Can Acute Neurological Disease Cause Cardiomyopathy in Horses?
Simple Summary
Abstract
1. Introduction
2. Case Summary
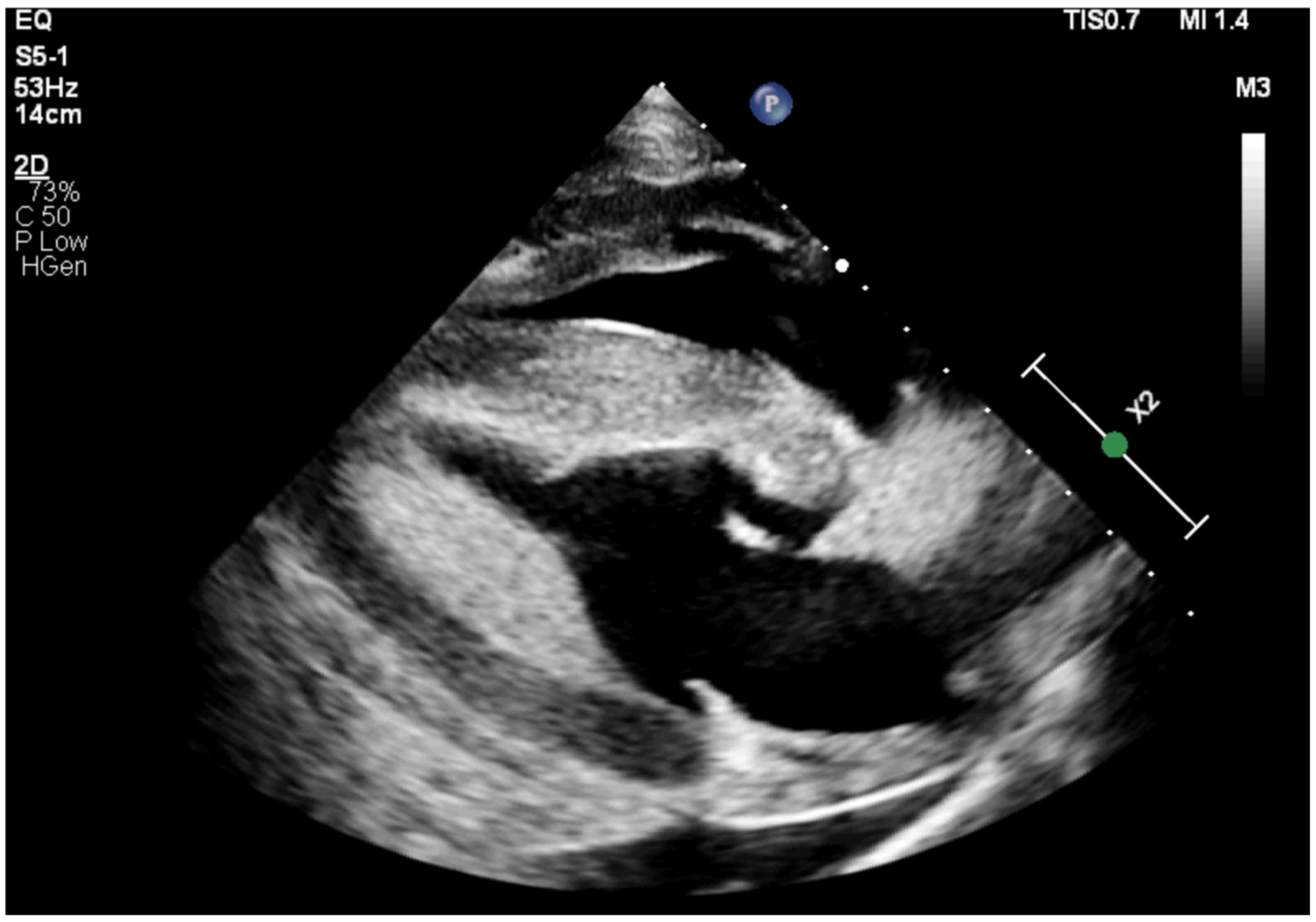
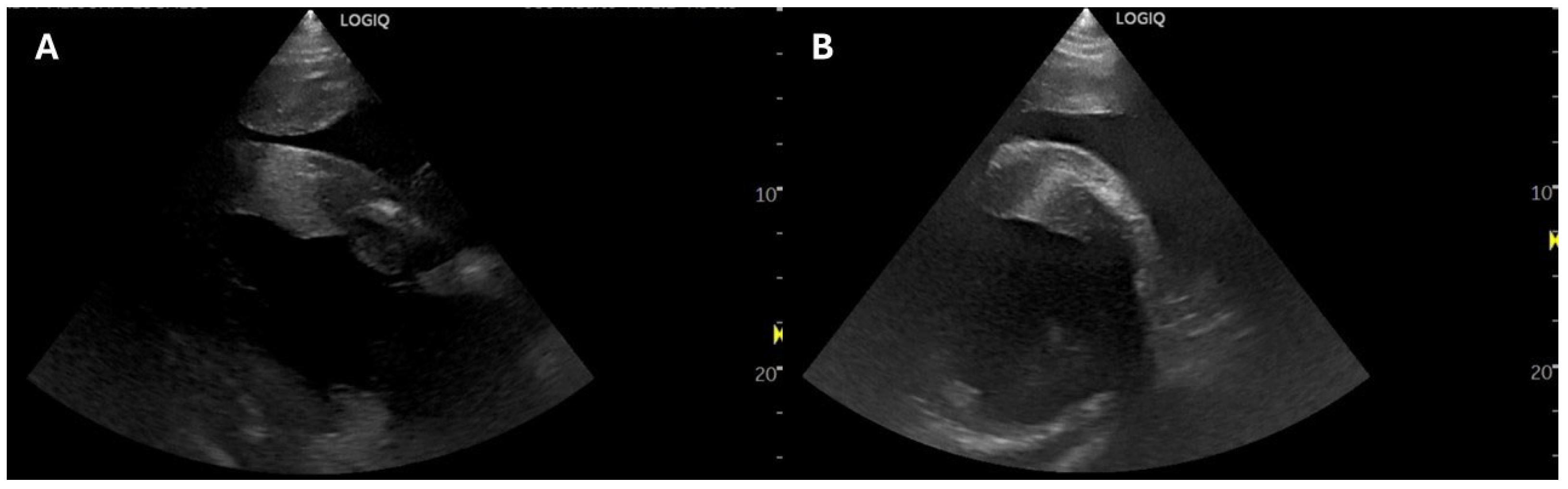
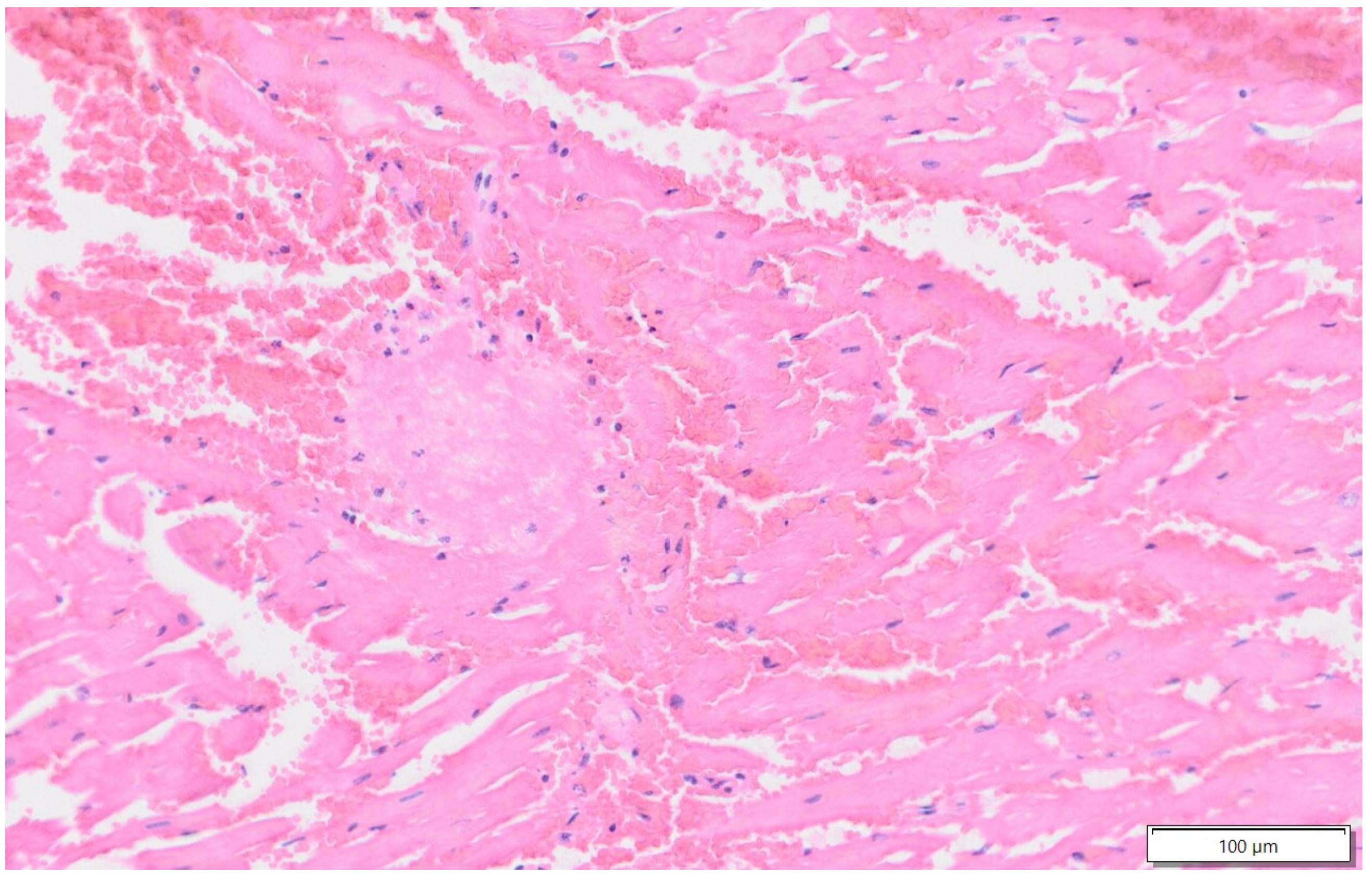
2.1. Case 1
2.2. Case 2
2.3. Case 3
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRI | continuous rate infusion |
| cTnI | cardiac troponin I |
| EF | ejection fraction |
| FS | fractional shortening |
| HR | heart rate |
| IVS | interventricular septum |
| LV | left ventricle |
| LVFW | left ventricular free wall |
| NSM | neurogenic stunned myocardium |
| RR | respiratory rate |
| SVT | supraventricular tachycardia |
| TP | total protein |
| US | ultrasound |
| VPC | ventricular premature complex |
| VT | ventricular tachycardia |
References
- Decloedt, A. Pericardial Disease, Myocardial Disease, and Great Vessel Abnormalities in Horses. Vet. Clin. N. Am. Equine Pract. 2019, 35, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, G. Inflammatory myocardial disease and toxicity in horses—A recent update. In Proceedings of the Veterinary Cardiovascular Meeting, Loughnorough, UK, 9–10 November 2012. [Google Scholar]
- Bulsara, K.R.; McGirt, M.J.; Liao, L.; Villavicencio, A.T.; Borel, C.; Alexander, M.J.; Friedman, A.H. Use of the peak troponin value to differentiate myocardial infarction from reversible neurogenic left ventricular dysfunction associated with aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2003, 98, 524–528. [Google Scholar] [CrossRef]
- Fodstad, H.; Kelly, P.J.; Buchfelder, M. History of the cushing reflex. Neurosurgery 2006, 59, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Qian, R.; Yang, W.; Wang, X.; Xu, Z.; Liu, X.; Sun, B. Evaluation of cerebral-cardiac syndrome using echocardiography in a canine model of acute traumatic brain injury. Am. J. Cardiovasc. Dis. 2015, 5, 72–76. [Google Scholar] [PubMed]
- Scheitz, J.F.; Sposato, L.A.; Schulz-Menger, J.; Nolte, C.H.; Backs, J.; Endres, M. Stroke-Heart Syndrome: Recent Advances and Challenges. J. Am. Heart Assoc. 2022, 11, e026528. [Google Scholar] [CrossRef]
- Piliponis, L.; Neverauskaitė-Piliponienė, G.; Kazlauskaitė, M.; Kačnov, P.; Glaveckaitė, S.; Barysienė, J.; Ročka, S. Neurogenic stress cardiomyopathy following aneurysmal subarachnoid haemorrhage: A literature review. Semin. Cardiovasc. Med. 2019, 25, 44–52. [Google Scholar] [CrossRef]
- Kenigsberg, B.B.; Barnett, C.F.; Mai, C.; Chang, J.J. Neurogenic Stunned Myocardium in Severe Neurological Injury. Curr. Neurol. Neurosci. Rep. 2019, 19, 90. [Google Scholar] [CrossRef]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef]
- Marr, C.M.; Patteson, M. Echocardiography. In Cardiology of the Horse, 2nd ed.; Marr, C.M., Bowen, I.M., Eds.; Saunders Ltd.: Edinburgh, NY, USA, 2010; pp. 105–126. [Google Scholar]
- Trachsel, D.S.; Calloe, K.; Rgensen, E.J.; Jørgensen, E.; Lunddahl, C.S.; Pedersen, P.J.; Kanters, J.K.; Buhl, R. Evaluation of electrocardiographic repolarization parameters after administration of trimethoprim-sulfadiazine, detomidine, or their combination in horses. Am. J. Vet. Res. 2021, 82, 207–217. [Google Scholar] [CrossRef]
- Van Loon, J.P.; Van Dierendonck, M.C. Monitoring equine head-related pain with the Equine Utrecht University scale for facial assessment of pain (EQUUS-FAP). Vet. J. 2017, 220, 88–90. [Google Scholar] [CrossRef]
- Gherasim, L. Takotsubo Syndrome vs. Neurogenic Stunned Myocardium. Maedica 2020, 15, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.A.; Wannamaker, B.B.; Malek, A.M.; Selassie, A.W. Myocardial infarction after epilepsy onset: A population-based retrospective cohort study. Epilepsy Behav. 2018, 88, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Bailén, M.; Rucabado Aguilar, L.; López Martínez, A. Aturdimiento miocárdico neurogénico. Med. Intensiv. 2006, 30, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; White, H.D. Universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 2173–2195. [Google Scholar] [CrossRef]
- Weis, R.; Carstensen, H.; Sattler, S.M.; Buhl, R.; Hesselkilde, E.M. Electrocardiographic Changes in a Horse with Induced Myocardial Infarction. Animals 2022, 12, 1272. [Google Scholar] [CrossRef]
- Reef, V.B.; Bonagura, J.; Buhl, R.; McGurrin, M.K.J.; Schwarzwald, C.C.; van Loon, G.; Young, L.E. Recommendations for management of equine athletes with cardiovascular abnormalities. J. Vet. Intern. Med. 2014, 28, 749–761. [Google Scholar] [CrossRef]
- Navas de Solis, C.; Dallap Schaer, B.L.; Boston, R.; Slack, J. Myocardial insult and arrhythmias after acute hemorrhage in horses. J. Vet. Emerg. Crit. Care 2015, 25, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, N.J.; McKenzie, H.C.; Barton, M.H.; Davis, J.L.; Dunkel, B.; Johnson, A.L.; MacDonald, E.S. Catheter-associated venous air embolism in hospitalized horses: 32 cases. J. Vet. Intern. Med. 2018, 32, 805–814. [Google Scholar] [CrossRef]
- Scott, R.A.; Rabinstein, A.A. Paroxysmal Sympathetic Hyperactivity. Semin. Neurol. 2020, 40, 485–491. [Google Scholar] [CrossRef]
- Verma, R.; Giri, P.; Rizvi, I. Paroxysmal sympathetic hyperactivity in neurological critical care. Indian J. Crit. Care Med. 2015, 19, 34–37. [Google Scholar]
- Gherasim, L.; Nistor, R. Neurogenic Stunned Myocardium as Part of Stress Cardiomyopathy. Maedica 2022, 17, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vekens, N.; Decloedt, A.; Ven, S.; De Clercq, D.; van Loon, G. Cardiac troponin I as compared to troponin T for the detection of myocardial damage in horses. J. Vet. Intern. Med. 2015, 29, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Bieber, M.; Werner, R.A.; Tanai, E.; Hofmann, U.; Higuchi, T.; Schuh, K.; Heuschmann, P.U.; Frantz, S.; Ritter, O.; Kraft, P.; et al. Stroke-induced chronic systolic dysfunction driven by sympathetic overactivity. Ann. Neurol. 2017, 82, 729–743. [Google Scholar] [CrossRef]
- Gehlen, H.; Marnette, S.; Rohn, K.; Stadler, P. Stress echocardiography in warmblood horses: Comparison of dobutamine/atropine with treadmill exercise as cardiac stressors. J. Vet. Intern. Med. 2006, 20, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Sandersen, C.; Detilleux, J.; Art, T.; Amory, H. Exercise and pharmacological stress echocardiography in healthy horses. Equine Vet. J. 2006, 38 (Suppl. S36), 159–162. [Google Scholar] [CrossRef]
- Vitale, V.; Vezzosi, T.; Di Franco, C.; Briganti, A.; Tognetti, R.; Conte, G.; Bucchioni, E.; Sgorbini, M. Equine echocardiography: Can dobutamine infusion correct alterations due to sedation with alpha-2 agonists? PLoS ONE 2022, 17, e0276256. [Google Scholar] [CrossRef]
- Durando, M. Exercise and stress testing. In Cardiology of the Horse, 2nd ed.; Marr, C.M., Bowen, I.M., Eds.; Saunders Ltd.: Edinburgh, NY, USA, 2010; pp. 139–149. [Google Scholar]
- Lyon, A.R.; Bossone, E.; Schneider, B.; Sechtem, U.; Citro, R.; Underwood, S.R.; Sheppard, M.N.; Figtree, G.A.; Parodi, G.; Akashi, Y.J.; et al. Current state of knowledge on Takotsubo syndrome: A Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 8–27. [Google Scholar] [CrossRef]
- Biso, S.; Wongrakpanich, S.; Agrawal, A.; Yadlapati, S.; Kishlyansky, M.; Figueredo, V. A Review of Neurogenic Stunned Myocardium. Cardiovasc. Psychiatry Neurol. 2017, 2017, 5842182. [Google Scholar] [CrossRef]
| Drugs | Case | Dose | Reference |
|---|---|---|---|
| Flunixin meglumine | 1 | 1.1 mg/kg q 24 h, IV | Ilium Flunixil, Glendenning, NSW, Australia |
| Penicillin procaine | 1 | 22,000 IU/kg q 12 h, IM | Ilium Propercillin, Glendenning, NSW, Australia |
| Hartmann’s intravenous fluids | 1 | 2 mL/kg/h | Baxter Healthcare, Toogabbie, NSW, Australia |
| Gentamicin | 1 | 6.6 mg/kg q 24 h, IV | Gentam-100, Glendenning, NSW, Australia |
| Dexamethasone | 1 | 0.2 mg/kg q 24 h, IV | Ilium Dexapent, Glendenning, NSW, Australia |
| Lidocaine | 1 | loading dose of 0.3 mg/kg IV over 15 min, followed by 0.025 mg/kg/min | Lignocaine 20, Glendenning, NSW, Australia |
| Magnesium sulphate | 1 | 2 mg/kg/min IV | Pfizer Australia Pty Ltd., Sydney, NSW, Australia |
| Prednisolone | 1 | 1 mg/kg q 24 h, PO | Pred-X 20 mg, Dechra Veterinary products PTY LTD, Somersby, NSW, Australia |
| Phenylbutazone | 2 | 4.4 mg/kg q 12 h, IV | Butasyl®, Zoetis Manufacturing & Research Spain, Girona, Spain |
| Detomidine | 2 | 0.006 mg/kg IV | Domidine®, Eurovet Animal Health B.V., Bladel, The Netherlands |
| Butorphanol | 2 | 0.03 mg/kg IV | Torbugesic®, Zoetis Manufacturing & Research Spain, Girona, Spain |
| Ringer Lactate | 2 | Initially a 10 L bolus, and subsequently at a rate of 2 mL/kg/h | B. Braun Medical SA, Rubí, Barcelona, Spain |
| Cefquinome | 2 | 1 mg/kg q 24 h, IM | Cobactan 2.5%®, Intervet International GmbH, Unterschleißheim, Germany |
| Dexamethasone | 2 | 0.1 mg/kg q 24 h, IV | Caliercortin 4 mg/mL®, Laboratorios Calier, S. A., Les Franqueses del Vallès, Barcelona, Spain |
| Tranexamic acid | 2 | 10 mg/kg IV | Amchafibrin 500 mg®, Meda Pharma SL, Madrid, Spain |
| Etamsylate | 2 | 5 mg/kg IV | Hemo 125 mg/mL®, Zoetis Manufacturing & Research Spain, Girona, Spain |
| Diazepam | 2 | 0.5 mg/kg IV | Valium®, Atnahs Pharma Netherlands B.V., København S, Denmark |
| Phenobarbital | 2 | 2 mg/kg q 12 h, IV | Luminal®, KERN PHARMA, Terrassa, Barcelona, Spain |
| Dimethyl sulfoxide | 2 | 0.2 g/kg q 12 h, IV diluted 10% in physiological saline solution | Dimetil sulfoxide, Fagron Ibérica SAU, Terrasa, Barcelona, Spain |
| Hypertonic saline | 2 | 5 mL/kg IV | B. Braun Medical SA, Rubí, Barcelona, Spain |
| Acepromazine | 2 | 0.03 mg/kg q 8 h, IM | Equipromazina®, Labiana Life Sciences, S.A., Terrassa, Barcelona, Spain |
| Gabapentin | 2 | 20 mg/kg q 12 h, PO | Gabapentina Sandoz®, Sandoz Farmacéutica, S.A., Madrid, Spain |
| Furosemide | 2 | 1 mg/kg q 12 h, IV | Seguril®, Sanofi-aventis, S.A., Barcelona, Spain |
| Synthetic colloid | 2 | 10 mL/kg q 24 h, IV | Gelaspan®, B. Braun, Melsungen, Germany |
| Trimethoprim-sulfadiazine | 2 | 30 mg/kg q 12 h, PO | Equibactin®, Dechra Regulatory B.V., Bladel, The Netherlands |
| Parameters | Horse 1 | Range for 80 kg | Horse 2 | Range for 454–620 kg |
|---|---|---|---|---|
| IVSd (cm) | 1.83 * | 1.32–1.68 | 3.24 * | 2.6–3.0 cm |
| LVIDd (cm) | 4.37 * | 5.33–6.47 | 10.54 | 10.4–12 cm |
| LVFWd (cm) | 1.75 * | 1.02–1.38 | 3.18 * | 2.2–2.8 cm |
| IVSs (cm) | 2.86 * | 2.08–2.52 | 5.33 * | 4.1–5.1 cm |
| LVIDs (cm) | 2.10 * | 3.54–4.66 | 5.10 * | 6.5–8.1 cm |
| LVFWs (cm) | 2.59 * | 1.6–2.2 | 4.2 * | 3.5–4.1 cm |
| SF (%) | 82 * | 32.3–40.1 | 51.6% * | 32.3–40.1% |
| RWT | 0.82 | - | 0.61 | 0.41–0.63 |
| Blood Parameter | Horse 1 | Horse 2 | Normal Range |
|---|---|---|---|
| RBC (×106/μL) | 5.94 * | 5.52 * | 6.5–12.5 |
| Haemoglobin (g/dL) | 9.5 * | 9.6 * | 11–19 |
| PCV (%) | 25 * | 26 * | 30–45 |
| WBC (×103/μL) | 8.69 | 17.09 * | 5.5–12 |
| Neutrophils (×103/μL) | 5.21 | 15.67 * | 2.5–8.1 |
| Lymphocytes (×103/μL) | 2.43 | 0.77 * | 1.63–3.40 |
| Monocytes (×103/μL) | 1.04 * | 0.53 | 0.0–0.7 |
| Eosinophils (×103/μL) | 0.00 | 0.09 | 0.00–0.96 |
| Basophils (×103/μL) | 0.00 | 0.02 | 0.00–0.36 |
| TP (g/dL) | 7 | 4.6 * | 5.3–7.3 |
| Fibrinogen (mg/dL) | 500 * | 200 mg/dL | <400 |
| Platelets (×103/μL) | 124 | 92 | 80–300 |
| Lactate (mmol/L) | 3.2 * | 6.9 * | <2 |
| cTnI (ng/mL) | >25 * | >25 * | <0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, V.; Velloso Álvarez, A.; de la Cuesta-Torrado, M.; Neira-Egea, P.; Vandecandelaere, M.; Tee, E.; Gimeno, M.; van Galen, G. Can Acute Neurological Disease Cause Cardiomyopathy in Horses? Animals 2025, 15, 1447. https://doi.org/10.3390/ani15101447
Vitale V, Velloso Álvarez A, de la Cuesta-Torrado M, Neira-Egea P, Vandecandelaere M, Tee E, Gimeno M, van Galen G. Can Acute Neurological Disease Cause Cardiomyopathy in Horses? Animals. 2025; 15(10):1447. https://doi.org/10.3390/ani15101447
Chicago/Turabian StyleVitale, Valentina, Ana Velloso Álvarez, María de la Cuesta-Torrado, Patricia Neira-Egea, Marie Vandecandelaere, Elizabeth Tee, Marina Gimeno, and Gaby van Galen. 2025. "Can Acute Neurological Disease Cause Cardiomyopathy in Horses?" Animals 15, no. 10: 1447. https://doi.org/10.3390/ani15101447
APA StyleVitale, V., Velloso Álvarez, A., de la Cuesta-Torrado, M., Neira-Egea, P., Vandecandelaere, M., Tee, E., Gimeno, M., & van Galen, G. (2025). Can Acute Neurological Disease Cause Cardiomyopathy in Horses? Animals, 15(10), 1447. https://doi.org/10.3390/ani15101447







