Decoding the Function of FGFBP1 in Sheep Adipocyte Proliferation and Differentiation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Differentiation
2.3. Cell Transfection
2.4. Real-Time Quantitative PCR
2.5. Cell Proliferation Analysis
2.6. Cell Cycle Analysis
2.7. Oil Red O Staining
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
3.1. Determination of FGFBP1 Overexpression and Knockdown Efficiency
3.2. Effects of FGFBP1 on the Proliferation of Sheep Preadipocytes
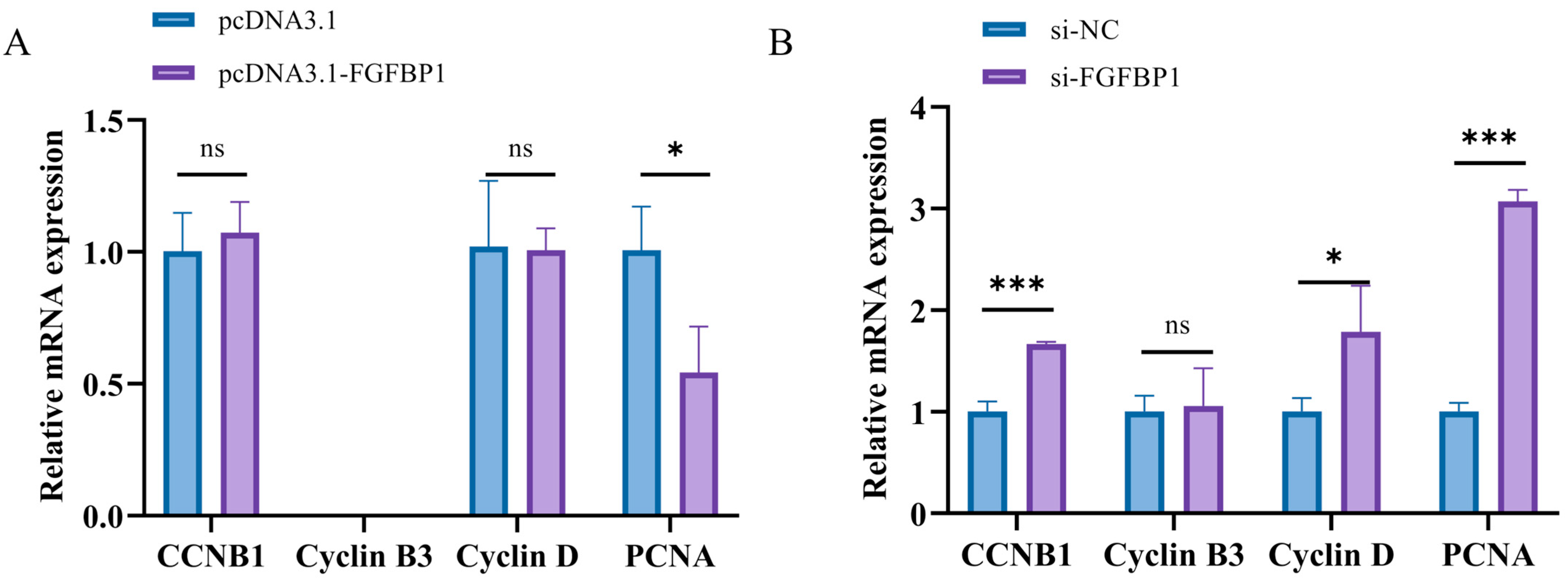
3.2.1. Determination of Proliferation Marker Genes After FGFBP1 Overexpression and Knockdown
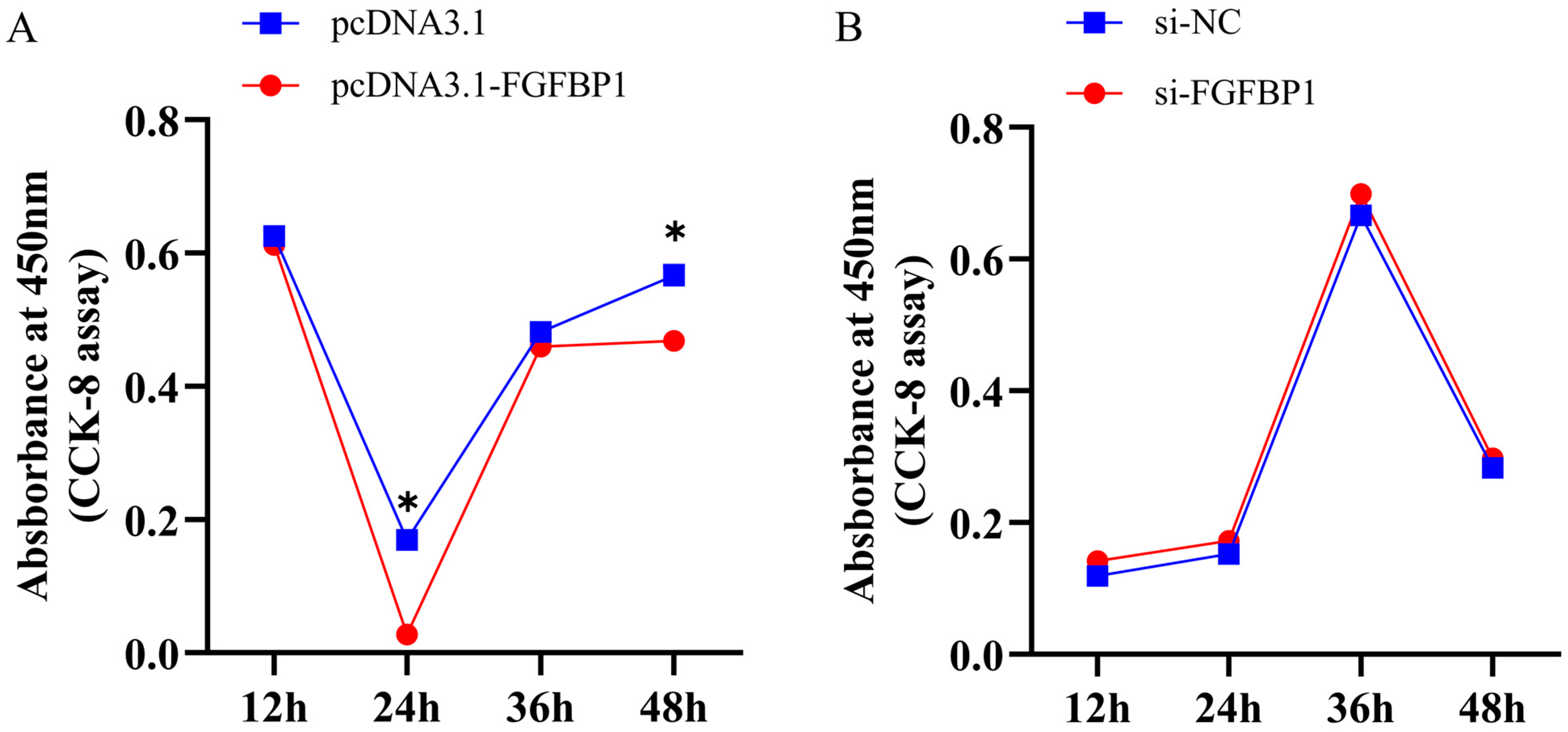
3.2.2. CCK-8 Assay Results After FGFBP1 Overexpression and Knockdown
3.2.3. EdU Assay Results After FGFBP1 Overexpression and Knockdown
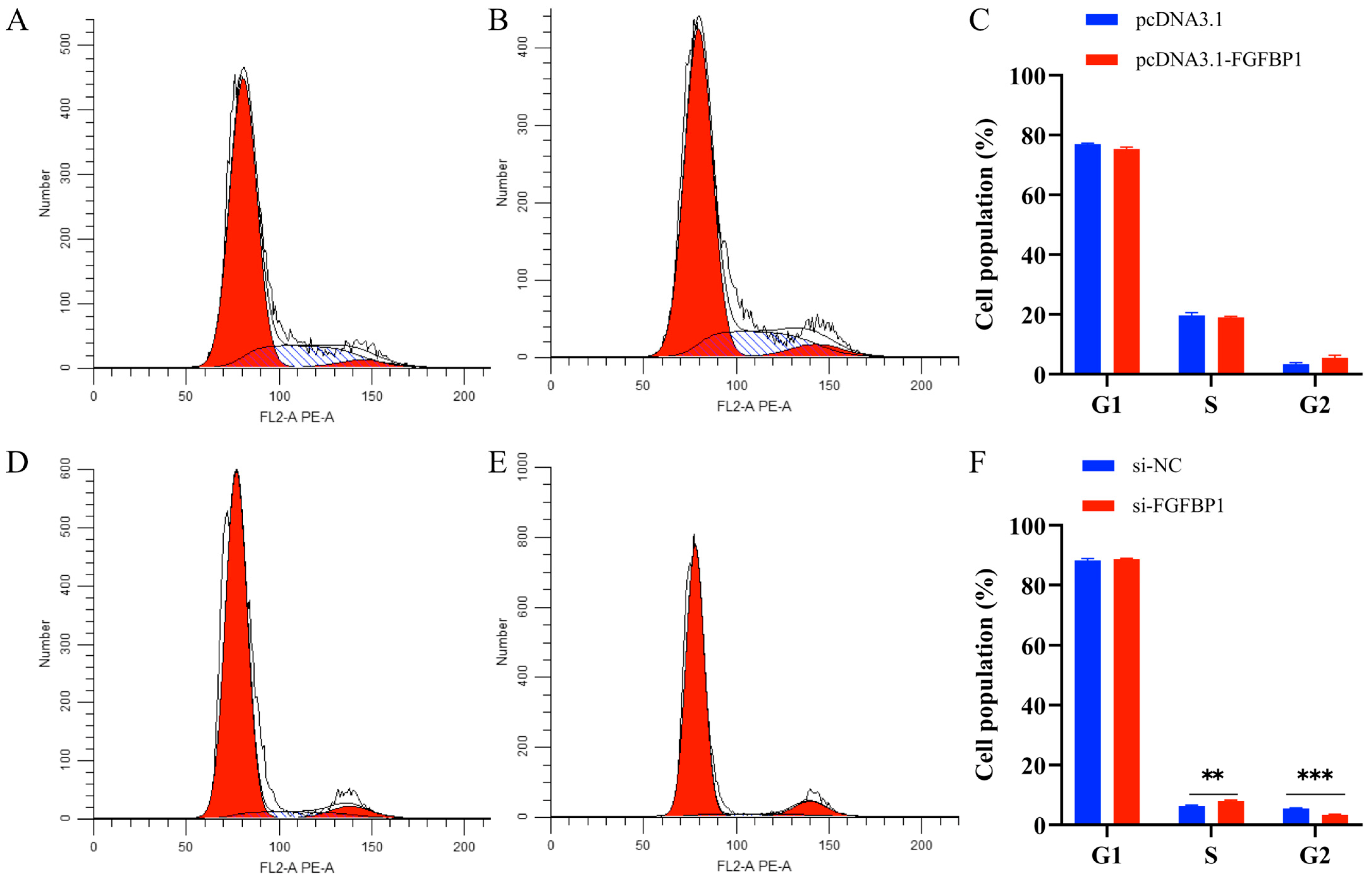
3.3. Effects of FGFBP1 Overexpression and Knockdown on the Cell Cycle
3.4. Effects of FGFBP1 on the Differentiation of Sheep Preadipocytes
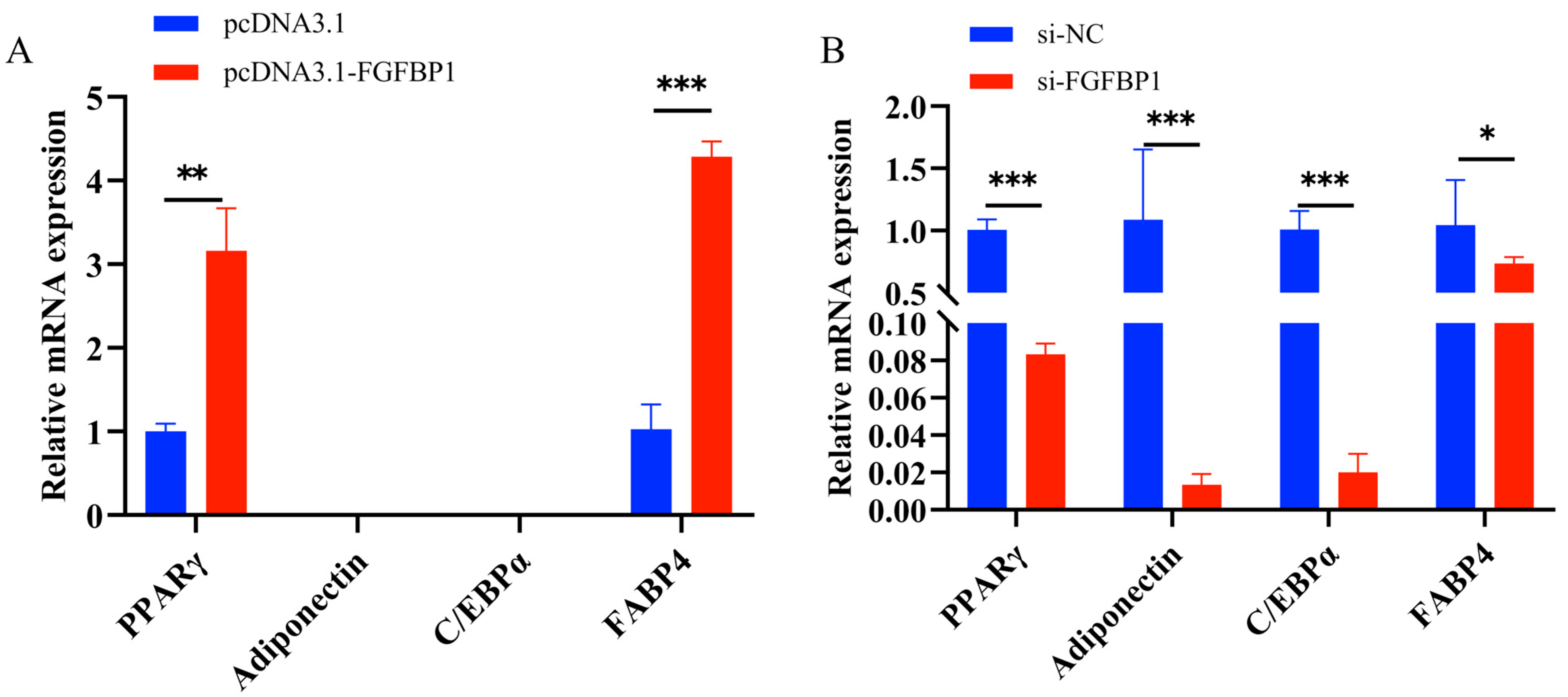
3.4.1. Effects of FGFBP1 Overexpression and Knockdown on Cell Differentiation
3.4.2. Oil Red O Staining Analysis After FGFBP1 Overexpression and Knockdown
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Z.; Wang, W.; Zhang, D.; Zhang, Y.; Zhao, Y.; Li, X.; Zhao, L.; Lin, C.; Wang, J.; Zhou, B.; et al. Ovine Rap1gap and Rbat Gene Polymorphisms and Their Association with Tail Fat Deposition in Hu Sheep. Front. Vet. Sci. 2022, 9, 974513. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Lu, Y.; Li, M.; Gao, Z.; Li, D.; Gao, Y.; Deng, W.; Wang, B. Whole-Genome Resequencing in Sheep: Applications in Breeding, Evolution, and Conservation. Genes 2025, 16, 363. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, J.; Han, J.; Yang, B. Association between Bmp2 Functional Polymorphisms and Sheep Tail Type. Animals 2020, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.H.; Nejati-Javaremi, A.; Moradi-Shahrbabak, M.; Dodds, K.G.; McEwan, J.C. Genomic Scan of Selective Sweeps in Thin and Fat Tail Sheep Breeds for Identifying of Candidate Regions Associated with Fat Deposition. BMC Genet. 2012, 13, 10. [Google Scholar] [CrossRef]
- Mansourizadeh, H.; Bakhtiarizadeh, M.R.; de Almeida Regitano, L.C.; Bruscadin, J.J. Fat-Tail Allele-Specific Expression Genes May Affect Fat Deposition in Tail of Sheep. PLoS ONE 2024, 19, e0316046. [Google Scholar] [CrossRef]
- Li, B.; Qiao, L.; An, L.; Wang, W.; Liu, J.; Ren, Y.; Pan, Y.; Jing, J.; Liu, W. Transcriptome Analysis of Adipose Tissues from Two Fat-Tailed Sheep Breeds Reveals Key Genes Involved in Fat Deposition. BMC Genom. 2018, 19, 338. [Google Scholar] [CrossRef]
- Amane, A.; Belay, G.; Kebede, A.; Dessie, T.; Worku, S.; Hanotte, O.; Vila, E. Analysis of Tail Morphology and Osteology in Ethiopian Indigenous Sheep. J. Archaeol. Sci. Rep. 2023, 47, 103776. [Google Scholar] [CrossRef]
- Ding, W.; Lu, Y.; Xu, B.; Chen, P.; Li, A.; Jian, F.; Yu, G.; Huang, S. Meat of Sheep: Insights into Mutton Evaluation, Nutritive Value, Influential Factors, and Interventions. Agriculture 2024, 14, 1060. [Google Scholar] [CrossRef]
- Chu, Y.; Gómez Rosso, L.; Huang, P.; Wang, Z.; Xu, Y.; Yao, X.; Bao, M.; Yan, J.; Song, H.; Wang, G. Liver Med23 Ablation Improves Glucose and Lipid Metabolism Through Modulating FOXO1 Activity. Cell Res. 2014, 24, 1250–1265. [Google Scholar] [CrossRef]
- Bandyopadhyay, G.K.; Lu, M.; Avolio, E.; Siddiqui, J.A.; Gayen, J.R.; Wollam, J.; Vu, C.U.; Chi, N.W.; O’Connor, D.T.; Mahata, S.K. Pancreastatin-Dependent Inflammatory Signaling Mediates Obesity-Induced Insulin Resistance. Diabetes 2015, 64, 104–116. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, M.; Chen, W.; Talbot, R.; Maddox, J.F.; Faraut, T.; Wu, C.; Muzny, D.M.; Li, Y.; Zhang, W.; et al. The Sheep Genome Illuminates Biology of the Rumen and Lipid Metabolism. Science 2014, 344, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Hussain, T.; Dai, C.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Huang, J.; Ji, F.; et al. Effects of Dietary Energy Level on Growth Performance, Blood Parameters and Meat Quality in Fattening Male Hu Lambs. J. Anim. Physiol. Anim. Nutr. 2020, 104, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; DelCurto-Wyffels, H.; Thomson, J.; Boles, J. Fat Deposition and Fat Effects on Meat Quality—A Review. Animals 2022, 12, 1550. [Google Scholar] [CrossRef]
- Schmidt, M.O.; Garman, K.A.; Lee, Y.G.; Zuo, C.; Beck, P.J.; Tan, M.; Aguilar-Pimentel, J.A.; Ollert, M.; Schmidt-Weber, C.; Fuchs, H.; et al. The Role of Fibroblast Growth Factor-Binding Protein 1 in Skin Carcinogenesis and Inflammation. J. Investig. Dermatol. 2018, 138, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Cottarelli, A.; Corada, M.; Beznoussenko, G.V.; Mironov, A.A.; Globisch, M.A.; Biswas, S.; Huang, H.; Dimberg, A.; Magnusson, P.U.; Agalliu, D.; et al. Fgfbp1 Promotes Blood-Brain Barrier Development by Regulating Collagen IV Deposition and Maintaining Wnt/β-Catenin Signaling. Development 2020, 147, dev185140. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Liebner, S. Developmental Regulation Of Barrier- and Non-Barrier Blood Vessels in the CNS. J. Intern. Med. 2022, 292, 31–46. [Google Scholar] [CrossRef]
- Taetzsch, T.; Brayman, V.L.; Valdez, G. FGF Binding Proteins (FGFBPs): Modulators of FGF Signaling in the Developing, Adult, and Stressed Nervous System. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2983–2991. [Google Scholar] [CrossRef]
- Hindson, J. Fgfbp1(+) Cells: Upper Crypt Intestinal Epithelial Regeneration. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 534. [Google Scholar] [CrossRef]
- Wang, F.; Luo, M.; Qu, H.; Cheng, Y. BAP1 Promotes Viability and Migration of ECA109 Cells Through KLF5/CyclinD1/FGF-BP1. FEBS Open Bio 2021, 11, 1497–1503. [Google Scholar] [CrossRef]
- Qiu, T.; Hou, L.; Zhao, L.; Wang, X.; Zhou, Z.; Yang, C.; Zhang, H.; Jiang, D.; Jiao, B.; Chen, C. SGCE Promotes Breast Cancer Stemness by Promoting the Transcription of FGF-BP1 by Sp1. J. Biol. Chem. 2023, 299, 105351. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Chen, X.; Yuan, S.; Xu, T.; Zhao, W.; Li, M.; Wang, F.; Hoffman, R.M.; Jia, L. Evodiamine Inhibits ESCC by Inducing M-Phase Cell-Cycle Arrest via CUL4A/p53/p21 Axis and Activating Noxa-Dependent Intrinsic and DR4-Dependent Extrinsic Apoptosis. Phytomedicine 2023, 108, 154493. [Google Scholar] [CrossRef] [PubMed]
- Varier, K.M.; Dan, G.; Li, X.; Liu, W.; Jiang, F.; Linghu, K.G.; Li, Y.; Ben-David, Y.; Zhang, N.; Xiao, C.; et al. B4 Suppresses Lymphoma Progression by Inhibiting Fibroblast Growth Factor Binding Protein 1 Through Intrinsic Apoptosis. Front. Pharmacol. 2024, 15, 1408389. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Fan, S.; Huang, M.; Pan, J.; Li, Y.; Miao, Q.; Lyu, W.; Li, X.; Deng, L.; Qiu, S.; et al. Targeting FAPα-Expressing Hepatic Stellate Cells Overcomes Resistance to Antiangiogenics in Colorectal Cancer Liver Metastasis Models. J. Clin. Investig. 2022, 132, e157399. [Google Scholar] [CrossRef] [PubMed]
- Ruan, R.; Li, L.; Li, X.; Huang, C.; Zhang, Z.; Zhong, H.; Zeng, S.; Shi, Q.; Xia, Y.; Zeng, Q.; et al. Unleashing the Potential of Combining FGFR Inhibitor and Immune Checkpoint Blockade for FGF/FGFR Signaling in Tumor Microenvironment. Mol. Cancer 2023, 22, 60. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Munyurangabo, G.; Zhou, Y.; He, S.; Zhang, P.; Yu, X.; Kong, G. Deficiency of the Histone H3K36 Methyltransferase SETD2 Inhibits the Proliferation and Migration of Hepatocellular Carcinoma Cells. J. Cancer 2024, 15, 6479–6489. [Google Scholar] [CrossRef]
- Lin, H.; Hassan Safdar, M.; Washburn, S.; Akhand, S.S.; Dickerhoff, J.; Ayers, M.; Monteiro, M.; Solorio, L.; Yang, D.; Wendt, M.K. Fibroblast Growth Receptor 1 is Regulated by G-Quadruplex in Metastatic Breast Cancer. Commun. Biol. 2024, 7, 963. [Google Scholar] [CrossRef]
- Huang, W.; Chen, Z.; Shang, X.; Tian, D.; Wang, D.; Wu, K.; Fan, D.; Xia, L. Sox12, a Direct Target of FoxQ1, Promotes Hepatocellular Carcinoma Metastasis Through Up-Regulating Twist1 and FGFBP1. Hepatology 2015, 61, 1920–1933. [Google Scholar] [CrossRef]
- Baba, H.; Kanda, M.; Sawaki, K.; Nakamura, S.; Ueda, S.; Shimizu, D.; Koike, M.; Kodera, Y.; Fujii, T. SLC7A9 as a Potential Biomarker for Lymph Node Metastasis of Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2022, 29, 2699–2709. [Google Scholar] [CrossRef]
- Edirisinghe, O.; Ternier, G.; Alraawi, Z.; Suresh Kumar, T.K. Decoding FGF/FGFR Signaling: Insights into Biological Functions and Disease Relevance. Biomolecules 2024, 14, 1622. [Google Scholar] [CrossRef]
- Edirisinghe, O.; Kumar, T.K.S. Pathology and Therapeutic Significance of Fibroblast Growth Factors. Targets 2025, 3, 5. [Google Scholar] [CrossRef]
- Sun, L.; Ji, S.; Xie, X.; Si, L.; Liu, S.; Lin, Y.; Wang, Y.; Song, Z.; Fang, N.; An, Y.; et al. Deciphering the Interaction between Twist1 and PPARγ during Adipocyte Differentiation. Cell Death Dis. 2023, 14, 764. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Park, S.; Choi, B.; Choi, W.; Lee, H.; Lee, J.M.; Lee, S.T.; Yoo, K.H.; Han, D.; Bang, G.; et al. Cultured Meat with Enriched Organoleptic Properties by Regulating Cell Differentiation. Nat. Commun. 2024, 15, 77. [Google Scholar] [CrossRef]
- Horwitz, A.; Birk, R. Adipose Tissue Hyperplasia and Hypertrophy in Common and Syndromic Obesity—The Case of BBS Obesity. Nutrients 2023, 15, 3445. [Google Scholar] [CrossRef] [PubMed]
- Pierezan, M.D.; Verruck, S. Cattle, Swine, Poultry and Fish Cells for Meat Production: Addressing Challenges of Co-Culture and the Innovative Approaches for Muscle and Fat Integration. Curr. Opin. Food Sci. 2024, 60, 101230. [Google Scholar] [CrossRef]
- Muir, L.A.; Neeley, C.K.; Meyer, K.A.; Baker, N.A.; Brosius, A.M.; Washabaugh, A.R.; Varban, O.A.; Finks, J.F.; Zamarron, B.F.; Flesher, C.G.; et al. Adipose Tissue Fibrosis, Hypertrophy, and Hyperplasia: Correlations with Diabetes in Human Obesity. Obesity 2016, 24, 597–605. [Google Scholar] [CrossRef]
- Qu, L.; Shen, M.; Guo, J.; Wang, X.; Dou, T.; Hu, Y.; Li, Y.; Ma, M.; Wang, K.; Liu, H. Identification of Potential Genomic Regions and Candidate Genes for Egg Albumen Quality by a Genome-Wide Association Study. Arch. Anim. Breed. 2019, 62, 113–123. [Google Scholar] [CrossRef]
- Struik, D.; Dommerholt, M.B.; Jonker, J.W. Fibroblast Growth Factors in Control of Lipid Metabolism: From Biological Function to Clinical Application. Curr. Opin. Lipidol. 2019, 30, 235–243. [Google Scholar] [CrossRef]
- Nies, V.J.; Sancar, G.; Liu, W.; van Zutphen, T.; Struik, D.; Yu, R.T.; Atkins, A.R.; Evans, R.M.; Jonker, J.W.; Downes, M.R. Fibroblast Growth Factor Signaling in Metabolic Regulation. Front. Endocrinol. 2015, 6, 193. [Google Scholar] [CrossRef]
- Yue, Y.; Cheng, X.; Zhao, S.G.; Liu, Z.; Liu, L.S.; Zhou, R.; Wu, J.P.; Brown, M.A. Effects of Tail Docking on the Expression of Genes Related to Lipid Metabolism in Lanzhou Fat-Tailed Sheep. Genet. Mol. Res. 2016, 15, gmr.15017323. [Google Scholar] [CrossRef]
- Janssen, J. The Causal Role of Ectopic Fat Deposition in the Pathogenesis of Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 13238. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Bai, W.D.; Liu, J.Q.; Zheng, Z.; Guan, H.; Zhou, Q.; Su, L.L.; Xie, S.T.; Wang, Y.C.; Li, J.; et al. Up-Regulation of FGFBP1 Signaling Contributes to miR-146a-Induced Angiogenesis in Human Umbilical Vein Endothelial Cells. Sci. Rep. 2016, 6, 25272. [Google Scholar] [CrossRef] [PubMed]
- Tassi, E.; McDonnell, K.; Gibby, K.A.; Tilan, J.U.; Kim, S.E.; Kodack, D.P.; Schmidt, M.O.; Sharif, G.M.; Wilcox, C.S.; Welch, W.J.; et al. Impact of Fibroblast Growth Factor-Binding Protein-1 Expression on Angiogenesis and Wound Healing. Am. J. Pathol. 2011, 179, 2220–2232. [Google Scholar] [CrossRef] [PubMed]



| Gene | Gene ID | Primer Sequence (5′–3′) |
|---|---|---|
| FGFBP1 | XM_004010004.5 | F: CCTCCTCCTTCTGGCTGTTCTG R: TGCTTGGTTGGCTGGCTCCT |
| CCNB1 | XM_027980034.1 | F: CCCTCCAGAAATCGGTGACT R: AGCTCAACATCAACCTCTCCA |
| Cyclin B3 | XM_012106700.4 | F: GCTTGTCCAACACCGTCACCAT R: ACCTCCACCAACCAGTCCACAA |
| Cyclin D | NM_001127289.1 | F: GGGATTGGGAGGTGCTGGTCTT R: AGGTCTGGGCGTGCTTCTTGA |
| PCNA | XM_004014340.5 | F: GCTCAAGTGGCGTGAACCTACA R: TACGGTCGCAGCGGTAAGTGT |
| PPARγ | NM_001100921.1 | F: TGCCGATTCCAGAAGTGCCTTG R: TCGCCCTCGCCTTTGCTTTG |
| Adiponectin | NM_001308565.1 | F: AACCACTATGACGGCACCACTG R: ATAGAGGAGCACGGAGCCAGAG |
| C/EBPα | NM_001308574.1 | F: ATGAGCAGCCACCTCCAGAG R: GCCAGGAACTCGTCGTTGAAG |
| FABP4 | NM_001114667.1 | F: AGGAAAGTGGCTGGCATGGC R: CTGGTAGCAGTGACACCGTTCA |
| β-actin | NM_001009784.3 | F: GCAGGTCATCACCATCGGCAAT R: CGTGTTGGCGTAGAGGTCCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; He, Z.; Wang, G.; Zhang, S.; Di, T.; Chang, M.; Han, W.; Gao, J.; Li, M.; Wang, Z.; et al. Decoding the Function of FGFBP1 in Sheep Adipocyte Proliferation and Differentiation. Animals 2025, 15, 1456. https://doi.org/10.3390/ani15101456
Tian L, He Z, Wang G, Zhang S, Di T, Chang M, Han W, Gao J, Li M, Wang Z, et al. Decoding the Function of FGFBP1 in Sheep Adipocyte Proliferation and Differentiation. Animals. 2025; 15(10):1456. https://doi.org/10.3390/ani15101456
Chicago/Turabian StyleTian, Liming, Zhaohua He, Guan Wang, Shuhong Zhang, Tenggang Di, Menghan Chang, Wei Han, Jingyi Gao, Meng Li, Ziyi Wang, and et al. 2025. "Decoding the Function of FGFBP1 in Sheep Adipocyte Proliferation and Differentiation" Animals 15, no. 10: 1456. https://doi.org/10.3390/ani15101456
APA StyleTian, L., He, Z., Wang, G., Zhang, S., Di, T., Chang, M., Han, W., Gao, J., Li, M., Wang, Z., Zhang, H., Li, S., & Yang, G. (2025). Decoding the Function of FGFBP1 in Sheep Adipocyte Proliferation and Differentiation. Animals, 15(10), 1456. https://doi.org/10.3390/ani15101456





