, R.; Maatman, R.G.H.J.; Davies, K.E.; van Ommen, G.-J.B.; van Deutekom, J.C.T.; den Dunnen, J.T. Generation and Characterization of Transgenic Mice with the Full-Length Human DMD Gene. J. Biol. Chem. 2008, 283, 5899–5907. [Google Scholar] [CrossRef]
- Chen, H.-C.; Chin, Y.-F.; Lundy, D.J.; Liang, C.-T.; Chi, Y.-H.; Kuo, P.; Hsieh, P.C.H. Utrophin Compensates Dys-trophin Loss during Mouse Spermatogenesis. Sci. Rep. 2017, 7, 7372. [Google Scholar]



| Significant SNP | Candidate Genes | Gene Position | Biological Process |
|---|---|---|---|
| CNCB10006390 | SLC10A6 | 8:131997359-132023739 | Spermatogenesis |
| CNC10020205 | MYRF | 2:9761230-9795873 | Gonadal development |
| CNC10050191 | GGA1 | 5:10186132-10213830 | Spermatogenesis |
| CNC10015560 | UTRN | 1:20514264-21047927 | Spermatogenesis |
| SNP Name | Genotypes | Number | p Value | Mean SRR ± SD (%) |
|---|---|---|---|---|
| CNC10015560 | TT | 59 | 0.298339167 | 65.0783 ± 9.2839 |
| CT | 74 | 62.5943 ± 9.1071 | ||
| CC | 31 | 63.5574 ± 11.0034 | ||
| CNC10020205 | AA | 14 | 0.068627451 | 64.1842 ± 9.9289 |
| AG | 75 | 61.8949 ± 9.1276 | ||
| GG | 75 | 65.3490 ± 9.7195 | ||
| CNC10050191 | TT | 4 | 0.002646241 | 48.4825 ± 10.2948 b |
| TC | 31 | 62.6477 ± 9.9919 a | ||
| CC | 129 | 64.3865 ± 9.0806 a | ||
| CNCB10006390 | TT | 50 | 0.267082987 | 65.0460 ± 9.7249 |
| TC | 77 | 62.3490 ± 9.7412 | ||
| CC | 37 | 64.5594 ± 8.7953 | ||
| CNC10062936 | GG | 32 | 0.007107671 | 59.6181 ± 9.8161 b |
| GA | 86 | 63.8147 ± 9.4879 a | ||
| AA | 46 | 66.2180 ± 8.7233 a | ||
| CNC10142631 | AA | 64 | 0.046342115 | 65.2662 ± 9.9858 a |
| GA | 71 | 61.3832 ± 9.6985 b | ||
| GG | 29 | 65.7458 ± 6.9775 a | ||
| CNC10031720 | AA | 38 | 0.016191411 | 60.1160 ± 11.5879 b |
| AG | 82 | 65.2585 ± 9.2777 a | ||
| GG | 44 | 63.7788 ± 7.2515 a | ||
| CNC10031721 | GG | 38 | 0.016191411 | 60.1160 ± 11.5879 b |
| GA | 82 | 65.2585 ± 9.2777 a | ||
| AA | 44 | 63.7788 ± 7.2515 a |
| SNP Name | CNC10050191 | CNC10062936 | CNC10031720 | CNC10031721 |
|---|---|---|---|---|
| Dominant genotype | CC | AA | AG/GG | GA/AA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, M.; Chen, G.; Tao, C.; Li, B.; Zhang, H.; Wang, H.; Wang, W. Genome-Wide Association Study That Identifies Molecular Markers with Freezing Resistance in Duroc Boar Sperm. Animals 2025, 15, 1474. https://doi.org/10.3390/ani15101474
Zhang J, Li M, Chen G, Tao C, Li B, Zhang H, Wang H, Wang W. Genome-Wide Association Study That Identifies Molecular Markers with Freezing Resistance in Duroc Boar Sperm. Animals. 2025; 15(10):1474. https://doi.org/10.3390/ani15101474
Chicago/Turabian StyleZhang, Jiajun, Meicheng Li, Guangxiang Chen, Chenyu Tao, Bushe Li, Hejun Zhang, Hongyang Wang, and Wenjun Wang. 2025. "Genome-Wide Association Study That Identifies Molecular Markers with Freezing Resistance in Duroc Boar Sperm" Animals 15, no. 10: 1474. https://doi.org/10.3390/ani15101474
APA StyleZhang, J., Li, M., Chen, G., Tao, C., Li, B., Zhang, H., Wang, H., & Wang, W. (2025). Genome-Wide Association Study That Identifies Molecular Markers with Freezing Resistance in Duroc Boar Sperm. Animals, 15(10), 1474. https://doi.org/10.3390/ani15101474




