7,8-DHF Modulates Aggressive Behavior in Sebastes schlegelii: Phenotype-Dependent Responses in Aggression-Dimorphic Individuals
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Husbandry
2.2. Experimental Design
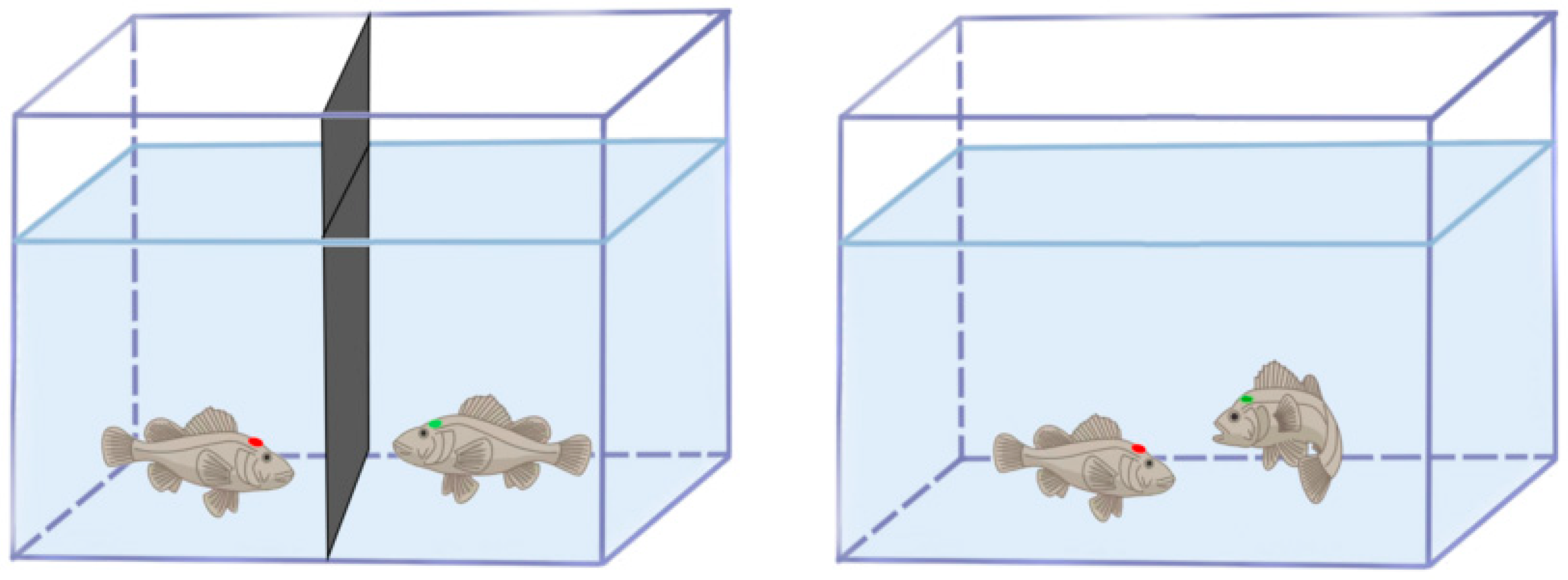
2.2.1. Experimental Fish Tagging
2.2.2. Experimental Setup and Distinguishing Aggressive Phenotypes
2.2.3. Experimental Fish Dosing
2.3. Behavioral Analysis
2.4. Statistical Analysis
3. Results
3.1. Aggressive Behavior Analysis in S. schlegelii
3.2. Locomotor Parameters of Aggression-Dimorphic Individuals
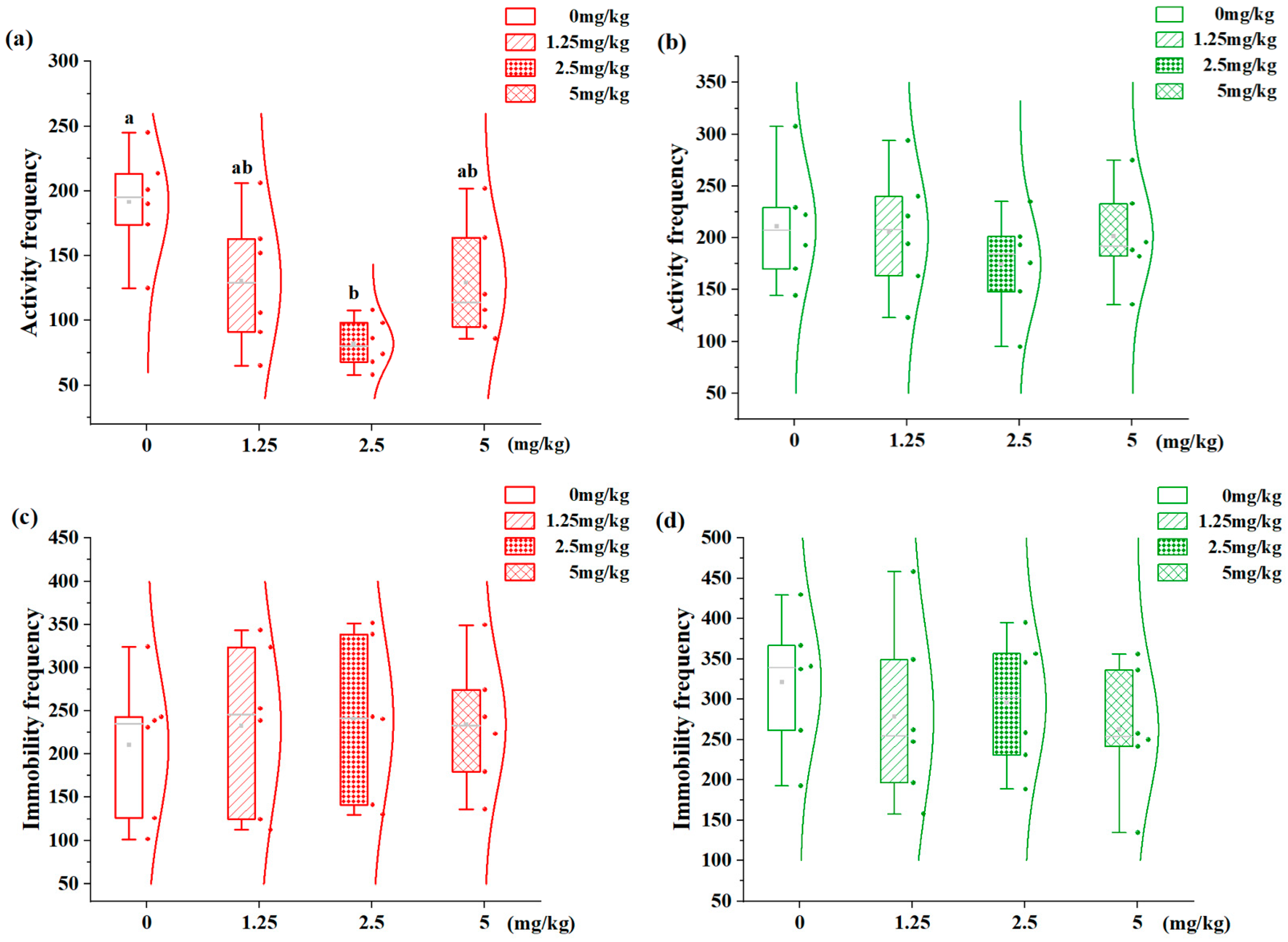
3.3. Effects of 7,8-DHF on Locomotor States in Aggression-Dimorphic Individuals
3.4. Effects of 7,8-DHF on Spatial Distribution in Aggression-Dimorphic Individuals
3.5. Effects of 7,8-DHF on Behavioral States in Aggression-Dimorphic Individuals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turnbull, J.F.; Adams, C.E.; Richards, R.H.; Robertson, D.A. Attack site and resultant damage during aggressive encounters in Atlantic salmon (Salmo salar L.) parr. Aquaculture 1998, 159, 345–353. [Google Scholar] [CrossRef]
- Carvalho, T.B.; Souza, E.C.M.; Pinheiro-da-Silva, J.; Villacorta-Correa, M.A. Effect of body size heterogeneity on the aggressive behavior of larvae of matrinxã, Brycon amazonicus (Characiformes, Bryconidae). Acta Amaz. 2018, 48, 304–310. [Google Scholar] [CrossRef]
- Cavallino, L.; Rincon, L.; Scaia, M.F. Social behaviors as welfare indicators in teleost fish. Front. Vet. Sci. 2023, 10, 1050510. [Google Scholar] [CrossRef]
- Zabegalov, K.N.; Kolesnikova, T.O.; Khatsko, S.L.; Volgin, A.D.; Yakovlev, O.A.; Amstislavskaya, T.G.; Friend, A.J.; Bao, W.; Alekseeva, P.A.; Lakstygal, A.M.; et al. Understanding zebrafish aggressive behavior. Behav. Process. 2019, 158, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Hellinger, J.; Jägers, P.; Spoida, K.; Weiss, L.C.; Mark, M.D.; Herlitze, S. Analysis of the territorial aggressive behavior of the bioluminescent flashlight fish Photoblepharon steinitzi in the red sea. Front. Mar. Sci. 2020, 7, 78. [Google Scholar] [CrossRef]
- Rosenau, M.L.; Mcphail, J. Inherited differences in agonistic behavior between two populations of coho salmon. Trans. Am. Fish. Soc. 1987, 116, 646–654. [Google Scholar] [CrossRef]
- Mutschler, C.; Telerman, S.B. Glial plasticity in the zebrafish central nervous system. Trends Cell Biol. 2024, 34, 531–534. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Meng, L.; Liu, B.; Ji, R.; Jiang, X.; Yan, X.; Xin, Y. Targeting the BDNF/TrkB pathway for the treatment of tumors. Oncol. Lett. 2019, 17, 2031–2039. [Google Scholar] [CrossRef]
- Niu, C.; Yue, X.; An, J.J.; Xu, H.; Xu, B. Genetic dissection of BDNF and TrkB expression in glial cells. Biomolecules 2023, 7, 549007. [Google Scholar] [CrossRef]
- Choo, M.; Miyazaki, T.; Yamazaki, M.; Kawamura, M.; Nakazawa, T.; Zhang, J.; Tanimura, A.; Uesaka, N.; Watanabe, M.; Sakimura, K.; et al. Retrograde BDNF to TrkB signaling promotes synapse elimination in the developing cerebellum. Nat. Commun. 2017, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Ito, W.; Chehab, M.; Thakur, S.; Li, J.; Morozov, A. BDNF-restricted knockout mice as an animal model for aggression. Genes Brain Behav. 2011, 10, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Li, Y.W.; Hu, W.; Chen, Q.L.; Shen, Y.J. Mechanisms involved in tributyltin-enhanced aggressive behaviors and fear responses in male zebrafish. Aquat. Toxicol. 2020, 220, 105408. [Google Scholar] [CrossRef]
- Wurzelmann, M.; Romeika, J.; Sun, D. Therapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury. Neural Regen. Res. 2017, 12, 7–12. [Google Scholar] [PubMed]
- Agrawal, R.; Noble, E.; Tyagi, E.; Zhuang, Y.; Ying, Z.; Gomez-Pinilla, F. Flavonoid derivative 7,8-DHF attenuates TBI pathology via TrkB activation. Biochim. Et Biophys. Acta 2015, 1852, 862–872. [Google Scholar] [CrossRef]
- Dhaliwal, N.; Dhaliwal, J.; Chopra, K. 7, 8-Dihydroxyflavone ameliorates cholinergic dysfunction, inflammation, oxidative stress, and apoptosis in a rat model of Vascular dementia. Neurochem. Res. 2024, 49, 1137–1149. [Google Scholar] [CrossRef]
- Nie, S.; Ma, K.; Sun, M.; Lee, M.; Tan, Y.; Chen, G.; Zhang, Z.; Zhang, Z.; Cao, X. 7,8-Dihydroxyflavone protects nigrostriatal dopaminergic neurons from rotenone-induced neurotoxicity in rodents. Park. Dis. 2019, 3, 9193534. [Google Scholar] [CrossRef]
- Yang, T.; Bayless, D.W.; Wei, Y.; Landayan, D.; Marcelo, I.M.; Wang, Y.; DeNardo, L.A.; Luo, L.; Druckmann, S.; Shah, N.M. Hypothalamic neurons that mirror aggression. Cell 2023, 186, 1195–1211.e19. [Google Scholar] [CrossRef]
- Loveland, J.L.; Fernald, R.D. Differential activation of vasotocin neurons in contexts that elicit aggression and courtship. Behav. Brain Res. 2017, 317, 188–203. [Google Scholar] [CrossRef]
- MacLean, A.; Metcalfe, N.B.; Mitchell, D. Alternative competitive strategies in juvenile Atlantic salmon (Salmo salar): Evidence from fin damage. Aquaculture 2000, 184, 291–302. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Zhang, X.; Hu, Y.; Liu, Y.; Ma, Z. Comparing behavioral performance and physiological responses of Sebastes schlegelii with different aggressiveness. Fish Physiol. Biochem. 2022, 48, 1333–1347. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.P.; Lima-Maximino, M.G.; Maximino, C. Acute fluoxetine differently affects aggressive display in zebrafish phenotypes. Aggress. Behav. 2019, 45, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Peeters, D.; Rietdijk, J.; Gerrits, D.; Rijpkema, M.; de Boer, S.F.; Verkes, R.J.; Homberg, J.R. Searching for neural and behavioral parameters that predict anti-aggressive effects of chronic SSRI treatment in rats. Neuropharmacology 2018, 143, 339–348. [Google Scholar] [CrossRef]
- Ariyasiri, K.; Choi, T.I.; Gerlai, R.; Kim, C.H. Acute ethanol induces behavioral changes and alters c-fos expression in specific brain regions, including the mammillary body, in zebrafish. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 109, 110264. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Cho, S.H. Fish meal replacement by chicken by-product meal in diet: Impacts on growth and feed availability of juvenile rockfish (Sebastes schlegelii), and economical analysis. Animals 2025, 15, 80. [Google Scholar] [CrossRef]
- Li, R.; Cho, S.H. Substitution impact of tuna by-product meal for fish meal in the diets of rockfish (Sebastes schlegelii) on growth and feed availability. Animals 2023, 13, 3586. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, X.; Wang, Y.; Zhang, X. Effects of environmental enrichment on growth performance, aggressive behavior and stress-induced changes in cortisol release and neurogenesis of black rockfish Sebastes schlegelii. Aquaculture 2020, 528, 735483. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Z.; Guo, H.; Qin, J.; Zhang, X. Changes in aggressive behavior, cortisol and brain monoamines during the formation of social hierarchy in black rockfish (Sebastes schlegelii). Animals 2020, 10, 2357. [Google Scholar] [CrossRef]
- Jia, Y.; Gao, Y.; Wan, J.; Gao, Y.; Li, J.; Guan, C. Altered physiological response and gill histology in black rockfish, Sebastes schlegelii, during progressive hypoxia and reoxygenation. Fish Physiol. Biochem. 2021, 47, 1133–1147. [Google Scholar] [CrossRef]
- GB/T 35892-2018; Laboratory Animal—Guideline for Ethical Review of Animal Welfare. The National Standards of the People’s Republic of China: Beijing, China, 2018.
- GB/T 35823-2018; Laboratory Animals—General Requirements for Animal Experiment. The National Standards of the People’s Republic of China: Beijing, China, 2018.
- Cooke, S.J.; Hinch, S.G.; Wikelski, M.; Andrews, R.D.; Kuchel, L.J.; Wolcott, T.G.; Butler, P.J. Biotelemetry: A mechanistic approach to ecology. Trends Ecol. Evol. 2004, 19, 334–343. [Google Scholar] [CrossRef]
- Benhaïm, D.; Ferrari, S.; Chatain, B.; Bégout, M.L. The shy prefer familiar congeners. Behav. Process. 2016, 126, 113–120. [Google Scholar] [CrossRef]
- Grunst, A.S.; Grunst, M.L. Animal personality in multiple stressor environments: The evolutionary ecology of among-individual differences in responses to stressor suites. Proc. R. Soc. B Biol. Sci. 2024, 291, 20241620. [Google Scholar]
- Geange, S.W. An evaluation of prior residency and habitat effects on the persistence of settling reef fishes. Ph.D. Thesis, Victoria University of Wellington, Wellington, Australia, 2010. [Google Scholar]
- Scarsella, G.E.; Duque, K.S.; Wong, S.C.; Sivaraman, B.; Earley, R.L. Hormonal responses to noncontact aggression in convict cichlid fish. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2016, 325, 219–230. [Google Scholar] [CrossRef]
- Hubená, P.; Horký, P.; Slavík, O. Test-dependent expression of behavioral syndromes: A study of aggressiveness, activity, and stress of chub. Aggress. Behav. 2020, 46, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Axling, J.; Vossen, L.E.; Peterson, E.; Winberg, S. Boldness, activity, and aggression: Insights from a large-scale study in Baltic salmon (Salmo salar L.). PLoS ONE 2023, 18, e0287836. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Xiao, Y.; Yin, H.H.; Chen, A.I.; Soong, T.W.; Je, H.S. Postnatal TrkB ablation in corticolimbic interneurons induces social dominance in male mice. Proc. Natl. Acad. Sci. USA. 2018, 115, e9909–e9915. [Google Scholar] [CrossRef]
- Adachi, M.; Autry, A.E.; Mahgoub, M.; Suzuki, K.; Monteggia, L.M. TrkB signaling in dorsal raphe nucleus is essential for antidepressant efficacy and normal aggression behavior. Neuropsychopharmacology 2017, 42, 886–894. [Google Scholar] [CrossRef]
- Chen, L.X.; Qi, Z.; Shao, Z.J.; Li, S.S.; Qi, Y.L.; Gao, K.; Liu, S.X.; Li, Z.; Sun, Y.S.; Li, P.Y. Study on antidepressant activity of pseudo-ginsenoside HQ on depression-Like behavior in mice. Molecules 2019, 24, 870. [Google Scholar] [CrossRef]
- Cortese, G.P.; Barrientos, R.M.; Maier, S.F.; Patterson, S.L. Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLCgamma1, and ERK in hippocampal synaptoneurosomes. J. Neurosci. 2011, 31, 4274–4279. [Google Scholar] [CrossRef]
- Guarino, A.; Bettegazzi, B.; Aziz, N.; Barbieri, M.; Bochicchio, D.; Crippa, L.; Marino, P.; Sguizzato, M.; Soukupova, M.; Zucchini, S.; et al. Low-dose 7,8-Dihydroxyflavone administration after status epilepticus prevents epilepsy development. Neurotherapeutics 2022, 19, 1951–1965. [Google Scholar] [CrossRef]
- Andreska, T.; Lüningschrör, P.; Sendtner, M. Regulation of TrkB cell surface expression-a mechanism for modulation of neuronal responsiveness to brain-derived neurotrophic factor. Cell Tissue Res. 2020, 382, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Hoy, K.C.; Strain, M.M.; Turtle, J.D.; Lee, K.H.; Huie, J.R.; Hartman, J.J.; Tarbet, M.M.; Harlow, M.L.; Magnuson, D.S.K.; Grau, J.W. Evidence that the central nervous system can induce a modification at the neuromuscular junction that contributes to the maintenance of a behavioral response. J. Neurosci. 2020, 40, 9186–9209. [Google Scholar] [CrossRef] [PubMed]
- Schlinger, B.A.; Paul, K.; Monks, D.A. Muscle, a conduit to brain for hormonal control of behavior. Horm. Behav. 2018, 105, 58–65. [Google Scholar] [CrossRef] [PubMed]
- García-Díaz Barriga, G.; Giralt, A.; Anglada-Huguet, M.; Gaja-Capdevila, N.; Orlandi, J.G.; Soriano, J.; Canals, J.M.; Alberch, J. 7,8-dihydroxyflavone ameliorates cognitive and motor deficits in a Huntington’s disease mouse model through specific activation of the PLCγ1 pathway. Hum. Mol. Genet. 2017, 26, 3144–3160. [Google Scholar] [CrossRef]
- Dai, B.; Zheng, B.; Dai, X.; Cui, X.; Yin, L.; Cai, J.; Zhuo, Y.; Tritsch, N.X.; Zweifel, L.S.; Li, Y.; et al. Experience-dependent dopamine modulation of male aggression. Nature 2025, 639, 430–437. [Google Scholar] [CrossRef]
- Kim, H.I.; Lee, S.; Lim, J.; Chung, S.; Koo, T.S.; Ji, Y.G.; Suh, Y.G.; Son, W.S.; Kim, S.H.; Choi, H.J. ERRγ ligand HPB2 upregulates BDNF-TrkB and enhances dopaminergic neuronal phenotype. Pharmacol. Res. 2021, 165, 105423. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, G. 7,8-Dihydroxyflavone and neuropsychiatric disorders: A translational perspective from the mechanism to drug development. Curr. Neuropharmacol. 2022, 20, 1479–1497. [Google Scholar] [CrossRef]
- Devi, L.; Ohno, M. 7,8-Dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2012, 37, 434–444. [Google Scholar] [CrossRef]
- Minichiello, L. TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009, 10, 850–860. [Google Scholar] [CrossRef]
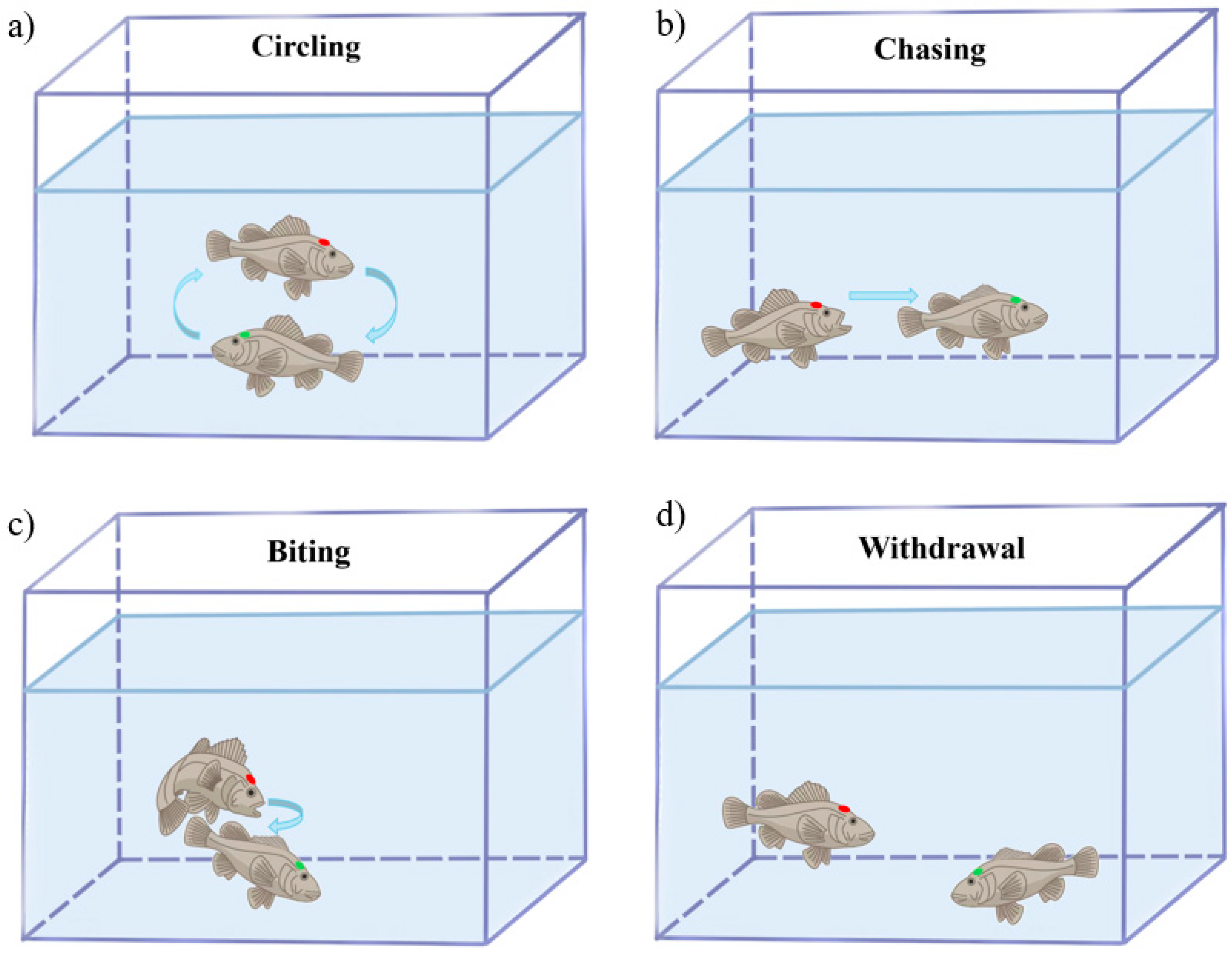


| Ethogram of Aggressive Behavior | Time (s) | Proportion of Phenotypes % | Total Proportion % | |
|---|---|---|---|---|
| Aggressive phenotype | Biting | 11.83 ± 1.72 | 11.71 | 1.98 |
| Chasing | 89.17 ± 6.23 | 88.29 | 14.86 | |
| Total | 101 | 100 | 16.84 | |
| Non-aggressive phenotype | Circling | 83.50 ± 3.43 | 16.73 | 13.91 |
| Withdrawal | 415.50 ± 5.53 | 83.27 | 69.25 | |
| Total | 499 | 100 | 83.16 |
| Movement Distance (cm) | Locomotor Acceleration (cm/s2) | Angular Velocity (°/s) | First Movement Latency (s) | Activity Frequency | Immobility Frequency | |
|---|---|---|---|---|---|---|
| H-agg | 10.91 ± 1.04 | 3607.54 ± 156.35 ** | 48.27 ± 5.62 | 1.19 ± 0.09 ** | 191.38 ± 16.50 | 210.61 ± 33.77 * |
| L-agg | 9.15 ± 0.89 | 2367.91 ± 169.29 | 36.15 ± 2.62 | 1.95 ± 0.16 | 210.91 ± 23.29 | 321.39 ± 33.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Ma, X.; Xiao, Y.; Zhang, T.; Ma, C.; Ma, Z. 7,8-DHF Modulates Aggressive Behavior in Sebastes schlegelii: Phenotype-Dependent Responses in Aggression-Dimorphic Individuals. Animals 2025, 15, 1463. https://doi.org/10.3390/ani15101463
Xu S, Ma X, Xiao Y, Zhang T, Ma C, Ma Z. 7,8-DHF Modulates Aggressive Behavior in Sebastes schlegelii: Phenotype-Dependent Responses in Aggression-Dimorphic Individuals. Animals. 2025; 15(10):1463. https://doi.org/10.3390/ani15101463
Chicago/Turabian StyleXu, Shufei, Xinna Ma, Yang Xiao, Tao Zhang, Chao Ma, and Zhen Ma. 2025. "7,8-DHF Modulates Aggressive Behavior in Sebastes schlegelii: Phenotype-Dependent Responses in Aggression-Dimorphic Individuals" Animals 15, no. 10: 1463. https://doi.org/10.3390/ani15101463
APA StyleXu, S., Ma, X., Xiao, Y., Zhang, T., Ma, C., & Ma, Z. (2025). 7,8-DHF Modulates Aggressive Behavior in Sebastes schlegelii: Phenotype-Dependent Responses in Aggression-Dimorphic Individuals. Animals, 15(10), 1463. https://doi.org/10.3390/ani15101463





