Overview of Patagonian Red Octopus (Enteroctopus megalocyathus) Fisheries in Chilean Regions and Their Food Safety Aspects
Simple Summary
Abstract
1. Introduction
2. Biology and Fishery Exploitation of the Patagonian Red Octopus (Enteroctopus megalocyathus)
2.1. Distribution, Life Cycle, and Capture
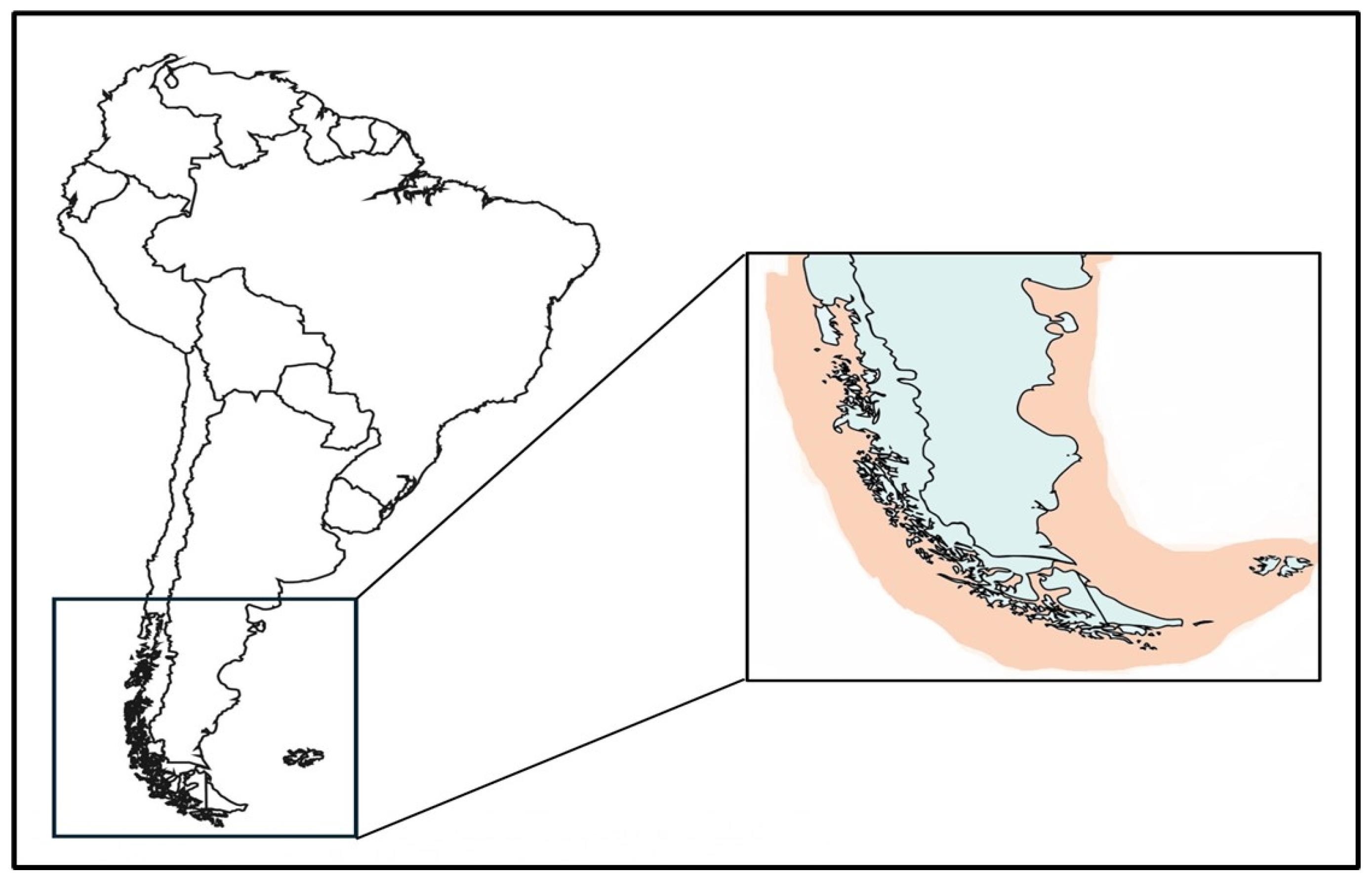
2.1.1. Geographical Distribution
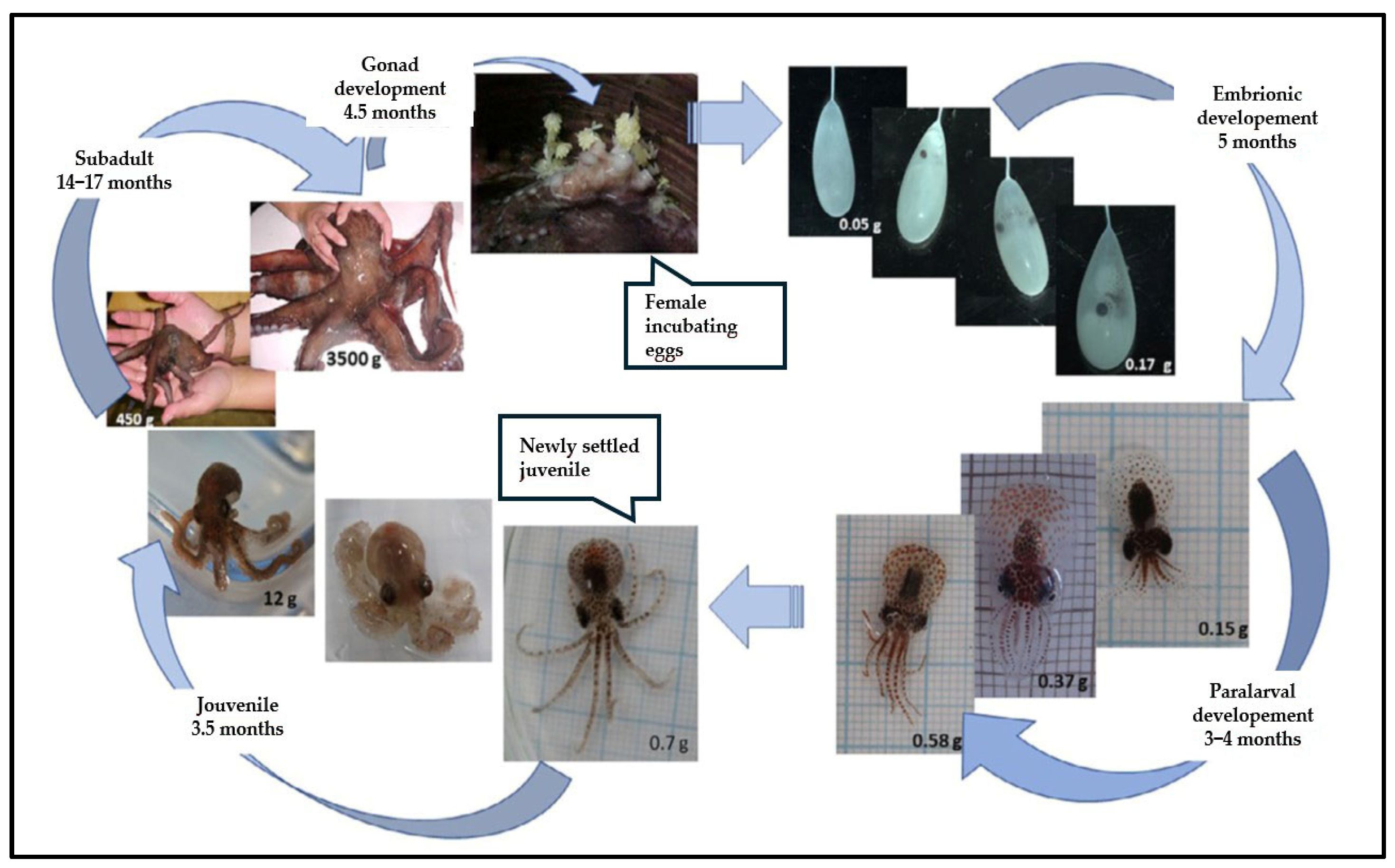
2.1.2. Life Cycle and Trophic Ecology
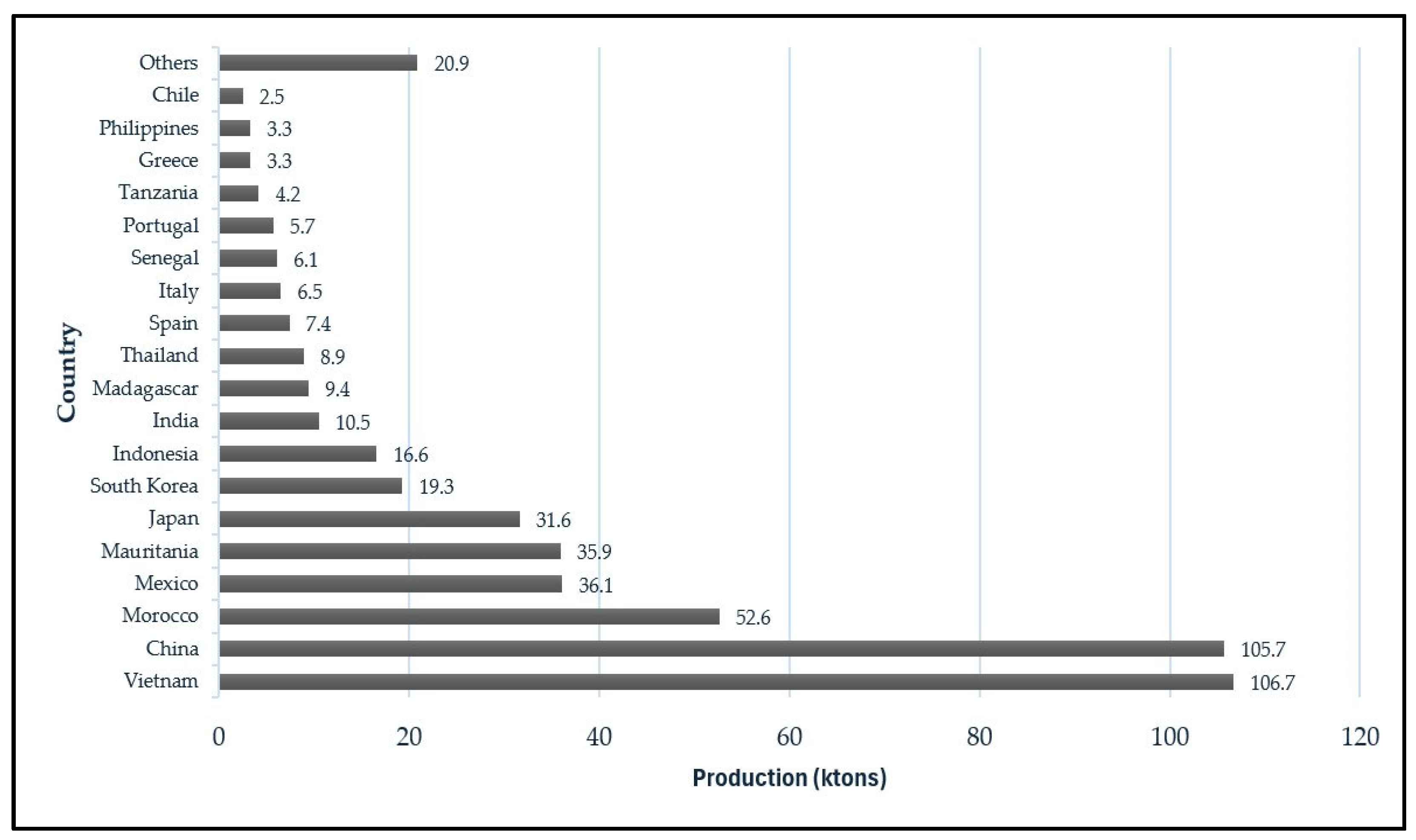
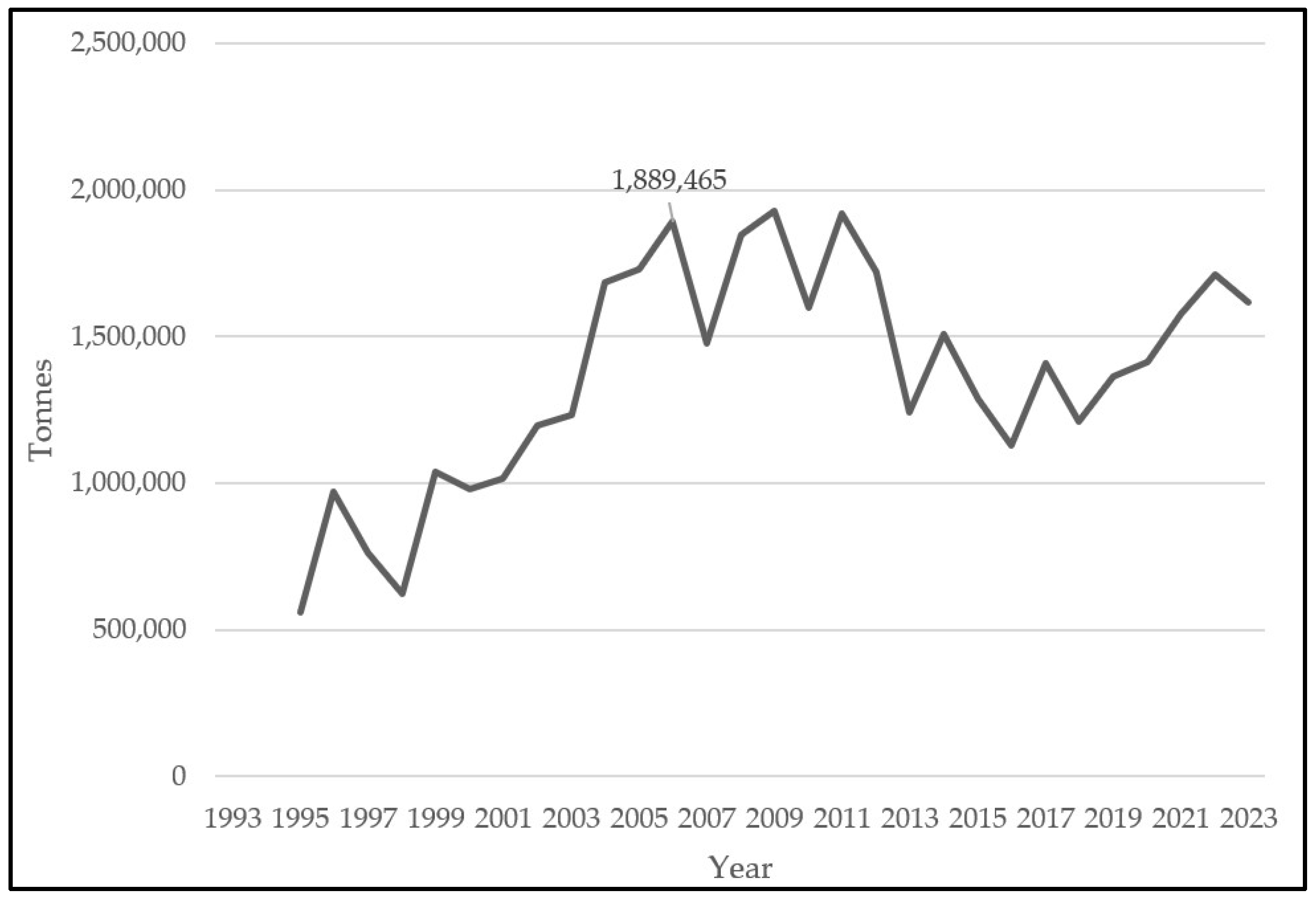
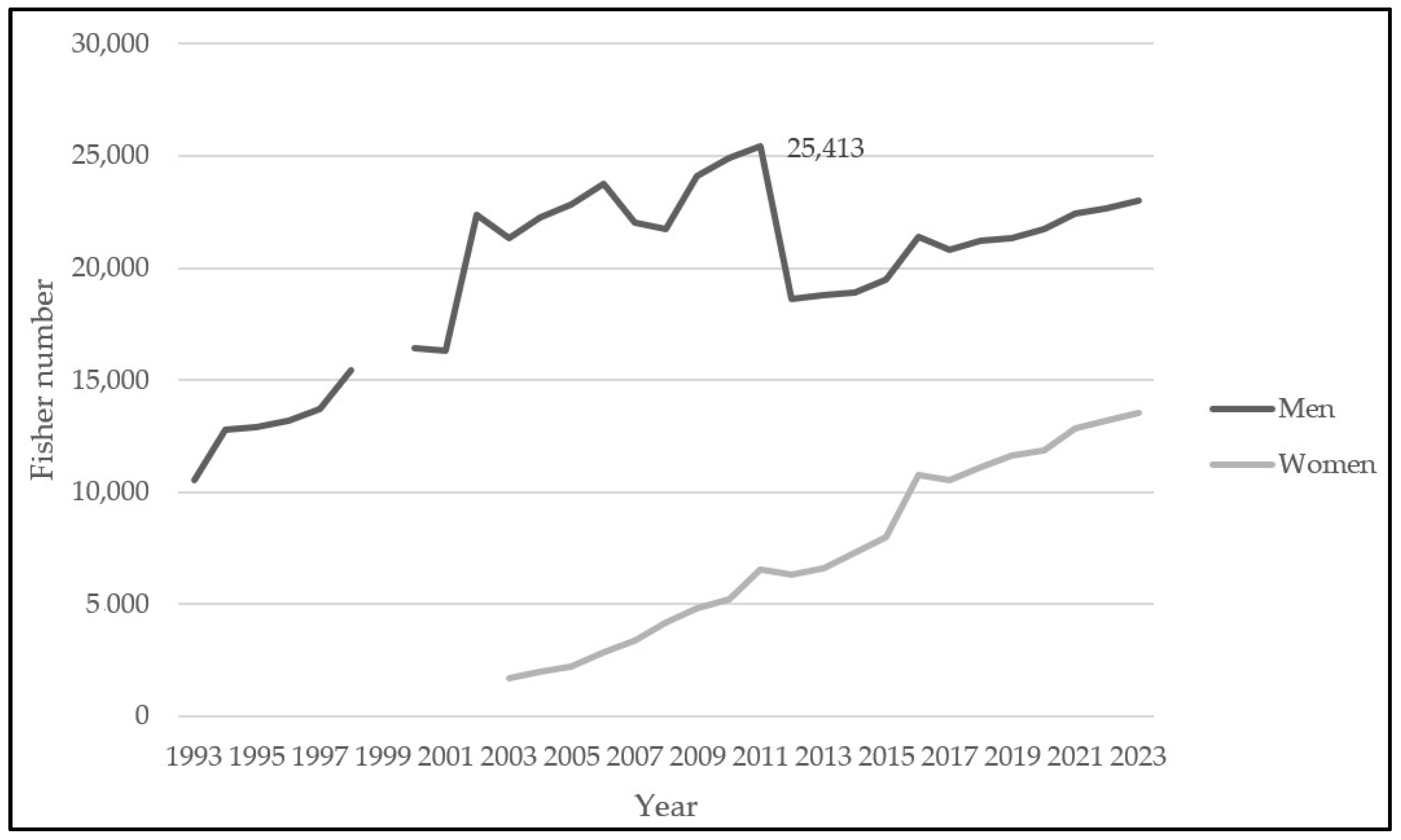
2.1.3. Landings and Fishery Relevance
3. Management Strategies for the Patagonian Red Octopus (Enteroctopus megalocyathus)
3.1. Artisanal Fishing Laws in Chile
3.2. Territorial Use Rights in Fisheries (TURFs), the Management Areas for Benthic Resource Exploitation
3.3. Internal Governance, Decision-Making Process, and Conflict Resolution
3.4. Positive Outcomes and Strengths of TURF Implementation in Chile
3.5. Challenges and Limitations of TURF Implementation
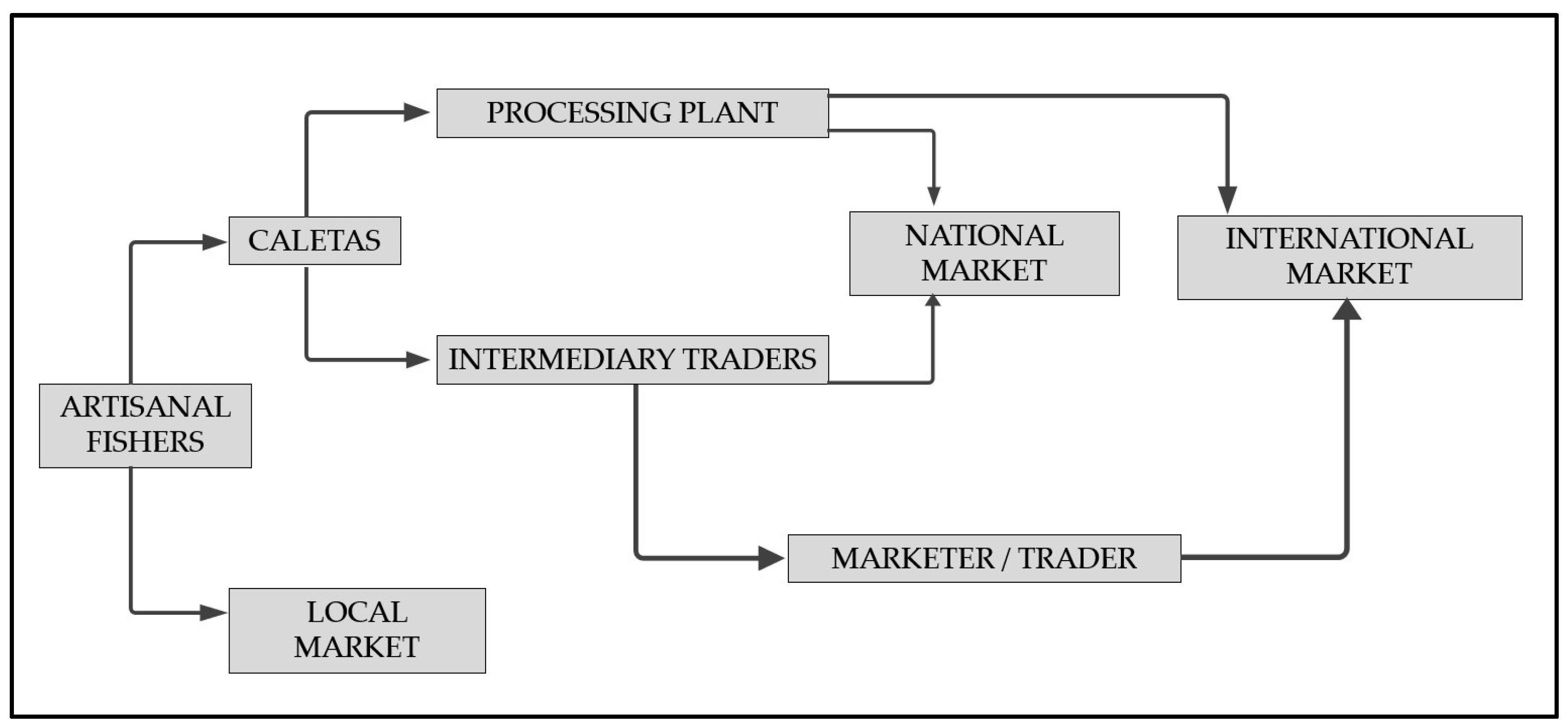
4. Octopus Fishing Practices and the Artisanal Productive Chain in Chile
5. Food Safety in the E. megalocyathus Artisanal Production Chain
5.1. Pre-Harvest Algal Toxin Contamination
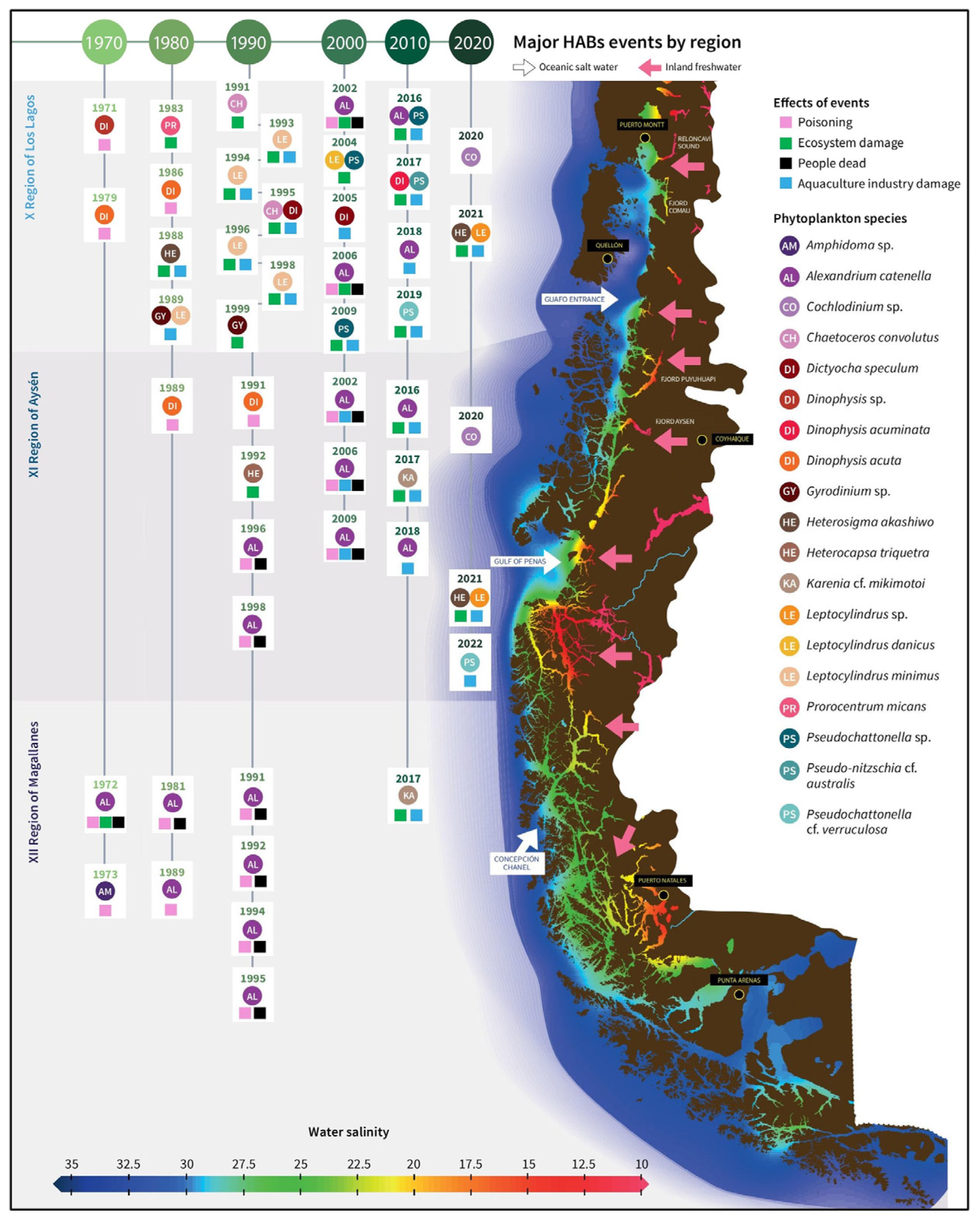
5.2. Pre-Harvest Microbial Contamination
5.3. Pre-Harvest Heavy Metal Contamination
5.4. Pre-Harvest Parasitic Contamination
5.5. Post-Harvest Microbial Contamination
5.6. Post-Harvest Biogenic Amine Contamination
6. Possible Improvements and Sustainable Practices
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossi, S. Fishing and Overfishing-Sustainable Harvest of the Sea. In SDG 14: Life Below Water 2023; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Pita, C.; Roumbedakis, K.; Fonseca, T.; Matos, F.L.; Pereira, J.; Villasante, S.; Pita, P.; Bellido, J.M.; Gonzalez, A.F.; García-Tasende, M.; et al. Fisheries for common octopus in Europe: Socioeconomic importance and management. Fish. Res. 2021, 235, 105820. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture 2024—Blue Transformation in Action; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Sauer, W.H.H.; Gleadall, I.G.; Downey-Breedt, N.; Doubleday, Z.; Gillespie, G.; Haimovici, M.; Ibáñez, C.M.; Katugin, O.N.; Leporati, S.; Lipinski, M.R.; et al. World Octopus Fisheries. Rev. Fish. Sci. Aquac. 2019, 29, 279–429. [Google Scholar] [CrossRef]
- Roa-Ureta, R.H.; Henríquez, J.; Molinet, C. Achieving sustainable exploitation through co-management in three Chilean small-scale fisheries. Fish. Res. 2020, 230, 105674. [Google Scholar] [CrossRef]
- Costello, C.; Ovando, D.; Hilborn, R.; Gaines, S.D.; Deschenes, O.; Lester, S.E. Status and solutions for the world’s unassessed fisheries. Science 2012, 338, 517–520. [Google Scholar] [CrossRef]
- Markaida, U.; Gilly, W.F. Cephalopods of Pacific Latin America. Fish. Res. 2016, 173, 113–121. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Rodhouse, P.G.; Pierce, G.J.; Sauer, W.; Sakai, M.; Allcock, L.; Zeidberg, L.D. World squid fisheries. Rev. Fish. Sci. Aquac. 2015, 23, 92–252. [Google Scholar] [CrossRef]
- Food and Agriculture Organization on the United Nations (FAO). The State of World Fisheries and Aquaculture 2022—Towards Blue Transformation; Food and Agriculture Organization on the United Nations (FAO): Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Pérez, J.A.; Haimovici, M. Cephalopod Fisheries in South America: General Overview and Current Status. In Cephalopod Culture; Iglesias, J., Fuentes, L., Villanueva, R., Eds.; Springer Dordrecht: Dordrecht, The Netherlands, 2014; pp. 199–215. [Google Scholar]
- Uriarte, Í.; Farías, A. Enteroctopus megalocyathus. In Cephalopod Culture; Iglesias, J., Fuentes, L., Villanueva, R., Eds.; Springer Dordrecht: Dordrecht, The Netherlands, 2014; pp. 199–215. [Google Scholar] [CrossRef]
- SERNAPESCA, 2023. Annual Report: Desembarque Artesanal por Región. Available online: https://www.sernapesca.cl/informacion-utilidad/anuarios-estadisticos-de-pesca-y-acuicultura/ (accessed on 14 May 2025).
- Ibáñez, C.M.; Sepúlveda, R.D.; Guerrero, J.; Chong, J. Redescription of Robsonella fontaniana (Cephalopoda: Octopodidae). J. Mar. Biol. Assoc. UK 2008, 88, 617–624. [Google Scholar] [CrossRef]
- Uriarte, I.; Astorga, M.; Navarro, J.C.; Viana, M.T.; Rosas, C.; Molinet, C.; Hernández, J.; Navarro, J.; Moreno-Villoslada, I.; Amthauer, R.; et al. Early life stage bottlenecks of carnivorous molluscs under captivity: A challenge for their farming and contribution to seafood production. Rev. Aquac. 2019, 11, 431–457. [Google Scholar] [CrossRef]
- Boyle, P.; Rodhouse, P. Cephalopods: Ecology and Fisheries; Blackwell Science: Oxford, UK, 2005. [Google Scholar] [CrossRef]
- Di Todaro, F. Acquacoltura senza crisi. Ma l’investimento sui polpi non convince fino in fondo. About Pharma 2024, 22, 34–36. [Google Scholar]
- Ortiz, N.; Ibáñez, C.M.; Farías, A.; Pardo-Gandarillas, M.C.; Uriarte, I. Enteroctopus megalocyathus, Patagonian red octopus. In Octopus Biology and Ecology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 397–417. [Google Scholar] [CrossRef]
- Chong, J.; Cortés, N.; Galleguillos, R.; Oyarzún, C. Estudio Biológico Pesquero del Recurso Pulpo en la X y XI Regiones. Informe final. FIP IT 99–20. 2001; 230p. Available online: https://www.subpesca.cl/portal/fipa/Consultor/Universidad-Catolica-de-la-Santisima-Concepcion/89659:Estudio-biologico-pesquero-del-recurso-pulpo-en-la-X-y-XI-Regiones (accessed on 14 May 2025).
- Ré, M.E. Pulpos Octopodidos (Cephalopoda: Octopodidae). In El Mar Argentino y sus Recursos Pesqueros; Boschi, E.E., Ed.; Tomo 2: Los moluscos de interés pesquero. Cultivos y estrategias reproductivas de bivalvos y equinoideos; Publicaciones Especiales INIDEP: Mar del Plata, Argentina, 1998; pp. 69–98. [Google Scholar]
- Ibáñez, C.M.; Chong, J.V. Feeding ecology of Enteroctopus megalocyathus (Cephalopoda: Octopodidae) in southern Chile. J. Mar. Biol. Assoc. UK 2008, 88, 793–798. [Google Scholar] [CrossRef]
- Pardo-Gandarillas, M.C.; Ibáñez, C.M.; Ruiz, J.F.; Bustos, C.A.; Peña, F.A.; Landaeta, M.F. Paralarvae of cephalopods in channels and fjords of the southern tip of Chile (46–53° S). Fish. Res. 2016, 173, 175–182. [Google Scholar] [CrossRef]
- Ortiz, N.; Re, M.E. First report of pseudohermaphroditism in cephalopods. J. Molluscan Stud. 2006, 72, 321–323. [Google Scholar] [CrossRef]
- Instituto de Fomento Pesquero (IFOP). Infome Final Corregido—Caracterización Biológico Pesquera de las Actividades Extractivas del Recurso Pulpo en la X Región. FIP Nº 2008-40. Valparaiso, Chile, Octubre 2010. Available online: www.ifop.cl (accessed on 14 May 2025).
- Ortiz, N.; Ré, M.E.; Márquez, F.; Glembocki, N.G. The reproductive cycle of the red octopus Enteroctopus megalocyathus in fishing areas of Northern Patagonian coast. Fishs Res. 2011, 110, 217–223. [Google Scholar] [CrossRef]
- Ortiz, N.; Ré, M.E.; Márquez, F. First description of eggs, hatchlings and hatchling behavior of Enteroctopus megalocyathus (Cephalopoda: Octopodidae). J. Plankton Res. 2006, 28, 881–890. [Google Scholar] [CrossRef]
- Villanueva, R.; Norman, M.D. Biology of the planktonic stages of benthic octopuses. Oceanogr. Mar. Biol. 2008, 46, 105–202. [Google Scholar] [CrossRef]
- Roura, Á.; Álvarez-Salgado, X.A.; González, Á.F.; Gregori, M.; Rosón, G.; Otero, J.; Guerra, Á. Life strategies of cephalopod paralarvae in a coastal upwelling system (NW Iberian Peninsula): Insights from zooplankton community and spatio-temporal analyses. Fish. Oceanogr. 2016, 25, 241–258. [Google Scholar] [CrossRef]
- Uriarte, I.; Enríquez, R.; Hernández, J.; Espinoza, V.; Montes de Oca, M.; Silva, A.; Monroig, O.; Sánchez, P.; Alvarez, D.; Farías, A.; et al. Artemia enrichment as modulator of gene expression patterns of Enteroctopus megalocyathus paralarvae at pre-settlement stage. Aquaculture 2024, 579, 740168. [Google Scholar] [CrossRef]
- Osorio, C.; Peña, R.; Ramajo, L.; Gracelon, N. Malacofauna bentonica de los canales oceanicos del sur de Chile (43°–45° S). Cienc. Y Tecnol. Del. Mar. 2006, 29, 103–114. [Google Scholar]
- Ibáñez, C.; Cortés, N.; Chong, J. Aspectos trofodinámicos del pulpo Enteroctopus megalocyathus en el Pacífico Suroriental. In Proceedings of the XXI Congreso Ciencias Mar, Viña del Mar, Chile, 19–23 September 2001. [Google Scholar]
- Pérez, M.; López, D.; Aguila, K.; Gonzalez, M. Feeding and growth in captivity of the octopus Enteroctopus megalocyathus Gould, 1852. Aquac. Res. 2006, 37, 550–555. [Google Scholar] [CrossRef]
- Ambrose, R.F.; Nelson, B.V. Predation by Octopus vulgaris in the Mediterranean. Mar. Ecol. 1983, 4, 251–261. [Google Scholar] [CrossRef]
- Ambrose, R.F. Effects of octopus predation on motile invertebrates in a rocky subtidal community. Mar. Ecol. Prog. Ser. 1986, 30, 261–273. [Google Scholar] [CrossRef]
- Espinoza, V.; Brokordt, K.; Farías, A.; Romero, A.; Uriarte, I. Assessment of culture density and prey availability for the management of cannibalism in paralarvae of Patagonian red octopus Enteroctopus megalocyathus. Aquac. Res. 2021, 52, 4629–4637. [Google Scholar] [CrossRef]
- Schiavini, A.; Goodall, R.N.P.; Lescrauwaet, A.K.; Koen Alonso, M. Food habits of the Peale’s dolphin, Lagenorhynchus australis; review and new information. Rep. Int. Whal. Comm. 1997, 47, 827–834. Available online: https://oceanrep.geomar.de/id/eprint/53025 (accessed on 14 May 2025).
- Laptikhovsky, V.V.; Arkhipkin, A.I.; Henderson, A.C. Feeding habits and dietary overlap in spiny dogfish Squalus acanthias (Squalidae) and narrowmouth catshark Schroederichthys bivius (Scyliorhinidae). J. Mar. Biol. UK 2001, 81, 1015–1018. [Google Scholar] [CrossRef]
- Koen Alonso, M.; Alberto Crespo, E.; Aníbal García, N.; Noemí Pedraza, S.; Ariel Mariotti, P.; Judith Mora, N. Fishery and ontogenetic driven changes in the diet of the spiny dogfish, Squalus acanthias, in Patagonian waters, Argentina. Environ. Biol. Fishes 2002, 63, 193–202. [Google Scholar] [CrossRef]
- Koen Alonso, M.; Crespo, E.A.; Pedraza, S.N.; Garcia, N.A.; Coscarella, M.A. Food habits of the South American sea lion, Otaria flavescens, off Patagonia, Argentina. Fish. Bull. 2000, 98, 250–263. Available online: http://hdl.handle.net/11336/70210 (accessed on 14 May 2025).
- Schiavini, A.; Rey, A.R. Long days, long trips: Foraging ecology of female rockhopper penguins Eudyptes chrysocome chrysocome at Tierra del Fuego. Mar. Ecol. Prog. Ser. 2004, 275, 251–262. [Google Scholar] [CrossRef]
- Rey, A.R.; Schiavini, A. Inter-annual variation in the diet of female southern rockhopper penguin (Eudyptes chrysocome chrysocome) at Tierra del Fuego. Polar Biol. 2005, 28, 132–141. [Google Scholar] [CrossRef]
- Clausen, A.P.; Arkhipkin, A.I.; Laptikhovsky, V.V.; Huin, N. What is out there: Diversity in feeding of gentoo penguins (Pygoscelis papua) around the Falkland Islands (Southwest Atlantic). Polar Biol. 2005, 28, 653–662. [Google Scholar] [CrossRef]
- Ré, M.E.; Kuba, L.; Ortiz, N.; Marquez, F.; Gosztonyi, A.; Nilsson, M. Ecología trófica de Enteroctopus megalocyathus en costas de la Patagonia Norte y Central, Argentina. In Book of Abstract of VII Jornadas Nacionales de Ciencias del mar y XIV Coloquio de Oceanografía; Bahia Blanca, Argentina, 2007; p. 378. Available online: https://bicyt.conicet.gov.ar/fichas/produccion/5270283 (accessed on 14 May 2025).
- Romero, M.; Dans, S.; González, R.; Svendsen, G.; García, N.; Crespo, E. Trophic overlap between the South American sea lion Otaria flavescens and the demersal trawl fishery in San Matías Gulf, Patagonia, Argentina. Lat. Am. J. Aquat. Res. 2011, 39, 344–358. [Google Scholar] [CrossRef]
- Pavés, H. Hábitos Alimentarios del Lobo fino Austral (Arctocephalus australis) en la Isla Guafo Durante las Temporadas Reproductivas de 2010 y 2012. Ph.D. Thesis, Universidad Austral de Chile, Valdivia, Chile, 2012. [Google Scholar]
- Romero, M.A.; Fernández, M.; Dans, S.L.; García, N.A.; González, R.; Crespo, E.A. Gastrointestinal parasites of bottlenose dolphins Tursiops truncatus from the extreme southwestern Atlantic, with notes on diet composition. Dis. Aquat. Org. 2014, 108, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Koen Alonso, M.; Crespo, E.A.; García, N.A.; Pedraza, S.N.; Coscarella, M.A. Diet of dusky dolphins, Lagenorhynchus obscurus, in waters off Patagonia, Argentina. Fish. Bull. 1998, 96, 366–374. [Google Scholar]
- Dans, S.L.; Crespo, E.A.; Koen-Alonso, M.; Markowitz, T.M.; Vera, B.B.; Dahood, A.D. Dusky Dolphin Trophic Ecology: Their Role in the Food Web. In The Dusky Dolphin; Academic Press: Cambridge, MA, USA, 2010; pp. 49–74. [Google Scholar] [CrossRef]
- Riccialdelli, L.; Newsome, S.D.; Dellabianca, N.A.; Bastida, R.; Fogel, M.; Goodall, R.N. Ontogenetic diet shift in Commerson’s dolphin (Cephalorhynchus commersonii commersonii) off Tierra del Fuego. Polar Biol. 2013, 36, 617–627. [Google Scholar] [CrossRef]
- Koen Alonso, M.; Crespo, E.A.; García, N.A.; Pedraza, S.N.; Mariotti, P.A.; Vera, B.B.; Mora, N.J. Food habits of Dipturus chilensis (Pisces: Rajidae) off Patagonia, Argentina. ICES J. Mar. Sci. 2001, 58, 288–297. [Google Scholar] [CrossRef]
- Arkhipkin, A.; Brickle, P.; Lptikhovsky, V.; Butcher, L.; Jones, E.; Potter, M.; Poulding, D. Variation in the diet of the red cod with size and season around the Falkland Islands (south-west Atlantic). J. Mar. Biol. UK 2001, 81, 1035–1040. [Google Scholar] [CrossRef]
- Elías, I.; Rajoy, C.R. Hábitos alimentarios del “Salmon de Mar” Pseudopercis semifasciata (Cuvier, 1829): Pinguipedidae, en Aguas Norpatagonicas Argentinas. Rev. Biol. Mar. 1992, 27, 133–146. [Google Scholar]
- Galván, D.E.; Botto, F.; Parma, A.M.; Bandieri, L.; Mohamed, N.; Iribarne, O.O. Food partitioning and spatial subsidy in shelter-limited fishes inhabiting patchy reefs of Patagonia. J. Fish. Biol. 2009, 75, 2585–2605. [Google Scholar] [CrossRef]
- Scioscia, G.; Raya Rey, A.; Saenz Samaniego, R.A.; Florentín, O.; Schiavini, A. Intra- and interannual variation in the diet of the Magellanic penguin (Spheniscus magellanicus) at Martillo Island, Beagle Channel. Polar Biol. 2014, 37, 1421–1433. [Google Scholar] [CrossRef]
- Michalik, A.; van Noordwijk, H.J.; Brickle, P.; Eggers, T.; Quillfeldt, P. The diet of the Imperial Shag Phalacrocorax atriceps at a colony on New Island, Falkland/Malvinas Islands combining different sampling techniques. Polar Biol. 2010, 33, 1537–1546. [Google Scholar] [CrossRef]
- Tobar, C.N.; Carmona, D.; Rau, J.R.; Cursach, J.A.; Vilugrón, J. Dieta invernal del cormorán imperial Phalacrocorax atriceps (Aves: Suliformes) en Bahía Caulín, Chiloé, sur de Chile. Rev. Bio Mar. Ocean. 2019, 54, 227–231. [Google Scholar] [CrossRef]
- Garbin, L.; Diaz, J.I.; Morgenthaler, A.; Millones, A.; Kuba, L.; Fuchs, D.; Navone, G.T. Cormorant pellets as a tool for the knowledge of parasite-intermediate host associations and nematode diversity in the environment. Helminthologia 2019, 56, 296. [Google Scholar] [CrossRef] [PubMed]
- Libenson, L.V. La dieta del Cormorán cuello negro (Phalacrocorax magellanicus) y el Cormorán real (P. albiventer) en el puerto de Comodoro Rivadavia (Chubut, Argentina). Nat. Pat. 1996, 4, 85–94. [Google Scholar]
- Harrington, K.J.; Bildstein, K.L. Predation of southern red octopus (Enteroctopus megalocyathus) by Striated Caracaras (Phalcoboenus australis) in the Falkland Islands. J. Raptor Res. 2019, 53, 220–222. [Google Scholar] [CrossRef]
- DS N° 137-85 Establece Tamaño Mínimo de Extracción Pulpo. (F.D.O. 05-07-1985). Available online: https://www.subpesca.cl/portal/normativa/Medidas-de-administracion-y-regimenes-de-acceso/Talla-minima-de-extraccion/644:D-S-N-137-85-Establece-Tamano-Minimo-de-Extraccion-Pulpo-F-D-O-05-07-1985 (accessed on 14 May 2025).
- SERNAPESCA, 2016. Anuario estadístico de pesca. Servicio Nacional de Pesca, Ministerio de Economía, Fomento y Reconstrucción, Chile. Available online: https://anuario.sernapesca.dataobservatory.net/desembarque/artesanal/ (accessed on 14 May 2025).
- Velázquez-Abunader, I.; Salas, S.; Cabrera, M.A. Differential catchability by zone, fleet, and size: The case of the red octopus (Octopus maya) and common octopus (Octopus vulgaris) fishery in Yucatan, Mexico. J. Shellfish. Res. 2013, 32, 845–854. [Google Scholar] [CrossRef]
- Gobierno de Chile. Ley General de Pesca y Acuicultura (Ley N° 18892/1989). Diario Oficial de la República de Chile, 1989. Available online: https://www.subpesca.cl/portal/615/w3-article-88020.html (accessed on 14 May 2025).
- SERNAPESCA, 2023. Annual Report: Desembarque Total. Available online: https://www.sernapesca.cl/informacion-utilidad/anuarios-estadisticos-de-pesca-y-acuicultura/ (accessed on 14 May 2025).
- Castilla, J.C.; Defeo, O. Latin American benthic shellfisheries: Emphasis on co-management and experimental practices. Rev. Fish. Biol. Fish. 2001, 11, 1–30. [Google Scholar] [CrossRef]
- SERNAPESCA, 2000. Anuario estadístico de pesca. Servicio Nacional de Pesca, Ministerio de Economía, Fomento y Reconstrucción, Chile. Available online: https://www.sernapesca.cl/anuarios-estadisticos-de-pesca-y-acuicultura-1960-2011/ (accessed on 14 May 2025).
- SERNAPESCA, 2010. Anuario estadístico de pesca. Servicio Nacional de Pesca, Ministerio de Economía, Fomento y Reconstrucción, Chile. Available online: https://www.sernapesca.cl/anuarios-estadisticos-de-pesca-y-acuicultura-1960-2011/ (accessed on 14 May 2025).
- SERNAPESCA, 2018. Anuario estadístico de pesca. Servicio Nacional de Pesca, Ministerio de Economía, Fomento y Reconstrucción, Chile. Available online: https://www.sernapesca.cl/informacion-utilidad/anuarios-estadisticos-de-pesca-y-acuicultura/ (accessed on 14 May 2025).
- SERNAPESCA, 2023. Subsector Pesquero Artesanal. Available online: https://www.sernapesca.cl/informacion-utilidad/anuarios-estadisticos-de-pesca-y-acuicultura/ (accessed on 14 May 2025).
- Jereb, P.; Roper, C.F.E.; Norman, M.D.; Finn, J.K. Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Volume 3. Octopods and Vampire Squids. FAO Species Cat. Fish. Purp. 2016, 4, 370. Available online: https://openknowledge.fao.org/handle/20.500.14283/i3489e (accessed on 14 May 2025).
- SUBPESCA, 2023. Report: Mujeres y Hombres en el Sector Pesquero y Acuicultor de Chile. Available online: https://www.subpesca.cl/portal/618/w3-article-121456.html (accessed on 14 May 2025).
- SUBPESCA, 2024b. Informe Sectorial de Pesca y Acuicultura¸ Octubre 2024. Available online: https://www.subpesca.cl/portal/618/w3-propertyvalue-788.html (accessed on 14 May 2025).
- SUBPESCA, 2024a. Áreas de Manejo de Recursos Bentónicos a Nivel Nacional. Unidad de Recursos Bentónicos, División de Administración Pesquera. Geoportal SUBPESCA. Available online: https://geoportal.subpesca.cl/portal/home/item.html?id=5235010559be4dc38770de363542768d#overview (accessed on 14 May 2025).
- Rocha, F.; Vega, M.A. Overview of cephalopod fisheries in Chilean waters. Fish. Res. 2003, 60, 151–159. [Google Scholar] [CrossRef]
- Galarza, E.; Kámiche Zegarra, J.N. Pesca Artesanal: Oportunidades Para el Desarrollo Regional; Universidad del Pacífico: Lima, Peru, 2015. [Google Scholar] [CrossRef]
- Hardin, G. The tragedy of the commons. Science 1968, 162, 1243–1248. [Google Scholar] [CrossRef]
- Ostrom, E. Governing the Commons: The Evolution of Institutions for Collective Action. In Political Economy of Institutions and Decisions; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar] [CrossRef]
- Castilla, J.C.; Gelcich, S. Management of the loco (Concholepas concholepas) as a driver for self-governance of small-scale benthic fisheries in Chile. FAO Tech. Rep. Report. 2007, 604, 1–11. [Google Scholar]
- Pascual-Fernández, J.J.; Florido-del-Corral, D.; De la Cruz-Modino, R.; Villasante, S. Small-Scale Fisheries in Spain: Diversity and Challenges. In Small-Scale Fisheries in Europe: Status, Resilience and Governance; Pascual-Fernández, J., Pita, C., Bavinck, M., Eds.; Springer: Cham, Switzerland, MARE Publication Series; 2020; Volume 23. [Google Scholar] [CrossRef]
- Castilla, J.C. Artisanal caletas: As units of production and co-managers of benthic invertebrates in Chile. Can. J. Fish. Aquat. Sci. Spec. Publ. 1997, 125, 407–413. [Google Scholar]
- Defeo, O.; Castilla, J.C. More than one bag for the world. Fishery crises and keys for co-management success in selected artisanal Latin American shell fisheries. Rev. Fish. Biol. Fish. 2005, 15, 265–283. [Google Scholar] [CrossRef]
- Gelcich, S.; Godoy, N.; Prado, L.; Castilla, J.C. Add-on conservation benefits of marine territorial user rights fishery policies in central Chile. Ecol. Appl. 2008, 18, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Gelcich, S.; Hughes, T.P.; Olsson, P.; Folke, C.; Defeo, O.; Fernández, M.; Castilla, J.C. Navigating transformations in governance of Chilean marine coastal resources. Proc. Natl. Acad. Sci. USA 2010, 107, 16794–16799. [Google Scholar] [CrossRef]
- Gelcich, S.; Fernández, M.; Godoy, N.; Canepa, A.; Prado, L.; Castilla, J.C. Territorial user rights for fisheries as ancillary instruments for marine coastal conservation in Chile. Conserv. Biol. 2012, 26, 1005–1015. [Google Scholar] [CrossRef]
- Jesus, M.D.; Zapelini, C.; Santana, R.O.D.; Schiavetti, A. Octopus Fishing and New Information on Ecology and Fishing of the Shallow-Water Octopus Callistoctopus furvus (Gould, 1852) Based on the Local Ecological Knowledge of Octopus Fishers in the Marine Ecoregions of Brazil. Front. Ecol. Evol. 2022, 10, 788879. [Google Scholar] [CrossRef]
- Gelcich, S.; Kaiser, M.J.; Castilla, J.C.; Edwards-Jones, G. Engagement in co-management of marine benthic resources influences environmental perceptions of artisanal fishers. Environ. Conserv. 2008, 35, 36–45. [Google Scholar] [CrossRef]
- Espinoza, C.; Gallardo, V.A.; Merino, C.; Pizarro, P.; Liu, K.M. Sustainability of the artisanal fishery in northern Chile: A case study of Caleta Pisagua. Sustainability 2020, 12, 7290. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Voluntary Guidelines for Securing Sustainable Small-Scale Fisheries in the Context of Food Security and Poverty Eradication; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2015; 35p, Available online: https://openknowledge.fao.org/handle/20.500.14283/i4356en (accessed on 14 May 2025).
- Arias, N.; Stotz, W. Sustainability analysis of the benthic fisheries managed in the TURF system in Chile. Int. J. Commons 2020, 14, 344–365. [Google Scholar] [CrossRef]
- Instituto de Fomento Pesquero (IFOP). Programa de Seguimiento Pesquerías Bajo Régimen de Áreas de Manejo 2022–2023; Boletín de Difusión: Valparaiso, Chile, 2023. [Google Scholar]
- Uriarte, I.; Iglesias, J.; Domingues, P.; Rosas, C.; Viana, M.T.; Navarro, J.C.; Seixas, P.; Vidal, E.; Ausburger, A.; Pereda, S.; et al. Current Status and Bottle Neck of Octopod Aquaculture: The Case of American Species. J. World Aquac. Soc. 2011, 42, 735–752. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Code of Conduct for Responsible Fisheries; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 1995. [Google Scholar] [CrossRef]
- Instituto de Fomento Pesquero (IFOP). Seguimiento del Estado de Situación de las Principales Pesquerías Nacionales. Investigación Situación Pesquerías Bentónicas, 2007. Published in June 2008. Available online: https://aquadocs.org/bitstream/handle/1834/7684/PE87MSFUGFXKKG86GFUQY9Q8E1PJSJ.pdf?sequence=1&isAllowed=y (accessed on 14 May 2025).
- Pita, C.; Pereira, J.; Lourenço, S.; Sonderblohm, C.; Pierce, G.J. The Traditional Small-Scale Octopus Fishery in Portugal: Framing Its Governability. In Interactive Governance for Small-Scale Fisheries; Jentoft, S., Chuenpagdee, R., Eds.; MARE Publication Series; Springer: Cham, Switzerland, 2015; Volume 13, pp. 117–132. [Google Scholar] [CrossRef]
- Sobrino, I.; Juárez, A.; Rey, J.; Romero, Z.; Baro, J. Description of the clay pot fishery in the Gulf of Cadiz (SW Spain) for Octopus vulgaris: Selectivity and exploitation pattern. Fish. Res. 2011, 108, 283–290. [Google Scholar] [CrossRef]
- Sonderblohm, C.P.; Guimarães, M.H.; Pita, C.; Rangel, M.; Pereira, J.; Gonçalves, J.M.; Erzini, K. Participatory assessment of management measures for Octopus vulgaris pot and trap fishery from southern Portugal. Mar. Policy 2017, 75, 133–142. [Google Scholar] [CrossRef]
- Tsangridis, A.; Sánchez Zalacaín, P.; Ioannidou, D. Exploitation patterns of Octopus vulgaris in two Mediterranean areas. Sci. Mar. 2002, 66, 59–68. [Google Scholar] [CrossRef]
- Hernández-García, V.; Hernández-López, J.L.; Castro, J.J. The octopus (Octopus vulgaris) in the small-scale trap fishery off the Canary Islands (Central-East Atlantic). Fish. Res. 1998, 35, 183–189. [Google Scholar] [CrossRef]
- Bañón, R.; Otero, J.; Campelos-Álvarez, J.M.; Garazo, A.; Alonso-Fernández, A. The traditional small-scale octopus trap fishery off the Galician coast (Northeastern Atlantic): Historical notes and current fishery dynamics. Fish. Res. 2018, 206, 115–128. [Google Scholar] [CrossRef]
- Olguín, A.; Jerez, G. Especies Bentónicas de Importancia Comercial. Chile; Recursos Pesqueros 2003, 1. IFOP, 30p. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.ifop.cl/wp–content/contenidos/uploads/biblioteca/libros_digitales/CATALOGO_BENTONICOS_2003_1.pdf&ved=2ahUKEwii2_DTl4uNAxUcHrkGHQrXNXQQFnoECBoQAQ&usg=AOvVaw2vZ6ZFdo-5EJFVGQxOYgGu (accessed on 14 May 2025).
- Sauer, W.H.H.; Potts, W.; Raberinary, D.; Anderson, J.; Sylvio Perrine, M.J. Assessment of current data for the octopus resource in Rodrigues, western Indian Ocean. Afr. J. Mar. Sci. 2011, 33, 181–187. [Google Scholar] [CrossRef]
- Nair, A.; Dutta, S.; Apte, D.; Kulkarni, B. Assessing abundance and catch selectivity of Octopus cyanea by the artisanal fishery in Lakshadweep islands, India. Aquat. Living Resour. 2018, 31, 10. [Google Scholar] [CrossRef]
- Alejo-Plata, M.D.C.; Gómez-Márquez, J.L.; Ramos Carrillo, S.; Herrera-Galindo, J.E. Reproducción, dieta y pesquería del pulpo Octopus hubbsorum (Mollusca: Cephalopoda) en la costa de Oaxaca, México. Rev. Biol. Trop. 2009, 57, 63–78. [Google Scholar] [CrossRef][Green Version]
- Haimovici, M.; Leite, T.S.; Marinho, R.A.; Batista, B.; Madrid, R.M.; Oliveira, J.E.L.; Lima, F.D.; Candice, L. As pesca-rias de polvos do Nordeste do Brasil. In A Pesca Marinha e Estuarina no Brasil: Estudos de caso Multidisciplinares; Vasconcellos, M., Haimovici, M., Ramos, K., Haimovici, M., Filho, J.M.A., Sunye, P.S., Eds.; Editoria da FURG: Rio Grande, TX, USA, 2014; ISBN 978-85-7566-335-6. [Google Scholar]
- Markaida, U.; Mendez-Loeza, I.; Rodríguez-Domínguez, A. Implementación de Señuelos Artificiales en la Pesca del Pulpo al Garete; El Colegio de la Frontera Sur: San Cristóbal de Las Casas, Mexico, 2015; p. 26. [Google Scholar]
- Norman, M.D.; Sweeney, M.J. The Shallow-water Octopuses (Cephalopoda: Octopodidae) of the Philippines. Invertebr. Taxon. 1997, 11, 89–140. [Google Scholar] [CrossRef]
- Narvarte, M.; González, R.; Fernández, M. Comparison of Tehuelche octopus (Octopus tehuelchus) abundance between an open-access fishing ground and a marine protected area: Evidence from a direct development species. Fish. Res. 2006, 79, 112–119. [Google Scholar] [CrossRef]
- Federación de Pescadores Artesanales de Chiloé (FEPROCH). Nuestras artes de pesca y su aporte a la conservación de la biodiversidad marina en la Comuna de Ancud. In Cuadernillo del Proyecto del Fondo de Protección Ambiental- XIII Concurso del Fondo de Protección Ambiental, Ministerio de Medio Ambiente; Federación de Pescadores Artesanales de Chiloé (FEPROCH): Ancud, Chile, 2010; 4p. [Google Scholar]
- Defeo, O.; Castilla, J.C. Harvesting and economic patterns in the artisanal Octopus mimus (Cephalopoda) fishery in a northern Chile cove. Fish. Res. 1998, 38, 121–130. [Google Scholar] [CrossRef]
- Armendáriz Villegas, E.J.; Ceballos-Vázquez, B.P.; Markaida, U.; Abitia-Cárdenas, A.; Medina-López, M.A.; Arellano-Martínez, M. Diet of Octopus bimaculatus Verril, 1883 (Cephalopoda: Octopodidae) in Bahía De Los Ángeles, Gulf of California. J. Shellfish. Res. 2014, 33, 305–314. [Google Scholar] [CrossRef]
- Markaida, U.; Flores, L.; Arias, E.; Mora, E. Reproduction and population structure of Octopus mimus fished in a Marine Protected Area of Ecuador. J. Mar. Biol. UK 2018, 98, 1383–1389. [Google Scholar] [CrossRef]
- Loulad, S.; Houssa, R.; Rhinane, H.; Boumaaz, A.; Benazzouz, A. Spatial distribution of marine debris on the seafloor of Moroccan waters. Mar. Pollut. Bull. 2017, 124, 303–313. [Google Scholar] [CrossRef]
- Sanchez, P.; Obarti, R. The Biology and Fishery of Octopus vulgaris Caught with Clay Pots on the Spanish. Mediterranean Coast. In Recent Advances in Cephalopod Fisheries Biology; Okutani, T., O’Dor, R.K., Kubodera, T., Eds.; Tokai University Press: Tokyo, Japan, 1993; pp. 477–487. Available online: https://searchworks.stanford.edu/view/6350403 (accessed on 14 May 2025).
- Jouffre, D.; Inejih, C.; Caverivière, A. Are the octopus pots used by the Mauritanian small-scale fishery dangerous for the resource? Bull. Mar. Sci. 2002, 71, 1081–1085. [Google Scholar]
- Ávila-Da-Silva, A.; Assunçao, R.; Tomas, A. Surgimento e evoluçao da pesca do polvo comum, Octopus vulgaris Cuvier, 1797, com potes no Estado de São Paulo, Brasil. In A Pesca Marinha e Estuarina no Brasil: Estudos de caso Multidisciplinares; Vasconcellos, M., Haimovici, M., Ramos, K., Haimovici, M., Filho, J.M.A., Sunye, P.S., Eds.; Editoria da FURG: Rio Grande, TX, USA, 2014; ISBN 978-85-7566-335-6. [Google Scholar]
- Oosthuizen, A. Economic feasibility of an experimental octopus fishery in South Africa. S Afr. J. Sci. 2004, 100, 595–602. [Google Scholar]
- Leporati, S.C.; Ziegler, P.E.; Semmens, J.M. Assessing the stock status of holobenthic octopus fisheries: Is catch per unit effort sufficient? ICES J. Mar. Sci. 2009, 66, 478–487. [Google Scholar] [CrossRef]
- Leporati, S.C.; Hart, A.M.; Larsen, R.; Franken, L.E.; De Graaf, M. Octopus life history relative to age, in a multi-geared developmental fishery. Fish. Res. 2015, 165, 28–41. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, K.H.; Park, S.W.; Lee, D.G. Study on the fishing performance of an alternative tubular-type pot for the common octopus, Octopus minor, in Korean coastal waters. Iran. J. Fish. Sci. 2015, 14, 73–86. [Google Scholar]
- Barry, P.D.; Tamone, S.L.; Tallmon, D.A. Evaluation of the capture efficiency and size selectivity of four pot types in the prospective fishery for North Pacific giant octopus (Enteroctopus dofleini). Fish. Bull. 2010, 108, 39. [Google Scholar]
- Collie, J.S.; Hall, S.J.; Kaiser, M.J.; Poiner, I.R. A quantitative analysis of fishing impacts on shelf-sea benthos. J. Anim. Ecol. 2000, 69, 785–798. [Google Scholar] [CrossRef]
- Clark, M.R.; Althaus, F.; Schlacher, T.A.; Williams, A.; Bowden, D.A.; Rowden, A.A. The impacts of deep-sea fisheries on benthic communities: A review. ICES J. Mar. Sci. 2016, 73, i51–i69. [Google Scholar] [CrossRef]
- Balguerías, E.; Quintero, M.E.; Hernández-González, C.L. The origin of the Saharan Bank cephalopod fishery. ICES J. Mar. Sci. 2000, 57, 15–23. [Google Scholar] [CrossRef]
- Regueira, M.; González, A.F.; Guerra, A. Habitat selection and population spreading of the horned octopus Eledone cirrhosa (Lamarck, 1798) in Galician waters (NW Atlantic). Fish. Res. 2014, 152, 66–73. [Google Scholar] [CrossRef]
- Silva, L.; Vila, Y.; Torres, M.Á.; Sobrino, I.; Acosta, J.J. Cephalopod assemblages, abundance and species distribution in the Gulf of Cadiz (SW Spain). Aquat. Living Resour. 2011, 24, 13–26. [Google Scholar] [CrossRef][Green Version]
- Chotiyaputta, C.; Nootmorn, P.; Jirapunpipat, K. Review of cephalopod fishery production and long-term changes in fish communities in the Gulf of Thailand. Bull. Mar. Sci. 2002, 71, 223–238. [Google Scholar]
- Quetglas, A.; Alemany, F.; Carbonell, A.; Merella, P.; Sánchez, P. Biology and fishery of Octopus vulgaris Cuvier, 1797, caught by trawlers in Mallorca (Balearic Sea, Western Mediterranean). Fish. Res. 1998, 36, 237–249. [Google Scholar] [CrossRef]
- Noro, K. Fishing gear and methods used for the octopus fisheries off Aomori prefecture. Bull. Fish. Res. Inst. 2013, 23, 123–128. Available online: http://www.aomori-itc.or.jp/index.php?id=5314 (accessed on 14 May 2025). (In Japanese).
- Kim, D.H.; An, H.C.; Lee, K.H.; Hwang, J.W. Optimal economic fishing efforts in Korean common octopus Octopus minor trap fishery. Fish. Sci. 2008, 74, 1215–1221. [Google Scholar] [CrossRef]
- Takeda, R. Octopus resources. Mar. Freshwr Behav. Physiol. 1990, 18, 111–148. [Google Scholar] [CrossRef]
- Taka, H.; Wada, M. Analysis of the drift speed and drift direction of fishing gear for pot drift fishing for Enteroctopus dofleini by obtaining positional information. J. Jap Soc. Fish. Sci. 2018, 84, 202–210. [Google Scholar] [CrossRef]
- Benítez, J.V.; Nava, A.F. Contribucción de la Pesca Artesanal a la Seguridad Alimentaria, el Empleo Rural y el Ingreso Familliar en Países de AMÉRICA del Sur; Organización de las Naciones Unidas para la Alimentación y la Agricultura (FAO): Santiago, Chile, 2016; ISBN 978-92-5-309289-5. Available online: http://www.fao.org/3/bi5768s.pdf (accessed on 14 May 2025).
- Zamuz, S.; Bohrer, B.M.; Shariati, M.A.; Rebezov, M.; Kumar, M.; Pateiro, M.; Lorenzo, J.M. Assessing the quality of octopus: From sea to table. Food Front. 2023, 4, 733–749. [Google Scholar] [CrossRef]
- Samarajeewa, U. Safety, Processing, and Utilization of Fishery Products. Fishes 2024, 9, 146. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, L. The microbial safety of fish and fish products: Recent advances in understanding its significance, contamination sources, and control strategies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 738–786. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.C.; Odu, N.N.; Anyamele, L.M.; Igwiloh, N.J.P.N.; Okonko, I.O. Microbial quality of frozen fish sold in Uyo Metropolis. Nat. Sci. 2012, 10, 71–77. [Google Scholar]
- Aragão, M.F.; Garruti, D.S.; Ogawa, N.B.P.; Bezerra, V.C.; Silva, E.M.C. Development of quality index method for eviscerated and non-eviscerated octopus (Octopus insularis). Cien Agron. 2019, 50, 242–250. [Google Scholar] [CrossRef]
- Barría, C.; Vásquez-Calderón, P.; Lizama, C.; Herrera, P.; Canto, A.; Conejeros, P.; Beltrami, O.; Suárez-Isla, B.A.; Carrasco, D.; Rubilar, I.; et al. Spatial Temporal Expansion of Harmful Algal Blooms in Chile: A Review of 65 Years Records. J. Mar. Sci. Eng. 2022, 10, 1868. [Google Scholar] [CrossRef]
- Díaz, P.A.; Figueroa, R.I. Toxic Algal Bloom Recurrence in the Era of Global Change: Lessons from the Chilean Patagonian Fjords. Microorganisms 2023, 11, 1874. [Google Scholar] [CrossRef]
- Díaz-Tapia, P.; Varela, D.; Pérez-Santos, I.; Díaz-Valdés, M.; Molinet, C.; Seguel, M.; Figueroa, R.I. Impacts of harmful algal blooms on the aquaculture industry: Chile as a case study. Cent. Ocean. A Coruña 2019, 6, 39–50. [Google Scholar] [CrossRef]
- Díaz, P.A.; Álvarez, G.; Pizarro, G.; Blanco, J.; Reguera, B. Lipophilic toxins in Chile: History, producers and impacts. Mar. Drugs 2022, 20, 122. [Google Scholar] [CrossRef]
- Suárez-Isla, B.; Guzmán-Méndez, L. Floraciones de Algas Nocivas. In Mareas Rojas y Toxinas Marinas; Orientaciones en Ciencias; Tecnología y Cultura; Editorial Universitaria: Santiago, Chile, 1998; 98p, Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.ifop.cl/marearoja/wp-content/uploads/sites/2/2016/01/8_-FLORACIONES-DE-ALGAS-NOCIVAS-Mareas-Rojas-y-Toxinas-Marinas-Guzman-y-Suarez-1998.pdf&ved=2ahUKEwjZr5Ws0IyNAxV_Q7gEHb1JHvMQFnoECBgQAQ&usg=AOvVaw1A0LiUfm-kuUMJ2OXgw-Sl (accessed on 14 May 2025).
- Lembeye, G. Distribución de quistes de Alexandrium catenella y otros dinoflagelados en sedimentos de la zona sur-austral de Chile. Cien Tecn Mar. 2004, 27, 21–31. [Google Scholar]
- Alvarez, G.; Uribe, E.; Quijano-Scheggia, S.; Lopez-Rivera, A.; Marino, C.; Blanco, J. Domoic acid production by Pseudo-nitzschia australis and Pseudo-nitzschia calliantha isolated from North Chile. Harmful Algae 2009, 8, 938–945. [Google Scholar] [CrossRef]
- Díaz, P.A.; Molinet, C.; Seguel, M.; Diaz, M.; Labra, G.; Figueroa, R.I. Coupling planktonic and benthic shifts during a bloom of Alexandrium catenella in southern Chile: Implications for bloom dynamics and recurrence. Harmful Algae 2014, 40, 9–22. [Google Scholar] [CrossRef]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Env. Environ. Health 2008, 7, 1–12. [Google Scholar] [CrossRef]
- Molinet, C.; Lafon, A.; Lembeye, G.; Moreno, C.A. Patrones de distribución espacial y temporal de floraciones de Alexandrium catenella (Whedon & Kofoid) Balech 1985, en aguas interiores de la Patagonia noroccidental de Chile. Rev. Chil. Hist. Nat. 2003, 76, 681–698. [Google Scholar] [CrossRef]
- Mardones, J.; Clément, A.; Rojas, X.; Aparicio, C. Alexandrium catenella during 2009 in Chilean waters, and recent expansion to coastal ocean. Harmful Algae News 2010, 41, 9. [Google Scholar]
- Hernández, C.; Díaz, P.A.; Molinet, C.; Seguel, M. Exceptional climate anomalies and northwards expansion of Paralytic Shellfish Poisoning outbreaks in Southern Chile. Harmful Algae News 2016, 54, 16. [Google Scholar]
- Guzmán, L.; Espinoza–González, O.; Pinilla, E.; Besoaín, V.; Calderón, M.J.; Cáceres, J.; Carbonell, P. Atmospheric and oceanographic processes on the distribution and abundance of Alexandrium catenella in the North of Chilean fjords. In Proceedings of the 18th International Conference on Harmful Algae, Nantes, France, 21–26 October 2018. [Google Scholar]
- Álvarez, G.; Díaz, P.A.; Godoy, M.; Araya, M.; Ganuza, I.; Pino, R.; Álvarez, F.; Rengel, J.; Hernández, C.; Uribe, E.; et al. Paralytic shellfish toxins in surf clams Mesodesma donacium during a large bloom of Alexandrium catenella dinoflagellates associated to an intense shellfish mass mortality. Toxins 2019, 11, 188. [Google Scholar] [CrossRef]
- Díaz, P.A.; Pérez-Santos, I.; Schwerter, C.; Arenas, S.; Navarro, P.; Mancilla-Gutiérrez, G.; Barrera, F. Multi-specific Harmful Algal Bloom in a Chilean Fjord: A dangerous phytoplankton cocktail. Harmful Algae News 2022, 70, 8–9. [Google Scholar]
- Alves-de-Souza, C.; Varela, D.; Contreras, C.; de La Iglesia, P.; Fernández, P.; Hipp, B.; Hernández, C.; Riobó, P.; Reguera, B.; Franco, J.M.; et al. Seasonal variability of Dinophysis spp. and Protoceratium reticulatum associated to lipophilic shellfish toxins in a strongly stratified Chilean fjord. Deep. Sea Res. Part II Top. Stud. Ocean. Oceanogr. 2014, 101, 152–162. [Google Scholar] [CrossRef]
- Pizarro, G.; Paz, B.; Alarcón, C.; Toro, C.; Frangópulos, M.; Salgado, P.; Olave, C.; Zamora, C.; Pacheco, H.; Guzmán, L. Winter distribution of toxic, potentially toxic phytoplankton, and shellfish toxins in fjords and channels of the Aysén region, Chile. Lat. Am. J. Aquat. Res. 2018, 46, 120–139. [Google Scholar] [CrossRef]
- León-Muñoz, J.; Urbina, M.A.; Garreaud, R.; Iriarte, J.L. Hydroclimatic conditions trigger record harmful algal bloom in western Patagonia (summer 2016). Sci. Rep. 2018, 8, 1330. [Google Scholar] [CrossRef]
- Mardones, J.I.; Paredes, J.; Godoy, M.; Suarez, R.; Norambuena, L.; Vargas, V.; Fuenzalida, G.; Pinilla, E.; Artal, O.; Rojas, X.; et al. Disentangling the environmental processes responsible for the world’s largest farmed fish-killing harmful algal bloom: Chile, 2016. Sci. Total Env. Environ. 2021, 766, 144383. [Google Scholar] [CrossRef]
- Eiriksson, T.; Moodley, L.; Lilliendahl, K.; Halldórsson, H.P.; Bamber, S.; Steinn Jónsson, G.; Thórdarson, J.; Agustsson, T.; Helgason, G.V. Estimate of organic load from aquaculture—A way to increased sustainability. RORUM 2017, 11. [Google Scholar] [CrossRef]
- Quiñones, R.A.; Fuentes, M.; Montes, R.M.; Soto, D.; León-Muñoz, J. Environmental issues in Chilean salmon farming: A review. Rev. Aquac. 2019, 11, 375–402. [Google Scholar] [CrossRef]
- Mardones, J.I.; Paredes-Mella, J.; Flores-Leñero, A.; Yarimizu, K.; Godoy, M.; Artal, O.; Corredor-Acosta, A.; Marcus, L.; Cascales, E.; Pablo Espinoza, J.; et al. Extreme harmful algal blooms, climate change, and potential risk of eutrophication in Patagonian fjords: Insights from an exceptional Heterosigma akashiwo fish-killing event. Prog. Oceanogr. 2023, 210, 102921. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Niklitschek, E.J.; Pereda, S.V. Aquaculture and Its Impacts on the Conservation of Chilean Patagonia. In Conservation in Chilean Patagonia. Integrated Science; Castilla, J.C., Armesto Zamudio, J.J., Martínez-Harms, M.J., Tecklin, D., Eds.; Springer: Cham, Switzerland, 2024; Volume 19, pp. 303–320. [Google Scholar] [CrossRef]
- Lopes, V.M.; Lopes, A.R.; Costa, P.; Rosa, R. Cephalopods as vectors of harmful algal bloom toxins in marine food webs. Mar. Drugs 2013, 11, 3381–3409. [Google Scholar] [CrossRef]
- Roldán-Wong, N.T.; Kidd, K.A.; Marmolejo-Rodríguez, A.J.; Ceballos-Vázquez, B.P.; Shumilin, E.; Arellano-Martínez, M. Bioaccumulation and biomagnification of potentially toxic elements in the octopus Octopus hubbsorum from the Gulf of California. Mar. Pollut. Bull. 2018, 129, 458–468. [Google Scholar] [CrossRef]
- Neves, R.A.F.; Nascimento, S.M.; Santos, L.N. Harmful algal blooms and shellfish in the marine environment: An overview of the main molluscan responses, toxin dynamics, and risks for human health. Environ. Sci. Pollut. Res. 2021, 28, 55846–55868. [Google Scholar] [CrossRef]
- Costa, P.R.; Rosa, R.; Sampayo, M.A.M. Tissue distribution of the amnesic shellfish toxin, domoic acid, in Octopus vulgaris from the Portuguese coast. Mar. Biol. 2004, 144, 971–976. [Google Scholar] [CrossRef]
- Monteiro, A.; Costa, P.R. Distribution and selective elimination of paralytic shellfish toxins in different tissues of Octopus vulgaris. Harmful Algae 2011, 10, 732–737. [Google Scholar] [CrossRef]
- Lopes, V.M.; Rosa, R.; Costa, P.R. Presence and persistence of the amnesic shellfish poisoning toxin, domoic acid, in octopus and cuttlefish brains. Mar. Environ. Res. 2018, 133, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.M.; Baptista, M.; Repolho, T.; Rosa, R.; Costa, P.R. Uptake, transfer and elimination kinetics of paralytic shellfish toxins in common octopus (Octopus vulgaris). Aquat. Toxicol. 2014, 146, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Lin, C.L.; Chen, C.H.; Hsieh, C.H.; Jen, H.C.; Jian, S.J.; Hwang, D.F. Toxin and species identification of toxic octopus implicated into food poisoning in Taiwan. Toxicon 2014, 91, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Oyaneder Terrazas, J.; Contreras, H.R.; García, C. Prevalence, variability and bioconcentration of saxitoxin-group in different marine species present in the food chain. Toxins 2017, 9, 190. [Google Scholar] [CrossRef]
- Ugarte, A.; Romero, J.; Farías, L.; Sapiains, R.; Aparicio-Rizzo, P.; Ramajo, L.; Aguirre, C.; Masotti, I.; Jacques, M.; Aldunce, P.; et al. Red tide and Global Change: Elements for the Construction of an Integrated Goverance of Harmful Algal Blooms (HABs). Center for Climate and Resilience Research (CR)2, (ANID/FONDAP/15110009); 2022; 84p. Available online: www.cr2.cl/eng/habs/ (accessed on 14 May 2025).
- Opitz-Ríos, C.; Burgos-Pacheco, A.; Paredes-Cárcamo, F.; Campanini-Salinas, J.; Medina, D.A. Metagenomics Insight into Veterinary and Zoonotic Pathogens Identified in Urban Wetlands of Los Lagos, Chile. Pathogens 2024, 13, 788. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Public health aspects of Vibrio spp. related to the consumption of seafood in the EU. EFSA J. 2024, 22, e8896. [Google Scholar] [CrossRef]
- Ishii, S.; Sadowsky, M.J. Escherichia coli in the environment: Implications for water quality and human health. Microbed Environ. 2008, 23, 101–108. [Google Scholar] [CrossRef]
- Acuña-Ramírez, S.; Jiménez-Badillo, M.L.; Galindo-Cortes, G.; Marval-Rodríguez, A.; Castañeda-Chávez, M.R.; Reyes-Velázquez, C.; Rodulfo-Carvajal, H.; de Donato-Capote, M. Environmental and Anthropogenic Influences on Coliform Concentrations in the Octopus insularis Production Chain in the Veracruz Reef System, Gulf of Mexico. Animals 2023, 13, 3049. [Google Scholar] [CrossRef]
- Lourenço, H.M.; Anacleto, P.; Afonso, C.; Ferraria, V.; Martins, M.F.; Carvalho, M.L.; Lino, A.R.; Nunes, M.L. Elemental composition of cephalopods from Portuguese continental waters. Food Chem. 2009, 113, 1146–1153. [Google Scholar] [CrossRef]
- Alves, R.N.; Maulvault, A.L.; Barbosa, V.L.; Fernandez-Tejedor, M.; Tediosi, A.; Kotterman, M.; van den Heuvel, F.H.M.; Robbens, J.; Fernandes, J.O.; Romme Rasmussen, R.; et al. Oral bioaccessibility of toxic and essential elements in raw and cooked commercial seafood species available in European markets. Food Chem. 2018, 267, 15–27. [Google Scholar] [CrossRef]
- Cano-Sancho, G.; Perelló, G.; Maulvault, A.L.; Marques, A.; Nadal, M.; Domingo, J.L. Oral bioaccessibility of arsenic, mercury and methylmercury in marine species commercialized in Catalonia (Spain) and health risks for the consumers. Food Chem. Toxicol. 2015, 86, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.; Salvagio Manta, D.; Mirto, S.; Quinci, E.M.; Ape, F.; Montalto, V.; Gristina, M.; Traina, A.; Sprovieri, M. Bioaccumulation of heavy metals in fish, crustaceans, molluscs and echinoderms from the Tuscany coast. Ecotoxicol. Environ. Saf. 2018, 162, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, I.; de Troch, M.; de Boeck, G. Potential health risks via consumption of six edible shellfish species collected from Piura—Peru. Ecotoxicol. Environ. Saf. 2018, 159, 249–260. [Google Scholar] [CrossRef]
- Bagul, V.R.; Shinde, D.N.; Chavan, R.P.; Patil, C.L.; Pawar, R.K. New perspective on heavy metal pollution of water. J. Chem. Pharm. Res. 2015, 7, 700–705. [Google Scholar]
- Vigneri, R.; Malandrino, P.; Gianì, F.; Russo, M.; Vigneri, P. Heavy metals in the volcanic environment and thyroid cancer. Mol. Cell Endocrinol. 2017, 457, 73–80. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Cerda, A. (Eds.) Heavy Metals in the Environment: Impact, Assessment, and Remediation; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-821656-9. [Google Scholar] [CrossRef]
- Carrera-Beltrán, L.; Gavilanes-Terán, I.; Idrovo-Novillo, J.; Valverde, V.H.; Rodríguez-Pinos, A.; Paredes, C.; Signer-Pastor, A.J.; Carbonell-Barrachina, A. Environmental Pollution by Heavy Metals from Volcanic Ash Due to the Eruption of the Tungurahua Volcano–Ecuador; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Rjeibi, M.; Metian, M.; Hajji, T.; Guyot, T.; Ben Chaouacha-Chekir, R.; Bustamante, P. Seasonal survey of contaminants (Cd and Hg) and micronutrients (Cu and Zn) in edible tissues of cephalopods from Tunisia: Assessment of risk and nutritional benefits. J. Food Sci. 2015, 80, 199–206. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Machado, R.; Afonso, C.; Lourenço, H.M.; Nunes, M.L.; Coelho, I.; Langerholc, T.; Marques, A. Bioaccessibility of Hg, Cd and As in cooked black scabbard fish and edible crab. Food Chem. Toxicol. 2011, 49, 2808–2815. [Google Scholar] [CrossRef]
- Torres-Escribano, S.; Ruiz, A.; Barrios, L.; Vélez, D.; Montoro, R. Influence of mercury bioaccessibility on exposure assessment associated with consumption of cooked predatory fish in Spain. J. Sci. Food Agric. 2011, 91, 981–986. [Google Scholar] [CrossRef]
- Chiocchetti, G.; Jadán-Piedra, C.; Vélez, D.; Devesa, V. Metal(loid) contamination in seafood products. Crit. Rev. Food Sci. Nutr. 2017, 57, 3715–3728. [Google Scholar] [CrossRef]
- Gao, S.; Wang, W.X. Oral bioaccessibility of toxic metals in contaminated oysters and relationships with metal internal sequestration. Ecotoxicol. Environ. Saf. 2014, 110, 261–268. [Google Scholar] [CrossRef]
- Amiard, J.C.; Amiard-Triquet, C.; Charbonnier, L.; Mesnil, A.; Rainbow, P.S.; Wang, W.X. Bioaccessibility of essential and non-essential metals in commercial shellfish from Western Europe and Asia. Food Chem. Toxicol. 2008, 46, 2010–2022. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, O.; Amyot, M. Effects of various cooking methods and food components on bioaccessibility of mercury from fish. Environ. Res. 2011, 111, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Kulp, K.S.; Fortson, S.L.; Knize, M.G.; Felton, J.S. An in vitro model system to predict the bioaccessibility of heterocyclic amines from a cooked meat matrix. Food Chem. Toxicol. 2003, 41, 1701–1710. [Google Scholar] [CrossRef]
- Storelli, M.M.; Barone, G.; Marcotrigiano, G.O. Cadmium in cephalopod mollusks: Implications for public health. J. Food Prot. 2005, 68, 577–580. [Google Scholar] [CrossRef]
- Mok, J.S.; Kwon, J.Y.; Son, K.T.; Choi, W.S.; Shim, K.B.; Lee, T.S.; Kim, J.H. Distribution of heavy metals in muscles and internal organs of Korean cephalopods and crustaceans: Risk assessment for human health. J. Food Prot. 2014, 77, 2168–2175. [Google Scholar] [CrossRef]
- Bonerba, E.; de Candia, G.; Ceci, E. Cadmium in Octopus vulgaris: An input to assess human health risk. Ital. J. Food Saf. 2009, 1, 73–76. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). EFSA Panel on Contaminants in the Food Chain (CONTAM). Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar] [CrossRef]
- Moon, C.S.; Zhang, Z.W.; Shimbo, S.; Watanabe, T.; Moon, D.H.; Lee, C.U.; Lee, B.K.; Ahn, D.; Lee, S.H.; Ikeda, M. Dietary intake of cadmium and lead among the general population in Korea. Environ. Res. 1995, 71, 46–54. [Google Scholar] [CrossRef]
- Cuadrado, C.; Kumpulainen, J.; Moreiras, O. Lead, cadmium and mercury contents in average Spanish market basket diets from Galicia, Valencia, Andalucía and Madrid. Food Addit. Contam. 1995, 12, 107–118. [Google Scholar] [CrossRef]
- Cuadrado, C.; Kumpulainen, J.; Carbajal, A.; Moreiras, O. Cereals contribution to the total dietary intake of heavy metals in Madrid, Spain. J. Food Compost. Anal. 2000, 13, 495–503. [Google Scholar] [CrossRef]
- Gestal, C.; Belcari, P.; Abollo, E.; Pascual, S. Parasites of cephalopods in the northern Tyrrhenian Sea (western Mediterranean): New host records and host specificity. Sci. Mar. 1999, 63, 39–43. [Google Scholar] [CrossRef]
- Guardone, L.; Bilska-Zając, E.; Giusti, A.; Malandra, R.; Cencek, T.; Armani, A. Larval ascaridoid nematodes in horned and musky octopus (Eledone cirrhosa and E. moschata) and longfin inshore squid (Doryteuthis pealeii): Safety and quality implications for cephalopod products sold as fresh on the Italian market. Int. J. Food Microbiol. 2020, 333, 108812. [Google Scholar] [CrossRef]
- Abollo, E.; Gestal, C.; Pascual, S. Anisakis infestation in marine fish and cephalopods from Galician waters: An updated perspective. Parasitol. Res. 2001, 87, 492–499. [Google Scholar] [CrossRef]
- Pico-Duran, G.; Pulleiro-Potel, L.; Abollo, E.; Pascual, S.; Munoz, P. Molecular identification of Anisakis and Hysterothylacium larvae in commercial cephalopods from the Spanish Mediterranean coast. Vet. Parasitoly 2016, 220, 47–53. [Google Scholar] [CrossRef]
- Gullian-Klanian, M.; Delgadillo Díaz, M.; Sánchez Solís, M.J. Molecular Characterization of Histamine-Producing Psychrotrophic Bacteria Isolated from Red Octopus (Octopus maya) in Refrigerated Storage. High-Throughput 2018, 7, 25. [Google Scholar] [CrossRef]
- Torres, D.T.; Keb, C.A.C.; Alcántara, J.G.; Balan, R.A.P.; Baldemar, A.C.; Rodríguez, G.V.; Alcántara, E.J.G. The prevalence of multidrug-resistant Salmonella in raw shrimp and octopus in Campeche, Mexico. J. Health Sci. 2022, 12, 193–197. [Google Scholar] [CrossRef]
- Costa, R.A. Escherichia coli in seafood: A brief overview. Adv. Biosci. Biotechnol. 2013, 04, 450–454. [Google Scholar] [CrossRef]
- Kumar, R.; Surendran, P.K.; Thampuran, N. Evaluation of culture, ELISA and PCR assays for the detection of Salmonella in seafood. Lett. Appl. Microbiol. 2008, 46, 221–226. [Google Scholar] [CrossRef]
- Heinitz, M.L.; Ruble, R.D.; Wagner, D.E. Incidence of Salmonella in Fish and Seafood. J. Food Prot. 2000, 63, 579–592. [Google Scholar] [CrossRef]
- Yam, W.C.; Chan, C.Y.; Bella, S.H.; Tam, T.Y.; Kueh, C.; Lee, T. Abundance of clinical enteric bacterial pathogens in coastal waters and shellfish. Water Res. 1999, 34, 51–56. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Expert Workshop on the Application of Biosecurity Measures to Control Salmonella Contamination in Sustainable Aquaculture, Mangalore, India, 19–21 January 2010; FAO Fisheries and Aquaculture Report No. 937; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2010. [Google Scholar]
- Gullian-Klanian, M.; Sànchez-Solís, M.J.; Terrats-Preciat, M.; Delgadillo-Díaz, M.; Aranda, J. Quality indicators and shelf life of red octopus (Octopus maya) in chilling storage. Food Sci Technol 2016, 36, 304–312. [Google Scholar] [CrossRef]
- Oh, S.K.; Lee, N.; Cho, Y.S.; Shin, D.B.; Choi, S.Y.; Koo, M. Occurrence of toxigenic Staphylococcus aureus in ready-to-eat food in Korea. J. Food Prot. 2007, 70, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Vaiyapuri, M.; Joseph, T.C.; Rao, B.M.; Lalitha, K.V.; Prasad, M.M. Methicillin-Resistant Staphylococcus aureus in Seafood: Prevalence, Laboratory Detection, Clonal Nature, and Control in Seafood Chain. J. Food Sci. 2019, 84, 3341–3351. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Microbiological Specifications for Foods (ICMSF). In Microorganisms in Foods 5: Characteristics of Microbial Pathogens; Springer Science and Business Media: Berlin, Germany, 1996.
- Hennekinne, J.A. Staphylococcus Aureus as a Leading Cause of Foodborne Outbreaks Worldwide. In Staphylococcus Aureus; Elsevier: Amsterdam, The Netherlands, 2018; pp. 129–146. [Google Scholar] [CrossRef]
- Adams, M.; Moss, M. Food Microbiology, 2nd ed.; The Royal Society of Chemistry: London, UK, 2000. [Google Scholar]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef]
- Ekici, K.; Omer, A.K. Biogenic amines formation and their importance in fermented foods. BIO Web Conf. 2020, 17, 232. [Google Scholar] [CrossRef]
- Wójcik, W.; Łukasiewicz, M.; Puppel, K. Biogenic amines: Formation, action and toxicity–a review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef]
- Ercolini, D.; Russo, F.; Nasi, A.; Ferranti, P.; Villani, F. Mesophilic and psychrotrophic bacteria from meat and their spoilage potential in vitro and in beef. Appl. Environ. Microbiol. 2009, 75, 1990–2001. [Google Scholar] [CrossRef]
- Prester, L.; Orct, T.; MacAn, J.; Vukušić, J.; Kipčić, D. Determination of biogenic amines and endotoxin in squid, musky octopus, Norway lobster, and mussel stored at room temperature. Arh. Hig. Rada Toksikol. 2010, 61, 389–397. [Google Scholar] [CrossRef]
- Torido, Y.; Takahashi, H.; Kuda, T.; Kimura, B. Analysis of the growth of histamine-producing bacteria and histamine accumulation in fish during storage at low temperatures. Food Control 2012, 26, 174–177. [Google Scholar] [CrossRef]
- Takahashi, H.; Ogai, M.; Miya, S.; Kuda, T.; Kimura, B. Effects of environmental factors on histamine production in the psychrophilic histamine-producing bacterium Photobacterium iliopiscarium. Food Control 2015, 52, 39–42. [Google Scholar] [CrossRef]
- Fichi, G.; Cardeti, G.; Perrucci, S.; Vanni, A.; Cersini, A.; Lenzi, C.; de Wolf, T.; Fronte, B.; Guarducci, M.; Susini, F. Skin lesion-associated pathogens from Octopus vulgaris: First detection of Photobacterium swingsii, Lactococcus garvieae and Betanodavirus. Dis. Aquat. Organ. 2015, 115, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Huang, Z.; Li, J.; Yang, H. Concentrations of biogenic amines in fish, squid and octopus and their changes during storage. Food Chem. 2012, 135, 2604–2611. [Google Scholar] [CrossRef]
- Gonzaga, V.E.; Lescano, A.G.; Huamán, A.A.; Salmón-Mulanovich, G.; Blazes, D.L. Histamine levels in fish from markets in Lima, Perú. J. Food Prot. 2009, 72, 1112–1115. [Google Scholar] [CrossRef]
- Shalaby, A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs (Text with EEA relevance). Off. J. Eur. Union. 2005, 338, 1–26. [Google Scholar]
- Gobierno de Chile. Decreto No 977/1996: Reglamento Sanitario de los Alimentos. Diario Oficial de la República de Chile, 1996. Available online: https://www.odepa.gob.cl/decreto-supremo-n-977-de-1996-reglamento-sanitario-de-los-alimentos (accessed on 14 May 2025).
- Prester, L. Biogenic amines in fish, fish products and shellfish: A review. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 1547–1560. [Google Scholar] [CrossRef]
- Hungerford, J.M. Scombroid poisoning: A review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Venkateswarlu, R.; Mohan, C.O.; Gopal, T.S. Biogenic amines in seafood: A review. J. Food Sci. Technol. 2016, 53, 2210–2218. [Google Scholar] [CrossRef]
- Dima, J.B.; Kowal, M.V.F.; Castañeda, J.; Ortiz, N. Changes on quality parameters and sensory attributes of the Patagonian red octopus (Enteroctopus megalocyathus) meat under different postharvest treatments. Mar. Fish Sci. (MAFIS) 2024, 37, 2. [Google Scholar] [CrossRef]
- Wallace, C.A.; Sperber, W.H.; Mortimore, S.E. Food Safety in the 21st Century: Public Health Perspective. Academic Press: San Diego, CA, USA, 2018. [Google Scholar]
- Greig, J.D.; Todd, E.C.; Bartleson, C.A.; Michaels, B.S. Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 1. Description of the problem, methods, and agents involved. J. Food Prot. 2007, 70, 1752–1761. [Google Scholar] [CrossRef]
- Ashie, I.N.A.; Smith, J.P.; Simpson, B.K. Spoilage and Shelf-Life Extension of Fresh Fish and Shellfish. Crit. Rev. Food Sci. Nutr. 1996, 36, 87–121. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O.G.; Styrbæk, K. Cephalopod gastronomy—A promise for the future. Front. Commun. 2018, 3, 38. [Google Scholar] [CrossRef]
- Sampels, S. The effects of storage and preservation technologies on the quality of fish products: A review. J. F Process Preserv. 2015, 39, 1206–1215. [Google Scholar] [CrossRef]
- Estrella-Gómez, N.; Escalante-Réndiz, D.; González-Burgos, A.; Sosa-Cordero, D.; Rojas-Herrera, R. Análisis microbiológico del pulpo rojo en puertos pesqueros de Campeche, México. Salud Publica Mex. 2016, 58, 453–460. [Google Scholar] [CrossRef][Green Version]
- Chin, J. Control of Communicable Diseases Manual, 17th ed.; American Public Health Association: Washington, DC, USA, 2000. [Google Scholar]
- Shawyer, M.; Medina Pizzali, A.F. The Use of Ice on Small Fishing Vessels; FAO: Rome, Italy, 2003; Fisheries Technical Paper; Available online: http://www.fao.org/documents/card/en/c/94bc9f36-072d-5b6e-a5a3-795af528c71a/ (accessed on 14 May 2025).
- SERNAPESCA. Manual de Buenas Prácticas Pesqueras Para la Pesca Artesanal; SERNAPESCA: Valparaiso, Chile, 2019; Available online: https://www.sernapesca.cl/manuales_y_publicaciones/manual-de-buenas-practicas-pesqueras-para-la-pesca-artesanal/ (accessed on 14 May 2025).
- Vázquez, H.R.; Nava, A.F. Acuicultura de Pequeña Escala y Recursos Limitados en América Latina y el Caribe: Hacia un Enfoque Integral de Políticas Públicas; FAO: Rome, Italy, 2014; 105p, Available online: https://openknowledge.fao.org/handle/20.500.14283/au437s (accessed on 14 May 2025).
- Gobierno de Chile. Ley N° 1333/1987: Requisitos de Calidad del Agua Para Diferentes Usos. Diario Oficial de la República de Chile, 1987. Available online: https://www.bcn.cl/catalogo/client/es_CL/publico/search/detailnonmodal/ent:$002f$002fSD_ILS$002f0$002fSD_ILS:100363/ada?qu=andnov=1 (accessed on 14 May 2025).
- SUBPESCA Comité de Manejo del Pulpo del sur de la Región de Los Lagos. Acta Extendida Comité de Manejo, Sesión Ordinaria Nº 01/2024: Región de Los Lagos, Chile 2024; SUBPESCA Comité de Manejo del Pulpo del sur de la Región de Los Lagos: Puerto Montt, Chile, 2024. [Google Scholar]
- Espinoza Espinoza, V.; Hernández Velásquez, J.; Farías Molina, A.; Barros Contreras, I.; Herrera Fuentes, L.M.; Díaz Pinto, D.; Godoy Ampuero, M.C.; Uriarte Merino, I. Manual de Pulpo Rojo Patagónico; Proyecto FICO75-GORE Los Lagos -Universidad Austral de Chile. “Preservando la tradición pesquera artesanal de las comunidades costeras para el siglo XXI a través de la acuicultura de pequeña escala”; Ediciones Universidad Austral de Chile: Valdivia, Chile, 2022; ISBN 978-956-390-185-6. Available online: https://isbnchile.cl/catalogo.php?mode=detalle&nt=138135 (accessed on 14 May 2025).

| Main Preys [20,42] | Main Predators |
|---|---|
| Squat lobster, Munida subrugosa Cod icefish species, Patagonothoten sp. Tractor crab, Peltarion spinosulum Cunningham’s triplefin, Helocogrammoides cunninghami Ribeiroclinus eigenmanni Rock crab species, Cyrtograpsus sp. True limpet species, Nacella sp. Crab eggs Octopus eggs Conspecifics | South American sea lion, Otaria flavescens [38,43] South American fur seal, Arctocephalus australis [44] Bottlenose dolphin, Tursiops truncatus [45] Dusky dolphin, Lagenorhynchus obscurus [46,47] Peale’s dolphin, Lagenorhynchus australis [35] Commerson’s dolphin, Cephalorhynchus commersonii commersonii [48] Beaked skate, Dipturus chilensis [49] Spiny dogfish, Squalus acanthias [36,37] Narrowmouth catshark, Schroederichtys bivius [36] Red cod, Salilota australis [50] Sandperch, Pseudopercis semifasciata [51,52] Argentine sea bass, Acanthistius patachonicus [53] Southern rockhopper penguin, Eudyptes chrysocome chrysocome [39,40] Gentoo penguin, Pygoscelis papua [41] Magellanic penguin, Spheniscus magellanicus [53] Imperial shag, Leucocarbo atriceps [54,55,56] Magellan cormorant, Leucocarbo magellanicus [57] King cormorant, Leucocarbo albiventer [57] Striated caracara’s, Phalcoboenus australis [58] |
| Anatomical Characteristic | Enteroctopus megalocyathus | Octopus mimus |
|---|---|---|
| Mantle length (max) | Up to 190 mm | Up to 190 mm |
| Total length (max) | Up to ~1.0 m | Up to ~1.2 m |
| Body weight (max) | Up to ~7 kg | Up to ~4 kg |
| Arm length (relative to mantle) | 3.5–5× mantle length | 4–6× mantle length |
| Arm formula 1 | 2 > 3 > 1 > 4 | 2 > 3 > 4 > 1 |
| Web depth 2 | 18–23% of arm length, equal on lateral and dorsal | 18–27% of arm length, deeper on lateral arms |
| Suckers per arm (adult) | ~180–210 | Up to ~330 |
| Enlarged suckers (male) | 8–10 on all arms, starting ~14th sucker (both sexes) | 2–4 enlarged suckers on 2nd and 3rd arms (males) |
| Gills (lamellae per demibranch) | 11–13 | 7–8 |
| Funnel organ | W-shaped, outer limbs shorter | W-shaped, limbs equal |
| Radula | 9 elements (7 rows of teeth + marginal plates) | 9 elements (7 rows of teeth + marginal plates) |
| Hectocotylized arm | Right third arm, 70–90% length of opposite arm | Right third arm, ~77% length of opposite arm |
| Ligula length (% of hectocotylized arm) | 11–22% | 0.7–1.8% |
| Calamus length (% of ligula) | 10–15% | 30–60% |
| Spermatophore length/number | Up to 3× mantle lengths (~3 produced) | 32–40% of mantle lengths/high number |
| Egg size/number | ~17 mm/few (~1000–5000) | 2–3 mm/numerous (~400,000) |
| Skin texture | Loose, longitudinal folds or flaps | Rugose, coarse papillae, 2–3 large over eyes |
| Color (live) | Reddish purple dorsally, pale ventrally | Orange to red–purple |
| False eye spots (ocelli) | Absent | Absent (some dark spots may appear) |
| Habitat depth range | 5–25 m (migrates deeper in breeding season or warmer months) | 0–30 m |
| Geographic distribution | Chilean and Argentinean Patagonia | Northern Peru to Northern Chile |
| Level | Institution | Main Functions (Artisanal Fisheries Focus) |
|---|---|---|
| 1 | Ministry of Economy, Development and Tourism | Defines national strategies for sustainable resource use, issues decrees, allocates budgets, and oversees institutional structure. |
| 2 | SUBPESCA (Undersecretariat for Fisheries and Aquaculture) | Issues special fishing permits, sets regulations, manages the Artisanal Fishers Register and AMERBs, and defines fishing quotas and authorized species. |
| 3 | SERNAPESCA (National Fisheries and Aquaculture Service) | Maintains the Artisanal Fishers Register, oversees sanitary and traceability programs, controls landings, and ensures food safety for export. |
| 4 | IFOP (Fisheries Development Institute) | Provides scientific data and technical assessments and supports monitoring programs and stock assessments. |
| INDESPA (Instituto de Desarrollo de la Pesca Artesanal y Acuicultura a Pequeña Escala) | Supports artisanal fisheries via funding, training, infrastructure, organizational strengthening, and policy implementation. | |
| 5 | Advisory Councils (National and Zonal) | Provides technical input on fisheries management plans, TURFs, and policy reforms. Includes artisanal representatives. |
| Scientific and Technical Committees | Advises on methodologies, sanitary standards, and policy development; uses IFOP data. | |
| 6 | National Artisanal Fisheries Register | Official record of artisanal fishers, vessels, and organizations authorized to operate. |
| 7 | Artisanal Fisher Organizations | Holds rights to AMERBs, co-manages resources, represents fishers, and implements local governance structures. |
| 8 | Individual Fishers | Fishers must be registered, operate within legal norms, and can be members of organizations. |
| Fishing Method | Description | Impact | Target Octopus Species | Geographical Area | References |
|---|---|---|---|---|---|
| Hand recollection | Operations are conducted on foot, via snorkeling, or using small boats; intertidal areas (1–30 m depth); traditional technique; and no specialized equipment. | Very selective and low-impact method. | See “Hook method” | See “Hook method” | [4,15,99] |
| Hook method | Harpoon or hook inside rock crevices (1–30 m depth); intertidal areas. | Very selective method; difficult to differentiate laying females from males; and poor quality of raw materials. | O. cyanea O. hubbsorum O. insularis O. maya O. mimus C. nocturnus O. tehuelchus | SW Indian Ocean NE Pacific SE Atlantic Western Central Atlantic SE Pacific SW Pacific SE Atlantic | [11,73,100,101,102,103,104,105,106,107] |
| Hookah diving | Same as the hook method, but with an air supply from a compressor on the fishing vessel (1–40 m depth). | Very selective method; difficult to differentiate laying females from males; and higher catches. | O. mimus O. hubbsorum | Ecuador Peru Chile Mexico | [108,109,110] |
| Pots | Pots of different materials that mimic refuges of octopus in sandy/muddy sea bottom; checked every 2-days (3–20 m depth). | Size selective for adults; avoids by-catch; low incidental mortalities and environmental impact; and possible plastic pollution resulting from poor management. | O. vulgaris O. insularis O. pallidus O. sinensis A. fangsiao O. tetricus O. minor E. dofleini | Mediterranean NE Atlantic Eastern Atlantic South Africa SE Atlantic Tasmania Japan Japan Western Australia Korea Alaska | [4,94,95,103,111,112,113,114,115,116,117,118,119] |
| Trawl nets | Broad-mesh net dragged by cables from fishing vessel; octopus is usually found in multispecies fishing or as by-catch (60–500 m depth depending on boat dimensions). | High impact on seabed and benthic shelf and lope communities. | O. vulgaris E. moschata E. cirrhosa Amphioctopus sp. | NW Africa (Saharan Bank) NE Atlantic NE Atlantic Gulf of Thailand | [120,121,122,123,124,125,126,127] |
| Fyke nets/Baited traps | 2–3 cylindrical-/cone-shaped chambers, placed in shallow coastal waters (8–30 m depth). | Efficient and selective method; can cause occasional by-catch; low environmental impact; possible ghost-fishing when traps are lost. | O. vulgaris O. minor E. dofleini | Eastern Mediterranean (mainly Greece) Canary Islands NW Pacific NW Pacific | [4,95,96,97,98,127,128,129] |
| Barrel longlines | Baited barrels suspended from a long line that attracts and traps octopuses seeking shelter (20–100 m depth). | High selective method; low by-catch; and low environmental impact | E. dofleini O. conispadiceus | Japan | [4,127] |
| Fishing lines | Fishing lines with hook-equipped end that is baited with different lures (10–80 m depth). | E. dofleini O. maya | Japan Mexico (typical “Al garete” fishing lines) | [104,130] | |
| Towed rakes | Rakes dragged over seabed to catch sand-hidden octopuses (10–30 m depth). | High environmental impact with strong seabed disturbance. | E. dofleini | Japan | [127] |
| No. | Organoleptic Criterion | Description | Grade 1 (First Quality) | Grade 2 (Second Quality) | Grade 3 (Third/Low Quality) |
|---|---|---|---|---|---|
| 1 | External appearance | Shape, physical damage, and integrity | Whole octopus, no cuts or damage, and vivid and uniform color | Minor damage to arms, slightly dull | Deep cuts, missing parts, and dull colour or black spots |
| 2 | Flesh texture (raw) | Firmness, consistency to the touch | Firm to the touch, resilient | Slightly soft but maintains shape | Flaccid, falls apart when touched |
| 3 | Smell | Freshness, marine aroma | Clean marine/seaweedy smell, no strange odors | Slight smell of decomposition or ammonia | Strong, unpleasant or rotten smell |
| 4 | Mantle and arm color | Uniformity, color intensity | Bright, uniform red | Pale red or some whitish areas | Intense discoloration, black or greenish spots |
| 5 | Mucus presence | Surface cleanliness | Low presence of transparent, watery mucus | Slight presence of mucus | Abundant presence of opalescent viscous mucus or sticky surface |
| 6 | Mantle turgor | Elasticity to touch | Turgid, elastic, and bounces back when pressed | Slight dent that doesn not fully recover, low elasticity | Deforms easily, wrinkled skin |
| 7 | Eyes and suckers | Clarity, firmness | Clear and translucent eyes, black pupil; firm and well-attached suckers | Slightly opaque eye, dark red/bloody pupil; soft suckers | Sunken eyes, opaque pupil; disintegrated or missing suckers |
| 8 | Flavor (if cooked) | Taste test (optional) | Sweet, ocean-like taste, and firm texture | Mild taste, slightly soft texture | Bitter taste, disintegrated texture |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truant, A.; Giacometti, F.; Hernández, J.; Espinoza, V.; Farías, A.; Uriarte, I.; Godoy, C.; Miotti Scapin, R.; Alberghini, L.; Catellani, P.; et al. Overview of Patagonian Red Octopus (Enteroctopus megalocyathus) Fisheries in Chilean Regions and Their Food Safety Aspects. Animals 2025, 15, 1464. https://doi.org/10.3390/ani15101464
Truant A, Giacometti F, Hernández J, Espinoza V, Farías A, Uriarte I, Godoy C, Miotti Scapin R, Alberghini L, Catellani P, et al. Overview of Patagonian Red Octopus (Enteroctopus megalocyathus) Fisheries in Chilean Regions and Their Food Safety Aspects. Animals. 2025; 15(10):1464. https://doi.org/10.3390/ani15101464
Chicago/Turabian StyleTruant, Alessandro, Federica Giacometti, Jorge Hernández, Viviana Espinoza, Ana Farías, Iker Uriarte, Cecilia Godoy, Riccardo Miotti Scapin, Leonardo Alberghini, Paolo Catellani, and et al. 2025. "Overview of Patagonian Red Octopus (Enteroctopus megalocyathus) Fisheries in Chilean Regions and Their Food Safety Aspects" Animals 15, no. 10: 1464. https://doi.org/10.3390/ani15101464
APA StyleTruant, A., Giacometti, F., Hernández, J., Espinoza, V., Farías, A., Uriarte, I., Godoy, C., Miotti Scapin, R., Alberghini, L., Catellani, P., & Giaccone, V. (2025). Overview of Patagonian Red Octopus (Enteroctopus megalocyathus) Fisheries in Chilean Regions and Their Food Safety Aspects. Animals, 15(10), 1464. https://doi.org/10.3390/ani15101464





