ATR Deficiency Impairs DNA Damage Repair and Accelerates Cellular Senescence in Bovine Mammary Epithelial Cells, Leading to Lactation Dysfunction
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Grouping
2.2. Preparation and Detection of Samples for Transcriptomics and Proteomics Analyses
2.3. Primary Bovine Mammary Cell (BMEC) Culture
2.4. ATR Knockdown in BMECs
2.5. RNA Extraction, cDNA Synthesis, and Quantitative PCR (qPCR)
2.6. Protein Extraction and Western Blotting
2.7. Flow Cytometric Analysis of Cell Cycle
2.8. Detection of Senescent Cells by β-Galactosidase
2.9. Detection of Secretory Phenotypes Related to Aging
2.10. Statistical Analysis
3. Results
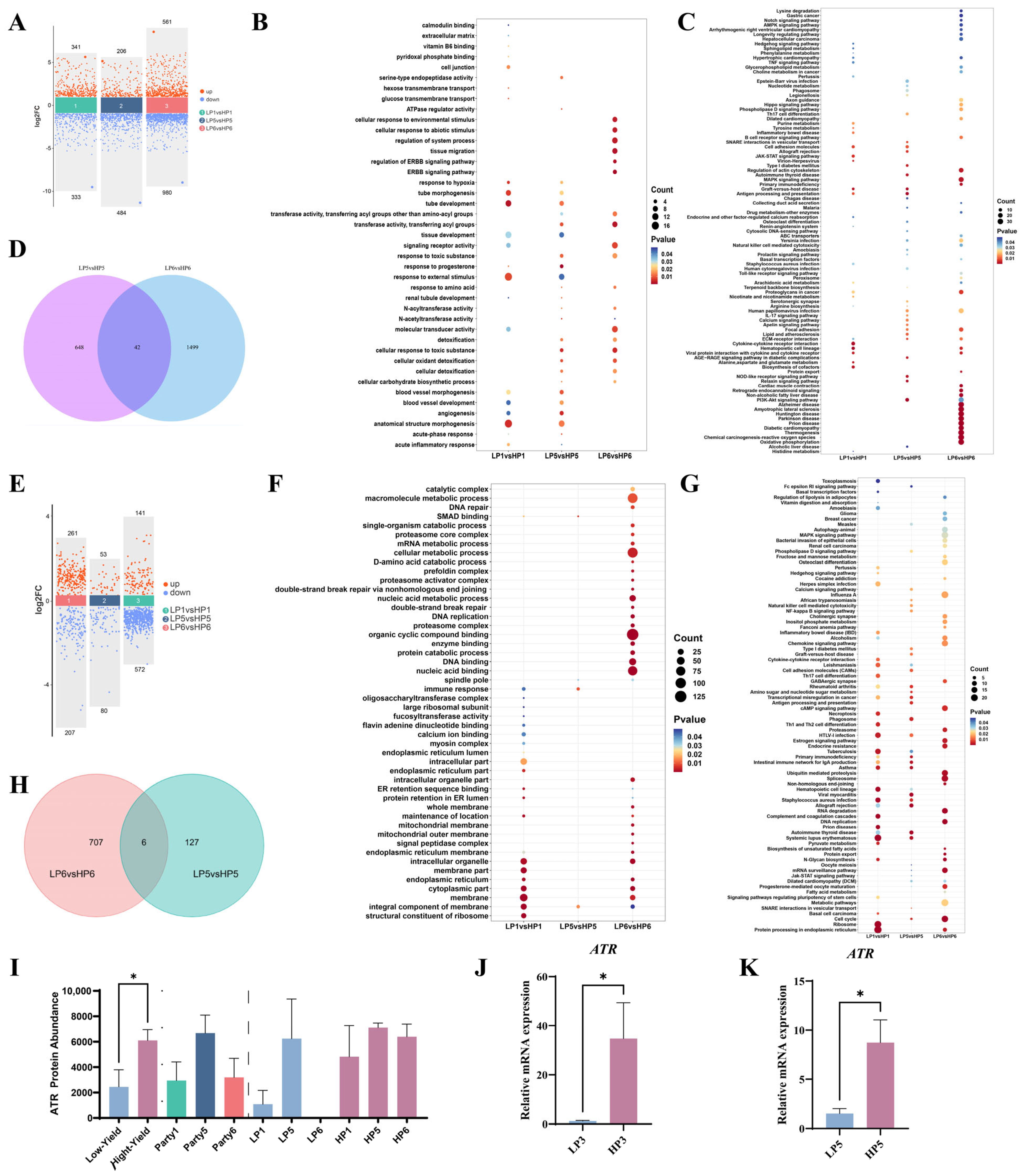
3.1. Multiomics Analysis and Verification
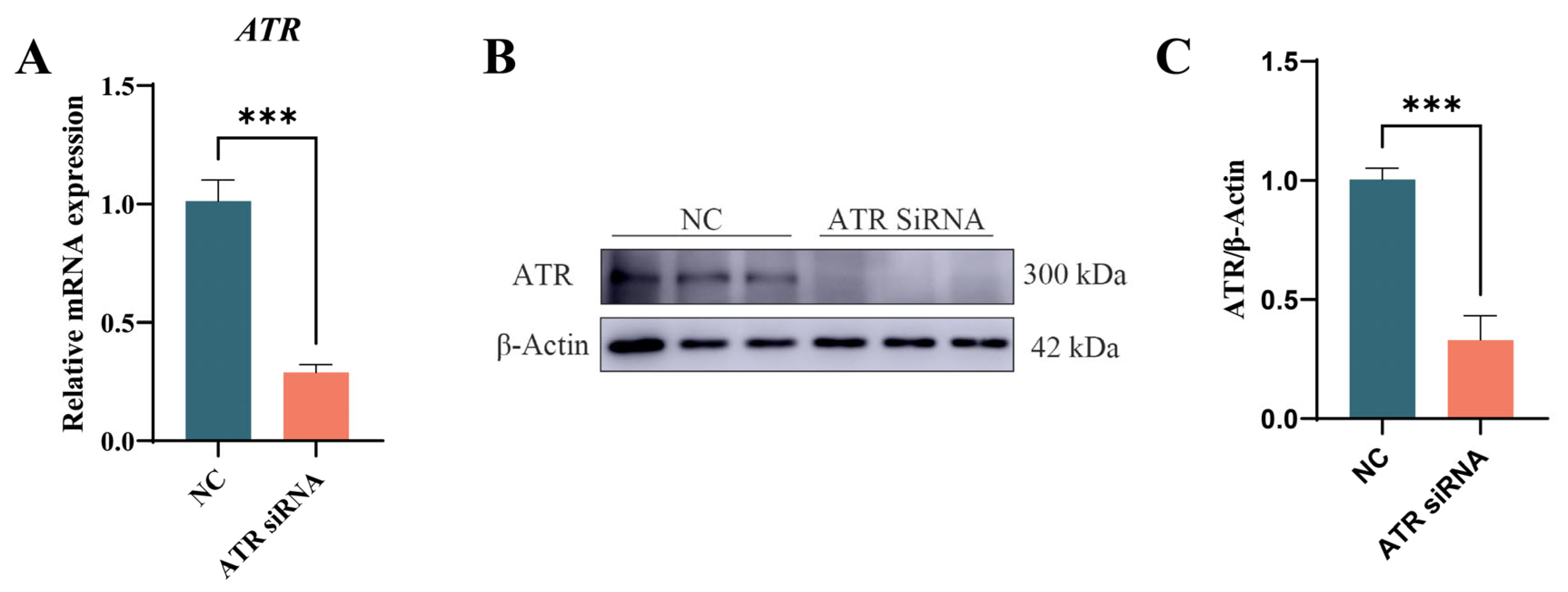
3.2. Verifying ATR Knockdown Efficiency in Primary BMECs
3.3. Effects of ATR Silencing on Senescence-Associated β-Galactosidase Expression and Cell Cycle in BMECs
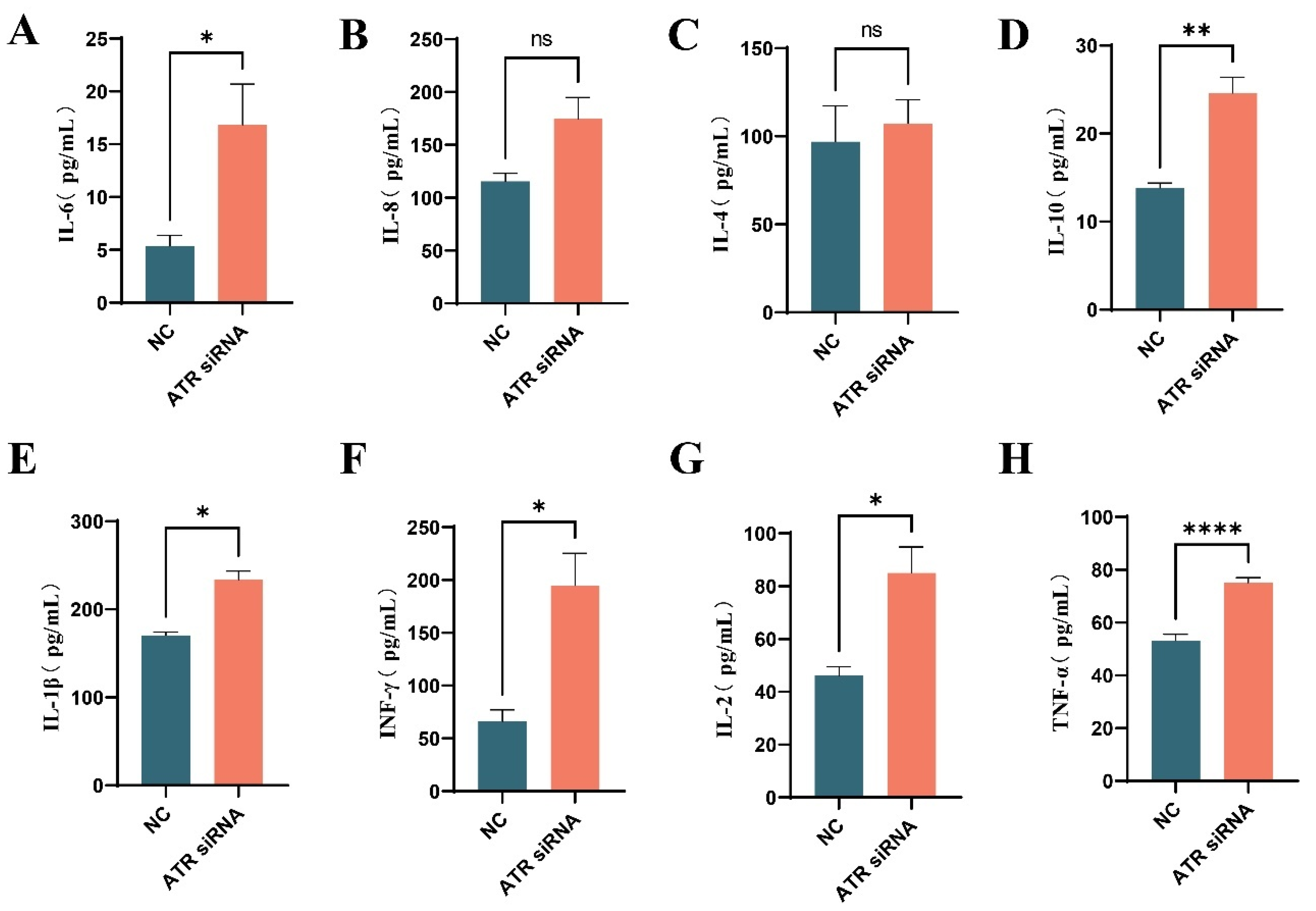
3.4. Effects of ATR Silencing on Senescence-Associated Secretory Phenotype (SASP) in BMECs
3.5. Effects of ATR Silencing on DNA Damage Repair and Apoptosis in BMECs
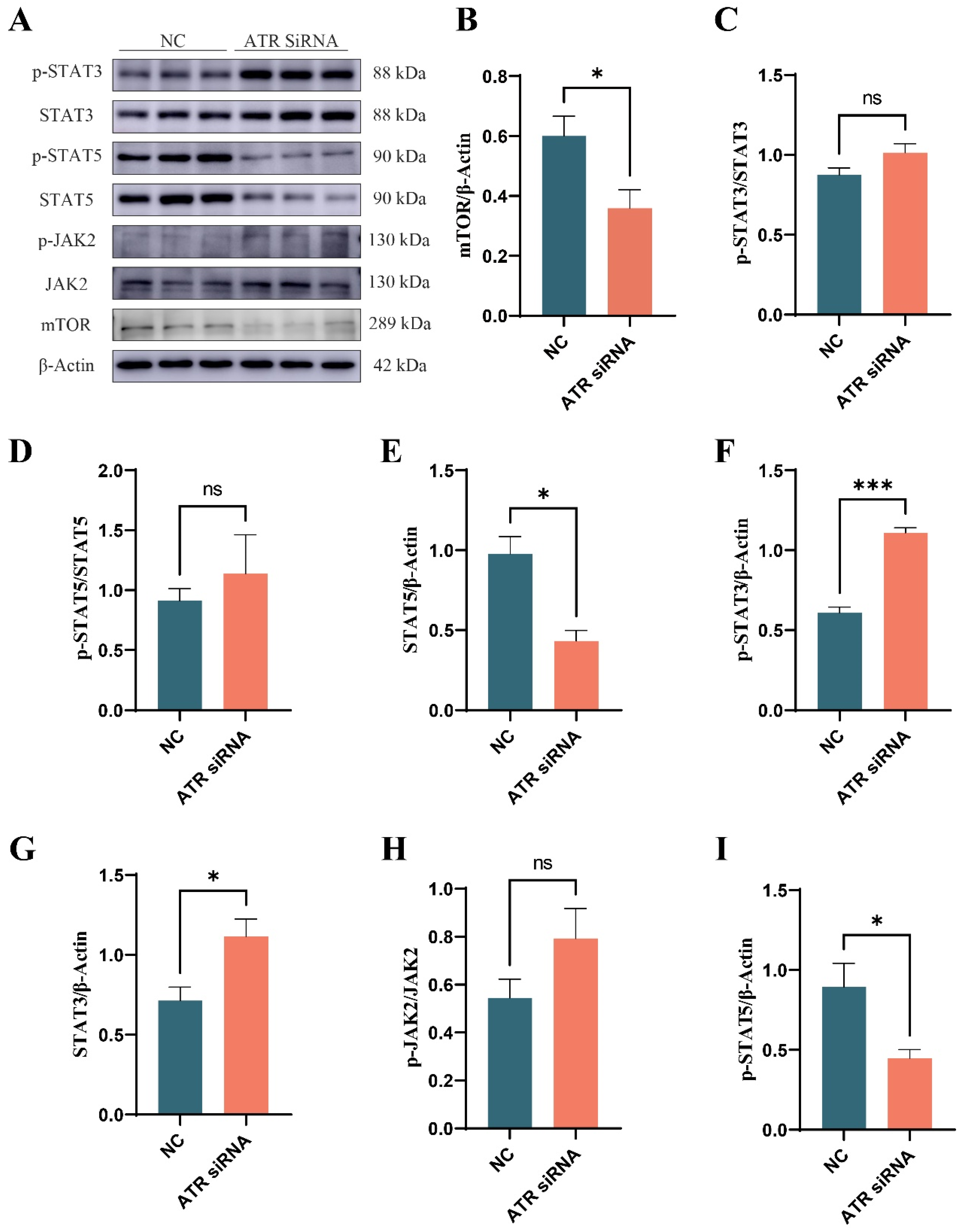
3.6. Effect of ATR Silencing on Lactation-Related Signaling Pathways in BMECs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Srivastava, V.; Huycke, T.R.; Phong, K.T.; Gartner, Z.J. Organoid models for mammary gland dynamics and breast cancer. Curr. Opin. Cell Biol. 2020, 66, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Periasamy, K.; Rodriguez-Zas, S.L.; Everts, R.E.; Lewin, H.A.; Hurley, W.L.; Loor, J.J. Old and new stories: Revelations from functional analysis of the bovine mammary transcriptome during the lactation cycle. PLoS ONE 2012, 7, e33268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Abbas, Z.; Hu, L.; Kang, L.; Tan, X.; Xu, Q.; Wang, Y. Extraction and Elevation of Cell-Free DNA under Mastitis and Heat Stress in Dairy Cattle. Animals 2023, 13, 1487. [Google Scholar] [CrossRef] [PubMed]
- Charrier, J.D.; Durrant, S.J.; Golec, J.M.; Kay, D.P.; Knegtel, R.M.; MacCormick, S.; Mortimore, M.; O’Donnell, M.E.; Pinder, J.L.; Reaper, P.M.; et al. Discovery of potent and selective inhibitors of ataxia telangiectasia mutated and Rad3 related (ATR) protein kinase as potential anticancer agents. J. Med. Chem. 2011, 54, 2320–2330. [Google Scholar] [CrossRef]
- Kim, H.; Aliar, K.; Tharmapalan, P.; McCloskey, C.W.; Kuttanamkuzhi, A.; Grünwald, B.T.; Palomero, L.; Mahendralingam, M.J.; Waas, M.; Mer, A.S.; et al. Differential DNA damage repair and PARP inhibitor vulnerability of the mammary epithelial lineages. Cell Rep. 2023, 42, 113256. [Google Scholar] [CrossRef]
- Maréchal, A.; Zou, L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 2006, 12, 440–450. [Google Scholar] [CrossRef]
- Joshi, P.A.; Jackson, H.W.; Beristain, A.G.; Di Grappa, M.A.; Mote, P.A.; Clarke, C.L.; Stingl, J.; Waterhouse, P.D.; Khokha, R. Progesterone induces adult mammary stem cell expansion. Nature 2010, 465, 803–807. [Google Scholar] [CrossRef]
- Huh, S.J.; Clement, K.; Jee, D.; Merlini, A.; Choudhury, S.; Maruyama, R.; Yoo, R.; Chytil, A.; Boyle, P.; Ran, F.A.; et al. Age- and pregnancy-associated DNA methylation changes in mammary epithelial cells. Stem Cell Rep. 2015, 4, 297–311. [Google Scholar] [CrossRef]
- Suryadevara, V.; Hudgins, A.D.; Rajesh, A.; Pappalardo, A.; Karpova, A.; Dey, A.K.; Hertzel, A.; Agudelo, A.; Rocha, A.; Soygur, B.; et al. SenNet recommendations for detecting senescent cells in different tissues. Nat. Rev. Mol. Cell Biol. 2024, 25, 1001–1023. [Google Scholar] [CrossRef]
- Sen, T.; Rodriguez, B.L.; Chen, L.; Corte, C.M.D.; Morikawa, N.; Fujimoto, J.; Cristea, S.; Nguyen, T.; Diao, L.; Li, L.; et al. Targeting DNA Damage Response Promotes Antitumor Immunity through STING-Mediated T-cell Activation in Small Cell Lung Cancer. Cancer Discov. 2019, 9, 646–661. [Google Scholar] [CrossRef]
- Toledo, L.I.; Altmeyer, M.; Rask, M.B.; Lukas, C.; Larsen, D.H.; Povlsen, L.K.; Bekker-Jensen, S.; Mailand, N.; Bartek, J.; Lukas, J. ATR prohibits replication catastrophe by preventing global exhaustion of RPA. Cell 2013, 155, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Bright, S.J.; Manandhar, M.; Flint, D.B.; Kolachina, R.; Ben Kacem, M.; Martinus, D.K.; Turner, B.X.; Qureshi, I.; McFadden, C.H.; Marinello, P.C.; et al. ATR inhibition radiosensitizes cells through augmented DNA damage and G2 cell cycle arrest abrogation. JCI Insight 2024, 9, e179599. [Google Scholar] [CrossRef] [PubMed]
- Kotsinas, A.; Aggarwal, V.; Tan, E.J.; Levy, B.; Gorgoulis, V.G. PIG3: A novel link between oxidative stress and DNA damage response in cancer. Cancer Lett. 2012, 327, 97–102. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, F.; Zhang, L. PARP1-DNA co-condensation: The driver of broken DNA repair. Signal Transduct. Target. Ther. 2024, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Swift, M.L.; Sell, C.; Azizkhan-Clifford, J. DNA damage-induced degradation of Sp1 promotes cellular senescence. Geroscience 2022, 44, 683–698. [Google Scholar] [CrossRef]
- Zada, D.; Sela, Y.; Matosevich, N.; Monsonego, A.; Lerer-Goldshtein, T.; Nir, Y.; Appelbaum, L. Parp1 promotes sleep, which enhances DNA repair in neurons. Mol. Cell 2021, 81, 4979–4993.E7. [Google Scholar] [CrossRef]
- Kołacz, K.; Robaszkiewicz, A. PARP1 at the crossroad of cellular senescence and nucleolar processes. Ageing Res. Rev. 2024, 94, 102206. [Google Scholar] [CrossRef]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef]
- Ovadya, Y.; Krizhanovsky, V. Senescent cells: SASPected drivers of age-related pathologies. Biogerontology 2014, 15, 627–642. [Google Scholar] [CrossRef]
- Hom, L.M.; Sun, S.; Campbell, J.; Liu, P.; Culbert, S.; Murphy, I.M.; Schafer, Z.T. A role for fibroblast-derived SASP factors in the activation of pyroptotic cell death in mammary epithelial cells. J. Biol. Chem. 2023, 299, 104922. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef]
- Liu, B.; Peng, Z.; Zhang, H.; Zhang, N.; Liu, Z.; Xia, Z.; Huang, S.; Luo, P.; Cheng, Q. Regulation of cellular senescence in tumor progression and therapeutic targeting: Mechanisms and pathways. Mol. Cancer 2025, 24, 106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yao, J.; Wu, D.; Huang, X.; Wang, Y.; Li, X.; Lu, Q.; Qiu, Y. Type 2 cytokine signaling in macrophages protects from cellular senescence and organismal aging. Immunity 2024, 57, 513–527.E6. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Shapiro, H.; Tsiobikas, C.; Selfors, L.M.; Chen, H.; Rosenbluth, J.; Moore, K.; Gupta, K.P.; Gray, G.K.; Oren, Y.; et al. Aging-Associated Alterations in Mammary Epithelia and Stroma Revealed by Single-Cell RNA Sequencing. Cell Rep. 2020, 33, 108566. [Google Scholar] [CrossRef]
- Sargeant, T.J.; Lloyd-Lewis, B.; Resemann, H.K.; Ramos-Montoya, A.; Skepper, J.; Watson, C.J. Stat3 controls cell death during mammary gland involution by regulating uptake of milk fat globules and lysosomal membrane permeabilization. Nat. Cell Biol. 2014, 16, 1057–1068. [Google Scholar] [CrossRef]
- Haricharan, S.; Li, Y. STAT signaling in mammary gland differentiation, cell survival and tumorigenesis. Mol. Cell Endocrinol. 2014, 382, 560–569. [Google Scholar] [CrossRef]
- Watson, C.J. Stat transcription factors in mammary gland development and tumorigenesis. J. Mammary Gland. Biol. Neoplasia 2001, 6, 115–127. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Ma, L.; Wang, J.; Guo, W.; Cheng, J.; Hu, G.; Fu, S.; Liu, J. Niacin stimulates EPH4EV mammary epithelial cell proliferation and mammary gland development in pubertal mice through activation of AKT/mTOR and ERK1/2 signaling pathways. Cell Tissue Res. 2021, 384, 313–324. [Google Scholar] [CrossRef]
- Danesh Pazhooh, R.; Rahnamay Farnood, P.; Asemi, Z.; Mirsafaei, L.; Yousefi, B.; Mirzaei, H. mTOR pathway and DNA damage response: A therapeutic strategy in cancer therapy. DNA Repair. 2021, 104, 103142. [Google Scholar] [CrossRef]



| Target Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| ATR | GATAGGTTGTGTTCTGCTAGAGTATGG | TGGCTGGTTGTGCTGGTAGTC |
| β-actin | TCTTCCAGCCTTCCTTCCTG | ACCGTGTTGGCGTAGAGGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Geng, Z.; Lian, S.; Wang, J.; Wu, R. ATR Deficiency Impairs DNA Damage Repair and Accelerates Cellular Senescence in Bovine Mammary Epithelial Cells, Leading to Lactation Dysfunction. Animals 2025, 15, 1419. https://doi.org/10.3390/ani15101419
Zhou Q, Geng Z, Lian S, Wang J, Wu R. ATR Deficiency Impairs DNA Damage Repair and Accelerates Cellular Senescence in Bovine Mammary Epithelial Cells, Leading to Lactation Dysfunction. Animals. 2025; 15(10):1419. https://doi.org/10.3390/ani15101419
Chicago/Turabian StyleZhou, Qijun, Zijian Geng, Shuai Lian, Jianfa Wang, and Rui Wu. 2025. "ATR Deficiency Impairs DNA Damage Repair and Accelerates Cellular Senescence in Bovine Mammary Epithelial Cells, Leading to Lactation Dysfunction" Animals 15, no. 10: 1419. https://doi.org/10.3390/ani15101419
APA StyleZhou, Q., Geng, Z., Lian, S., Wang, J., & Wu, R. (2025). ATR Deficiency Impairs DNA Damage Repair and Accelerates Cellular Senescence in Bovine Mammary Epithelial Cells, Leading to Lactation Dysfunction. Animals, 15(10), 1419. https://doi.org/10.3390/ani15101419





