Quantitative Proteomics Revealed the Molecular Regulatory Network of Lysine and the Effects of Lysine Supplementation on Sunit Skeletal Muscle Satellite Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Cells
2.2. Lys Concentration Screening
2.3. Cell Proliferation Experiment
2.4. Western Blot
2.5. Immunocytochemistry
2.6. TMT Quantitative Proteomics
2.7. Real-Time Fluorescent Quantitative PCR Validation Assay for Differentially Expressed Proteins
2.8. Statistical Analysis and Processing of the Data
3. Results
3.1. Effect of Lys Treatment on Survival Rate of SMSCs
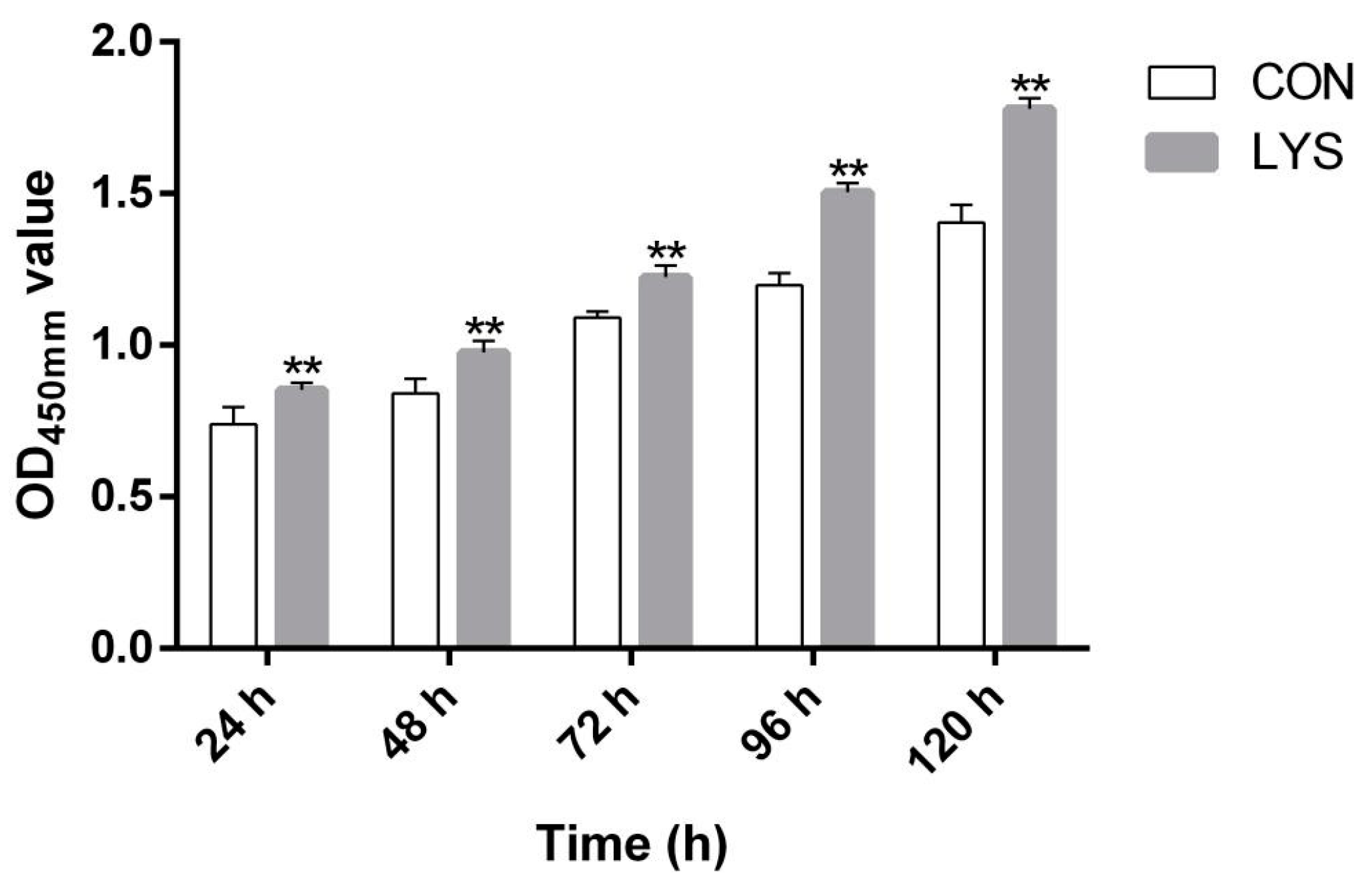
3.2. Effect of Lys on the Proliferation of SMSCs
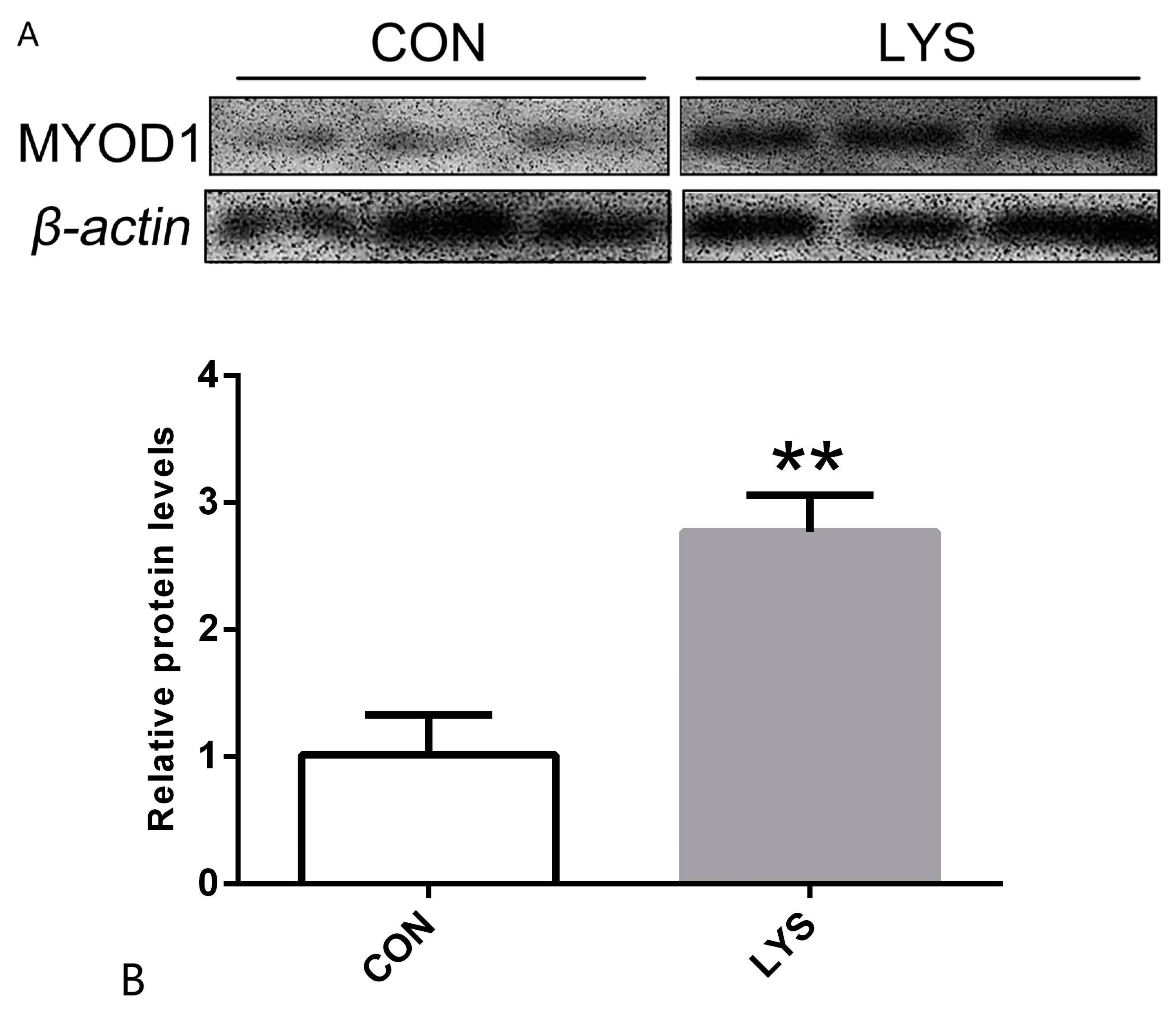
3.3. The Effect of Lys on Myogenic Differentiation Protein Expression
3.4. The Effect of Lys on the Expression of α-Actinin and Myostatin in SMSCs
3.5. TMT Quantitative Proteomics Analysis
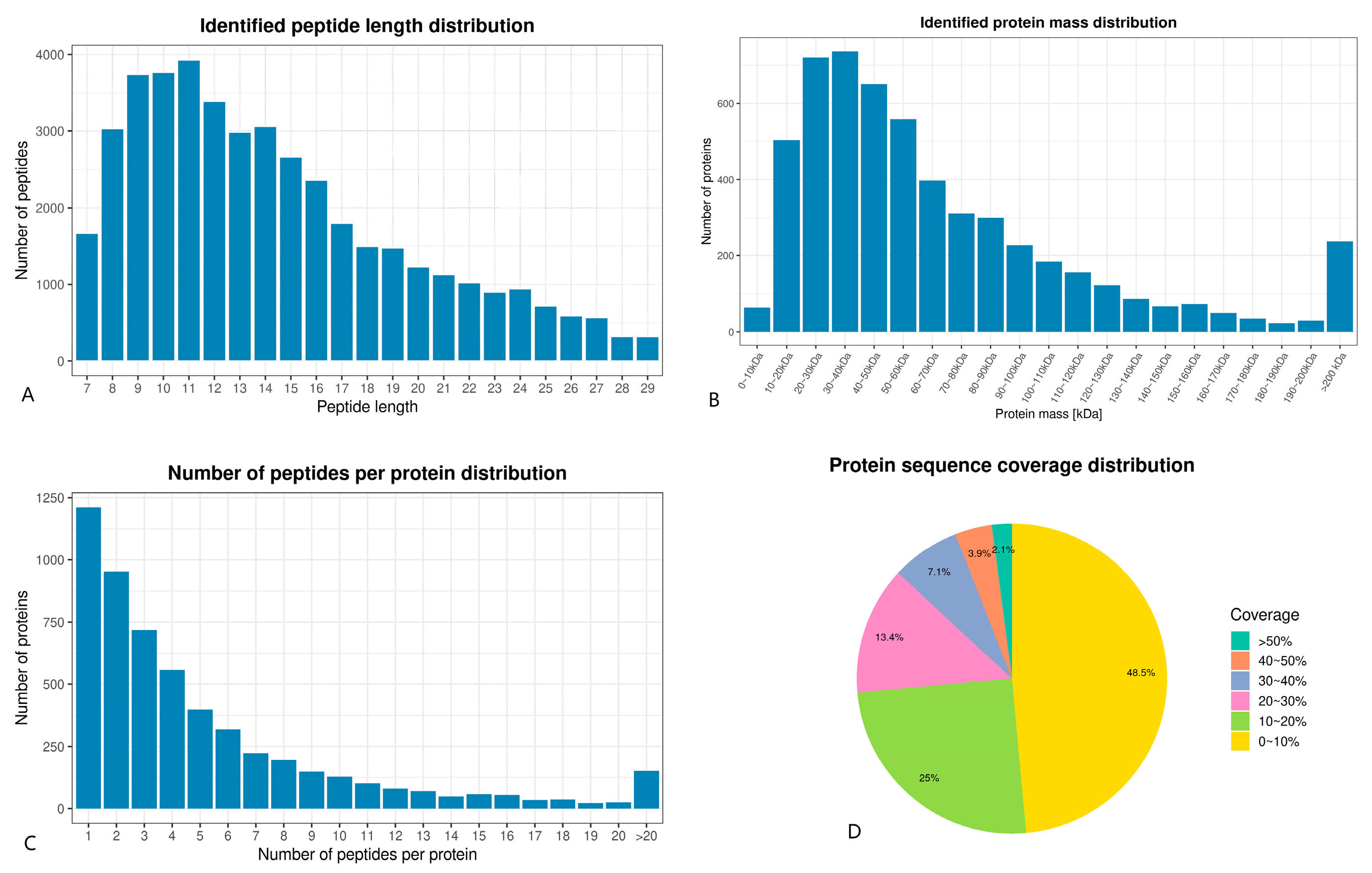
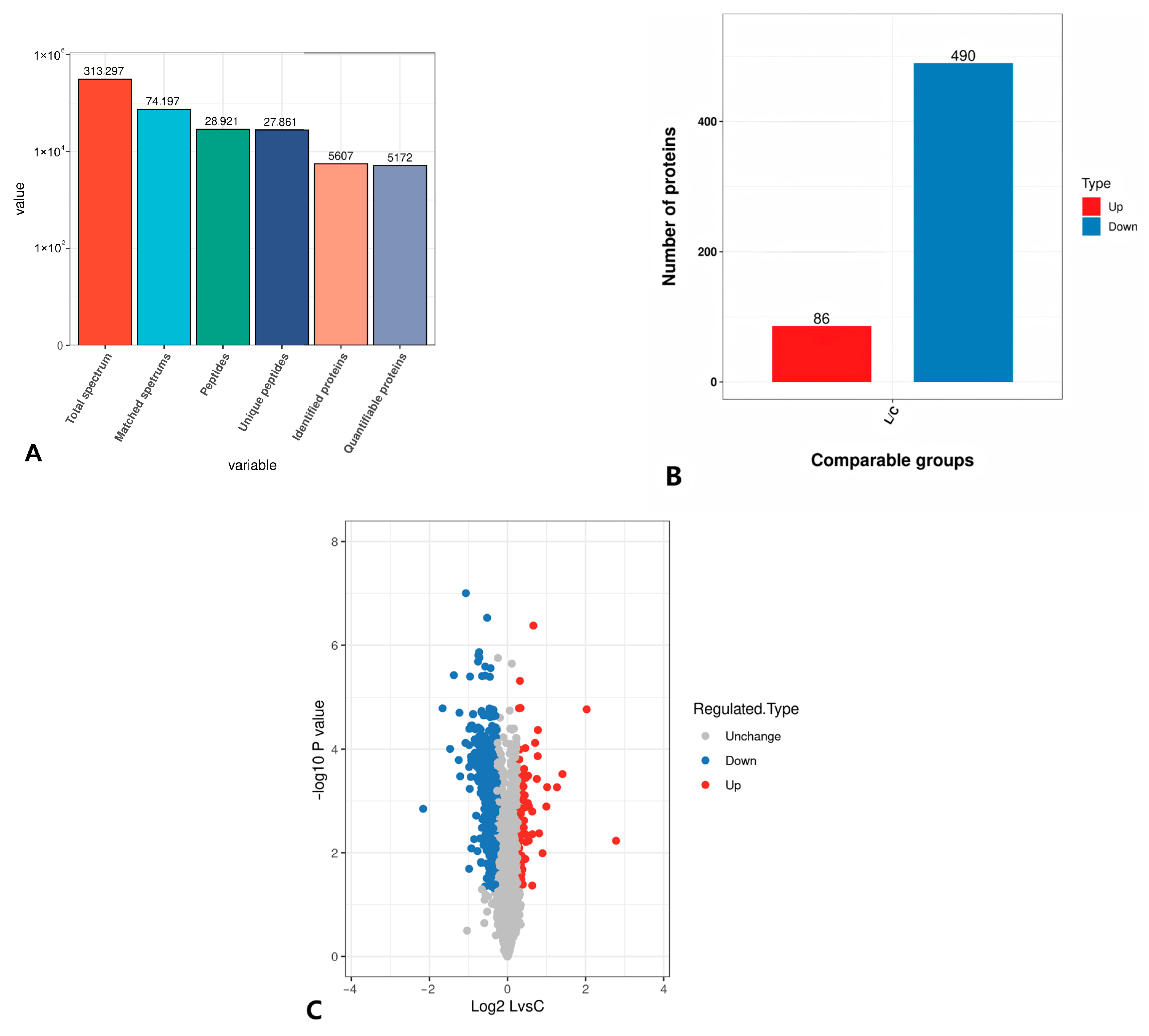
3.5.1. TMT Quantitative Proteomics Identification
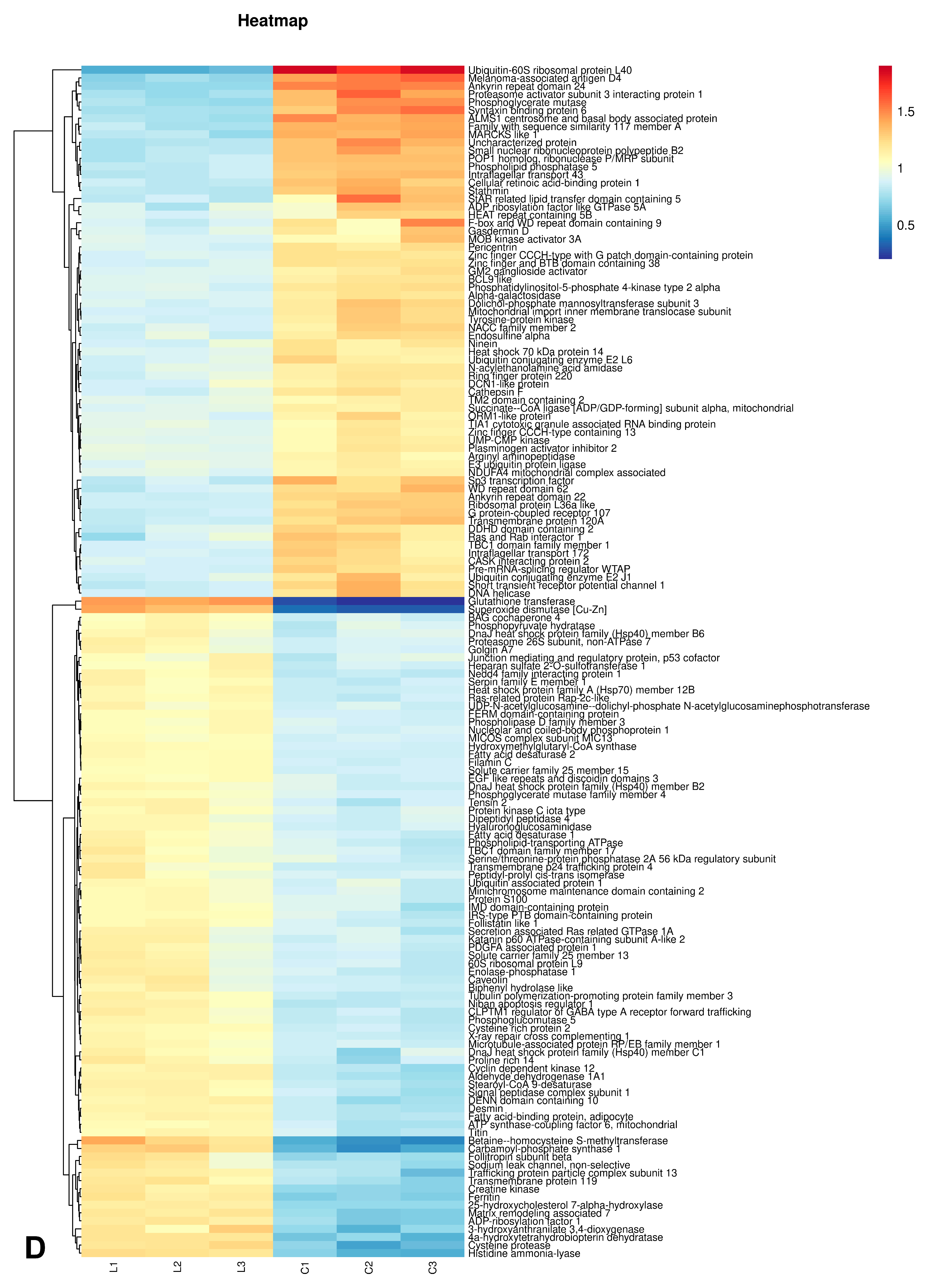
3.5.2. Statistical Analysis of Differential Proteins
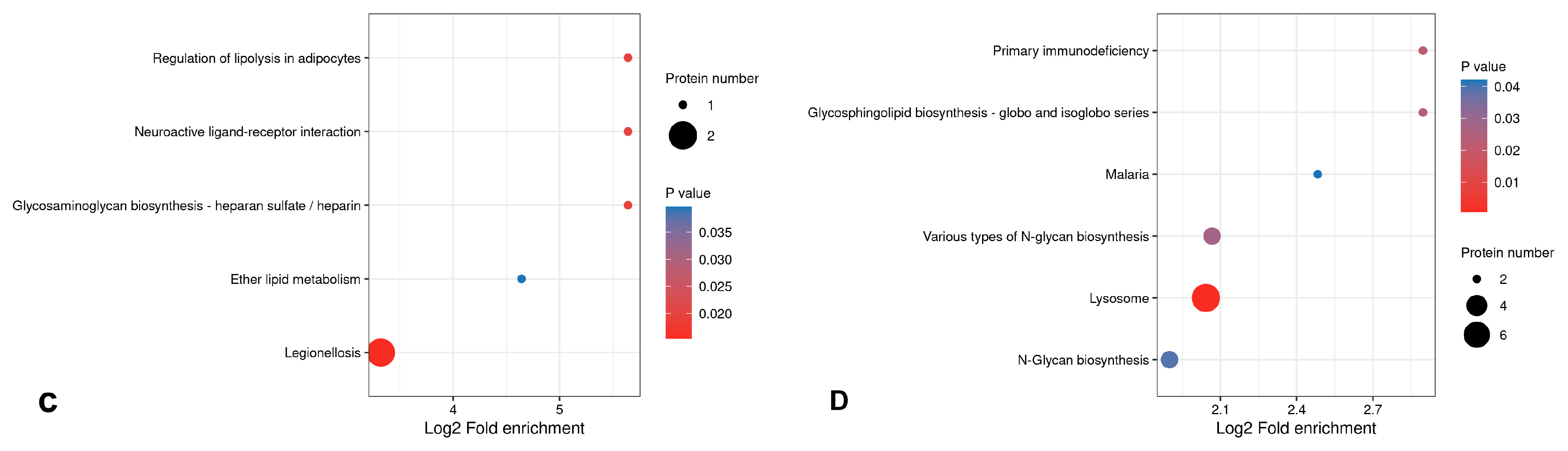
3.5.3. Bioinformatics Analysis of Differential Proteins
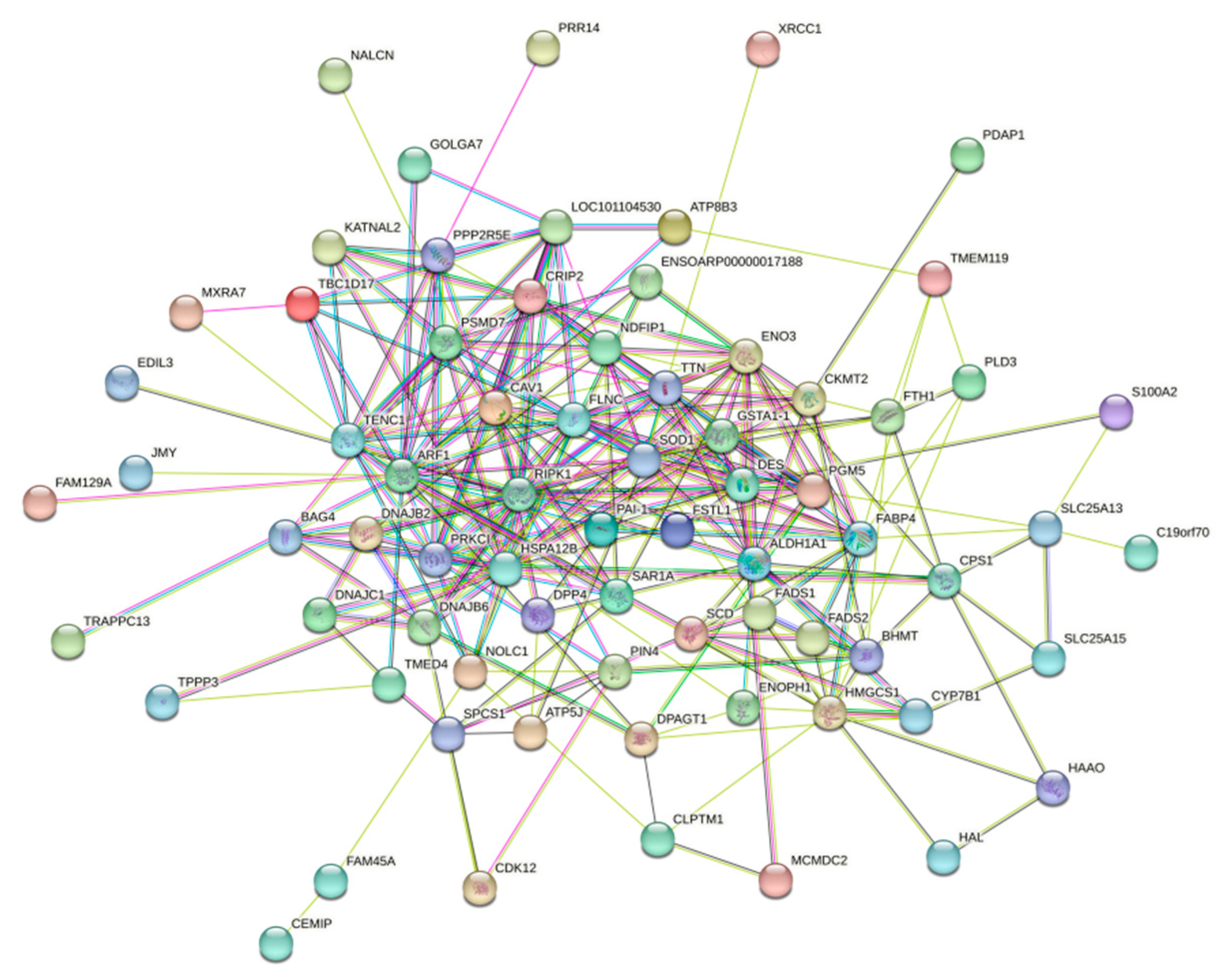
3.5.4. PPi Protein Interactions Analysis
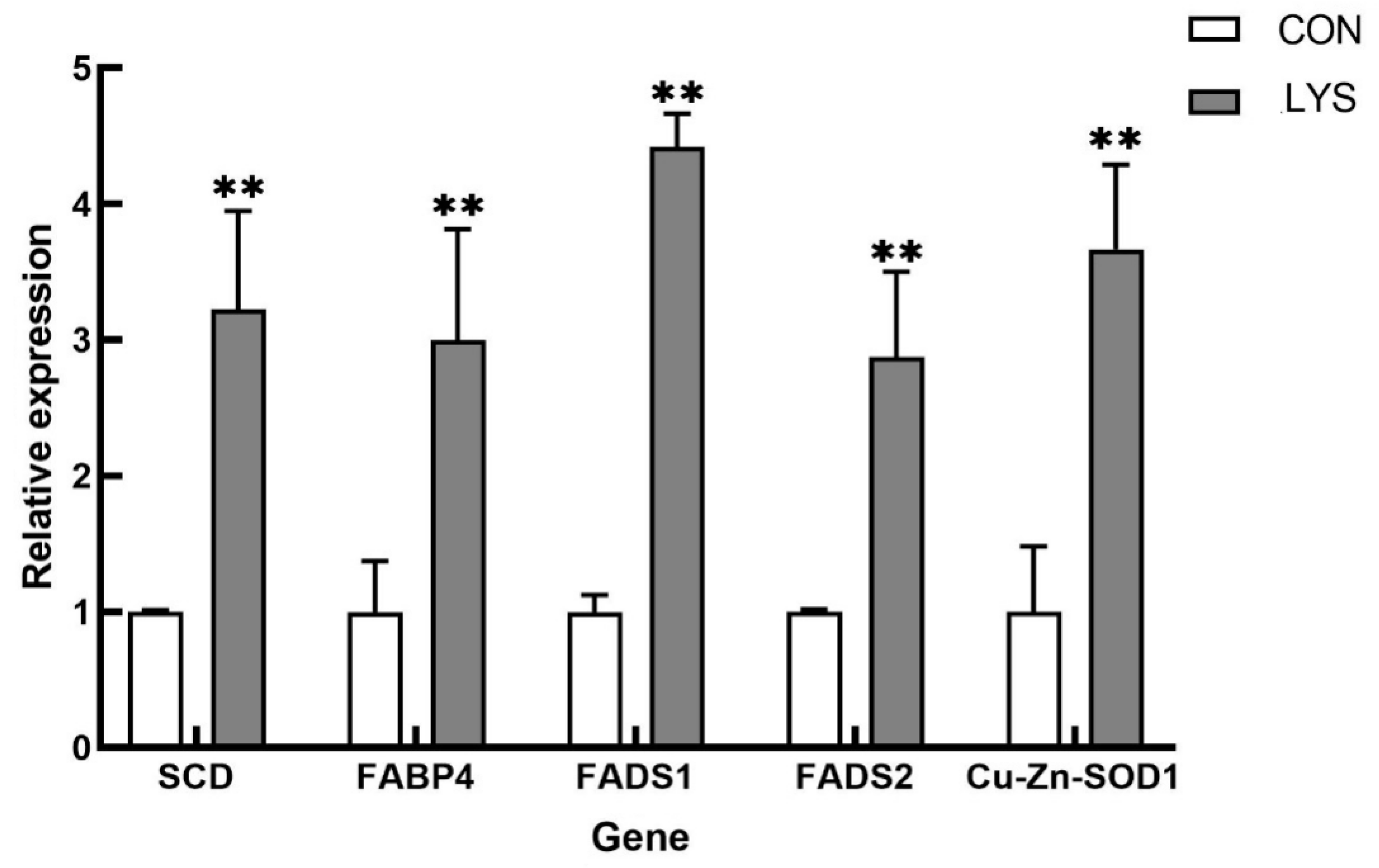
3.5.5. RT-qPCR Validation of Differentially Expressed Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safaei, S.M.H.; Dadpasand, M.; Mohammadabadi, M.; Atashi, H.; Stavetska, R.; Klopenko, N.; Kalashnyk, O. An Origanum majorana Leaf Diet Influences Myogenin Gene Expression, Performance, and Carcass Characteristics in Lambs. Animals 2022, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Askari, N.; Mohammad Abadi, M.; Baghizadeh, A. ISSR markers for assessing DNA polymorphism and genetic characterization of cattle, goat and sheep populations. Iran. J. Biotechnol. 2011, 9, 222–229. [Google Scholar]
- He, X.; Wu, R.; Yun, Y.; Qin, X.; Huang, Y.; Chen, L.; Han, Y.; Wu, J.; Sha, L.; Borjigin, G. MicroRNA and circular RNA profiling in the deposited fat tissue of Sunite sheep. Front. Vet. Sci. 2022, 9, 954882. [Google Scholar] [CrossRef]
- Ishida, A.; Kyoya, T.; Nakashima, K.; Katsumata, M. Muscle protein metabolism during compensatory growth with changing dietary lysine levels from deficient to sufficient in growing rats. J Nutr. Sci. Vitaminol. 2011, 57, 401–408. [Google Scholar] [CrossRef]
- Mastellar, S.L.; Coleman, R.J.; Urschel, K.L. Controlled trial of whole body protein synthesis and plasma amino acid concentrations in yearling horses fed graded amounts of lysine. Vet. J. 2016, 216, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, K. Muscle memory and a new cellular model for muscle atrophy and hypertrophy. J. Exp. Biol. 2016, 219 Pt 2, 235–242. [Google Scholar] [CrossRef]
- Jin, C.L.; Ye, J.L.; Yang, J.; Gao, C.Q.; Yan, H.C.; Li, H.C.; Wang, X.Q. mTORC1 Mediates Lysine-Induced Satellite Cell Activation to Promote Skeletal Muscle Growth. Cells 2019, 8, 1549. [Google Scholar] [CrossRef]
- Jin, C.L.; Zhang, Z.M.; Song, Z.W.; Gao, C.Q.; Yan, H.C.; Wang, X.Q. mTORC1-Mediated Satellite Cell Differentiation Is Required for Lysine-Induced Skeletal Muscle Growth. J. Agric. Food Chem. 2020, 68, 4884–4892. [Google Scholar] [CrossRef]
- Wang, T.; Feugang, J.M.; Crenshaw, M.A.; Regmi, N.; Blanton, J.R.; Liao, S.F. A Systems Biology Approach Using Transcriptomic Data Reveals Genes and Pathways in Porcine Skeletal Muscle Affected by Dietary Lysine. Int. J. Mol. Sci. 2017, 18, 885. [Google Scholar] [CrossRef]
- Zeng, P.L.; Yan, H.C.; Wang, X.Q.; Zhang, C.M.; Zhu, C.; Shu, G.; Jiang, Q.Y. Effects of dietary lysine levels on apparent nutrient digestibility and serum amino Acid absorption mode in growing pigs. Asian-Australas. J. Anim. Sci. 2013, 26, 1003–1011. [Google Scholar] [CrossRef]
- Cornelison, D.; Wold, B. Single-Cell Analysis of Regulatory Gene Expression in Quiescent and Activated Mouse Skeletal Muscle Satellite Cells. Dev. Biol. 1997, 191, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yu, F.; Margrethe, T.; Xing-Min, L.; Rui-Tong, D. Molecular Understanding of Meat Quality Through Application of Proteomics. Food Rev. Int. 2014, 31, 13–28. [Google Scholar] [CrossRef]
- Costache, M.; Van, I. Avian Proteomics: Potential Tool for Pre- and Post-Slaughter Poultry Muscle Quality Evaluation-A Review. Sci. Pap. Ser. D Anim. Sci. 2018, 11, 283–294. [Google Scholar]
- Cheng, S.; Wang, X.; Zhang, Q.; He, Y.; Zhang, X.; Yang, L.; Shi, J. Comparative Transcriptome Analysis Identifying the Different Molecular Genetic Markers Related to Production Performance and Meat Quality in Longissimus Dorsi Tissues of MG × STH and STH Sheep. Genes 2020, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, Y.; Jia, G.; Zhao, H.; Liu, G.; Huang, Z. Arginine Promotes Slow Myosin Heavy Chain Expression via Akirin2 and the AMP-Activated Protein Kinase Signaling Pathway in Porcine Skeletal Muscle Satellite Cells. J. Agric. Food Chem. 2018, 66, 4734–4740. [Google Scholar] [CrossRef]
- Vadivel Gnanasundram, S.; Fåhraeus, R. Translation Stress Regulates Ribosome Synthesis and Cell Proliferation. Int. J. Mol. Sci. 2018, 19, 3757. [Google Scholar] [CrossRef]
- Zheng, J.; Du, M.; Zhang, J.; Liang, Z.; Ahmad, A.A.; Shen, J.; Salekdeh, G.H.; Ding, X. Transcriptomic and Metabolomic Analyses Reveal Inhibition of Hepatic Adipogenesis and Fat Catabolism in Yak for Adaptation to Forage Shortage During Cold Season. Front. Cell Dev. Biol. 2022, 9, 759521. [Google Scholar] [CrossRef]
- Board, P.G.; Coggan, M.; Watson, S.; Gage, P.W.; Dulhunty, A.F. CLIC-2 modulates cardiac ryanodine receptor Ca2+ release channels. Int. J. Biochem. Cell Biol. 2004, 36, 1599–1612. [Google Scholar] [CrossRef]
- Dulhunty, A.; Gage, P.; Curtis, S.; Chelvanayagam, G.; Board, P. The glutathione transferase structural family includes a nuclear chloride channel and a ryanodine receptor calcium release channel modulator. J. Biol. Chem. 2001, 276, 3319–3323. [Google Scholar] [CrossRef]
- Dulhunty, A.F.; Pouliquin, P.; Coggan, M.; Gage, P.W.; Board, P.G. A recently identified member of the glutathione transferase structural family modifies cardiac RyR2 substate activity, coupled gating and activation by Ca2+ and ATP. Biochem. J. 2005, 390 Pt 1, 333–343. [Google Scholar] [CrossRef]
- Abdellatif, Y.; Liu, D.; Gallant, E.M.; Gage, P.W.; Board, P.G.; Dulhunty, A.F. The Mu class glutathione transferase is abundant in striated muscle and is an isoform-specific regulator of ryanodine receptor calcium channels. Cell Calcium. 2007, 41, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Nagahisa, H.; Okabe, K.; Iuchi, Y.; Fujii, J.; Miyata, H. Characteristics of Skeletal Muscle Fibers of SOD1 Knockout Mice. Oxid. Med. Cell. Longev. 2016, 2016, 9345970. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.L.; Song, W.; Liu, Y.; Chaudhuri, A.; Pieke-Dahl, S.; Strong, R.; Huang, T.T.; Epstein, C.J.; Roberts, L.J., 2nd; Csete, M.; et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic. Biol. Med. 2006, 40, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Ergun, U. The Role of Cu, Zn Superoxide Dismutase (SOD1) in Muscle Stem Cell Function. Master’s Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2021. [Google Scholar]
- Turrens, J.F.; Crapo, J.D.; Freeman, B.A. Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J. Clin. Investig. 1984, 73, 87–95. [Google Scholar] [CrossRef]
- Li, G.; Feng, X.; Wang, S. Effects of Cu/Zn superoxide dismutase on strain injury-induced oxidative damage to skeletal muscle in rats. Physiol. Res. 2005, 54, 193–199. [Google Scholar] [CrossRef]
- Theil, E.C. The ferritin family of iron storage proteins. Adv. Enzym. Relat. Areas Mol. Biol. 1990, 63, 421–449. [Google Scholar]
- Chasteen, N.D. Ferritin. Uptake, storage, and release of iron. Met. Ions. Biol. Syst. 1998, 35, 479–514. [Google Scholar]
- Rucker, P.; Torti, F.M.; Torti, S.V. Role of H and L subunits in mouse ferritin. J. Biol. Chem. 1996, 271, 33352–33357. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- Alves, F.M.; Kysenius, K.; Caldow, M.K.; Hardee, J.P.; Crouch, P.J.; Ayton, S.; Bush, A.I.; Lynch, G.S.; Koopman, R. Iron accumulation in skeletal muscles of old mice is associated with impaired regeneration after ischaemia-reperfusion damage. J. Cachexia Sarcopenia Muscle 2021, 12, 476–492. [Google Scholar] [CrossRef]
- Voillet, V.; San Cristobal, M.; Père, M.C.; Billon, Y.; Canario, L.; Liaubet, L.; Lefaucheur, L. Integrated Analysis of Proteomic and Transcriptomic Data Highlights Late Fetal Muscle Maturation Process. Mol. Cell. Proteom. 2018, 17, 672–693. [Google Scholar] [CrossRef]
- Cassar-Malek, I.; Boby, C.; Picard, B.; Reverter, A.; Hudson, N.J. Molecular regulation of high muscle mass in developing Blonde d’Aquitaine cattle foetuses. Biol. Open 2017, 6, 1483–1492. [Google Scholar] [PubMed]
- Bottje, W.G.; Lassiter, K.; Dridi, S.; Hudson, N.; Kong, B.W. Enhanced expression of proteins involved in energy production and transfer in breast muscle of pedigree male broilers exhibiting high feed efficiency. Poult. Sci. 2017, 96, 2454–2458. [Google Scholar] [CrossRef]
- Goldblatt, H. The effect of high salt intake on the blood pressure of rabbits. Lab. Investig. 1969, 21, 126–128. [Google Scholar]
- Asakura, A.; Komaki, M.; Rudnicki, M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 2001, 68, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Shefer, G.; Wleklinski-Lee, M.; Yablonka-Reuveni, Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J. Cell Sci. 2004, 117 Pt 22, 5393–5404. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Gomez, F.E.; Fox, B.G.; Ntambi, J.M. Differential regulation of the stearoyl-CoA desaturase genes by thiazolidinediones in 3T3-L1 adipocytes. J. Lipid Res. 2000, 41, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hao, J.; Zeng, J.; Sauter, E.R. SnapShot: FABP Functions. Cell 2020, 182, 1066–1066.e1. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, M.J.; Jeon, G.J.; Chung, H.Y. Association of genetic variants for FABP3 gene with back fat thickness and intramuscular fat content in pig. Mol. Biol. Rep. 2011, 3, 2161–2166. [Google Scholar] [CrossRef]
- Wolfrum, C.; Borrmann, C.M.; Borchers, T.; Spener, F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha–and gamma-mediated gene expression via liver fatty acid binding protein: A signaling path to the nucleus. Proc. Natl. Acad. Sci. USA 2001, 98, 2323–2328. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Ulmer, J.; Langella, P.; Bermúdez-Humarán, L.G. Gut ecosystem: How microbes help us. Benef. Microbes. 2014, 5, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Gao, G.; Du, L.; Wang, J.; Wang, Q.; Yang, F.; Zhou, X.; Long, D.; Huang, J.; Liu, Z.; et al. Time-Series Clustering of lncRNA-mRNA Expression during the Adipogenic Transdifferentiation of Porcine Skeletal Muscle Satellite Cells. Curr. Issues Mol. Biol. 2022, 44, 2038–2053. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.P.; Liang, Z.Q.; Gu, Z.L.; Qin, Z.H. Molecular mechanism and regulation of autophagy. Acta Pharmacol. Sin. 2005, 26, 1421–1434. [Google Scholar] [CrossRef]
- Sato, T.; Ito, Y.; Nagasawa, T. Regulation of skeletal muscle protein degradation and synthesis by oral administration of lysine in rats. J. Nutr. Sci. Vitaminol. 2013, 59, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ito, Y.; Nagasawa, T. L-Lysine suppresses myofibrillar protein degradation and autophagy in skeletal muscles of senescence-accelerated mouse prone 8. Biogerontology 2017, 18, 85–95. [Google Scholar] [CrossRef]
- Leil, T.A.; Chen, Z.W.; Chang, C.S.; Olsen, R.W. GABAA receptor-associated protein traffics GABAA receptors to the plasma membrane in neurons. J. Neurosci. 2004, 24, 11429–11438. [Google Scholar] [CrossRef] [PubMed]
- Fernández, Á.F.; López-Otín, C. The functional and pathologic relevance of autophagy proteases. J. Clin. Investig. 2015, 125, 33–41. [Google Scholar] [CrossRef]
- Fujita, N.; Hayashi-Nishino, M.; Fukumoto, H.; Omori, H.; Yamamoto, A.; Noda, T.; Yoshimori, T. An Atg4B mutant hampers the lipidation of LC3 paralogues and causes defects in autophagosome closure. Mol. Biol. Cell 2008, 19, 4651–4659. [Google Scholar] [CrossRef]
- Velikkakath, A.K.; Nishimura, T.; Oita, E.; Ishihara, N.; Mizushima, N. Mammalian Atg2 proteins are essential for autophagosome formation and important for regulation of size and distribution of lipid droplets. Mol. Biol. Cell 2012, 23, 896–909. [Google Scholar] [CrossRef]
- Bozic, M.; van den Bekerom, L.; Milne, B.A.; Goodman, N.; Roberston, L.; Prescott, A.R.; Macartney, T.J.; Dawe, N.; McEwan, D.G. A conserved ATG2-GABARAP family interaction is critical for phagophore formation. EMBO Rep. 2020, 21, e48412. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, X.; Li, B.; Yang, H.J.; Miller, M.; Yang, A.; Dhar, A.; Pavletich, N.P. Mechanisms of mTORC1 activation by RHEB and inhibition by PRAS40. Nature 2017, 552, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Zhang, F.; Zhang, L.; Wei, W. The advances in pyroptosis initiated by inflammasome in inflammatory and immune diseases. Inflamm. Res. 2020, 69, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Cui, C.; Zhao, X.; Zhang, Y.; Zhang, Y.; Zhao, J.; Shen, X.; He, H.; Wang, J.; Ma, M.; et al. Filamin C regulates skeletal muscle atrophy by stabilizing dishevelled-2 to inhibit autophagy and mitophagy. Mol. Ther. Nucleic Acids 2021, 27, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fang, X.; Ithychanda, S.S.; Wu, T.; Gu, Y.; Chen, C.; Wang, L.; Bogomolovas, J.; Qin, J.; Chen, J. Interaction of Filamin C With Actin Is Essential for Cardiac Development and Function. Circ. Res. 2023, 133, 400–411. [Google Scholar] [CrossRef]




| Target Genes | Primers Sequences | Length of Product/Bp | GenBank Accession No. |
|---|---|---|---|
| SCD | F ACCATCACAGCACCTCCTTCC R CTCATTTCAGGGCGGATGTCTTC | 98 | NM_001009254.1 |
| FABP4 | F AAGAAGTGGGTGTGGGCTTTGC R TCCTGGCCCAATTTGAAGGACATC | 145 | NM_001114667.1 |
| FADS1 | F CCTGTGTTCCGTGCTGCTCAG R TGGTTCCACTTAGAGGTCCCGAAG | 99 | XM_004019593.6 |
| FADS2 | F GGAAGACTGCTGAGGACATGAACC R GATAGTGAACCAGGCGATGCTCTC | 104 | XM_015103138.4 |
| [Cu-Zn]-SOD1 | F GCCGTCTGCGTGCTGAAGG R CCAAACTGATGGACGTGGAACCC | 137 | NM_001145185.2 |
| β-actin | F CCACAGCCGAGCGGGAAATTG R AGGAGGACGACGCAGCAGTAG | 99 | XM_004013078.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Bai, F.; Zhao, Q.; Shi, J.; Hao, Y.; Wu, J. Quantitative Proteomics Revealed the Molecular Regulatory Network of Lysine and the Effects of Lysine Supplementation on Sunit Skeletal Muscle Satellite Cells. Animals 2025, 15, 1425. https://doi.org/10.3390/ani15101425
Wang M, Bai F, Zhao Q, Shi J, Hao Y, Wu J. Quantitative Proteomics Revealed the Molecular Regulatory Network of Lysine and the Effects of Lysine Supplementation on Sunit Skeletal Muscle Satellite Cells. Animals. 2025; 15(10):1425. https://doi.org/10.3390/ani15101425
Chicago/Turabian StyleWang, Mingxu, Fan Bai, Qinan Zhao, Jianan Shi, Yutian Hao, and Jindi Wu. 2025. "Quantitative Proteomics Revealed the Molecular Regulatory Network of Lysine and the Effects of Lysine Supplementation on Sunit Skeletal Muscle Satellite Cells" Animals 15, no. 10: 1425. https://doi.org/10.3390/ani15101425
APA StyleWang, M., Bai, F., Zhao, Q., Shi, J., Hao, Y., & Wu, J. (2025). Quantitative Proteomics Revealed the Molecular Regulatory Network of Lysine and the Effects of Lysine Supplementation on Sunit Skeletal Muscle Satellite Cells. Animals, 15(10), 1425. https://doi.org/10.3390/ani15101425






