Assessing the Effect of Polyethylene Microplastics in the Freshwater Leech Erpobdella johanssoni (Annelida, Hirudinida) Through Integrated Biomarkers and Histopathological Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
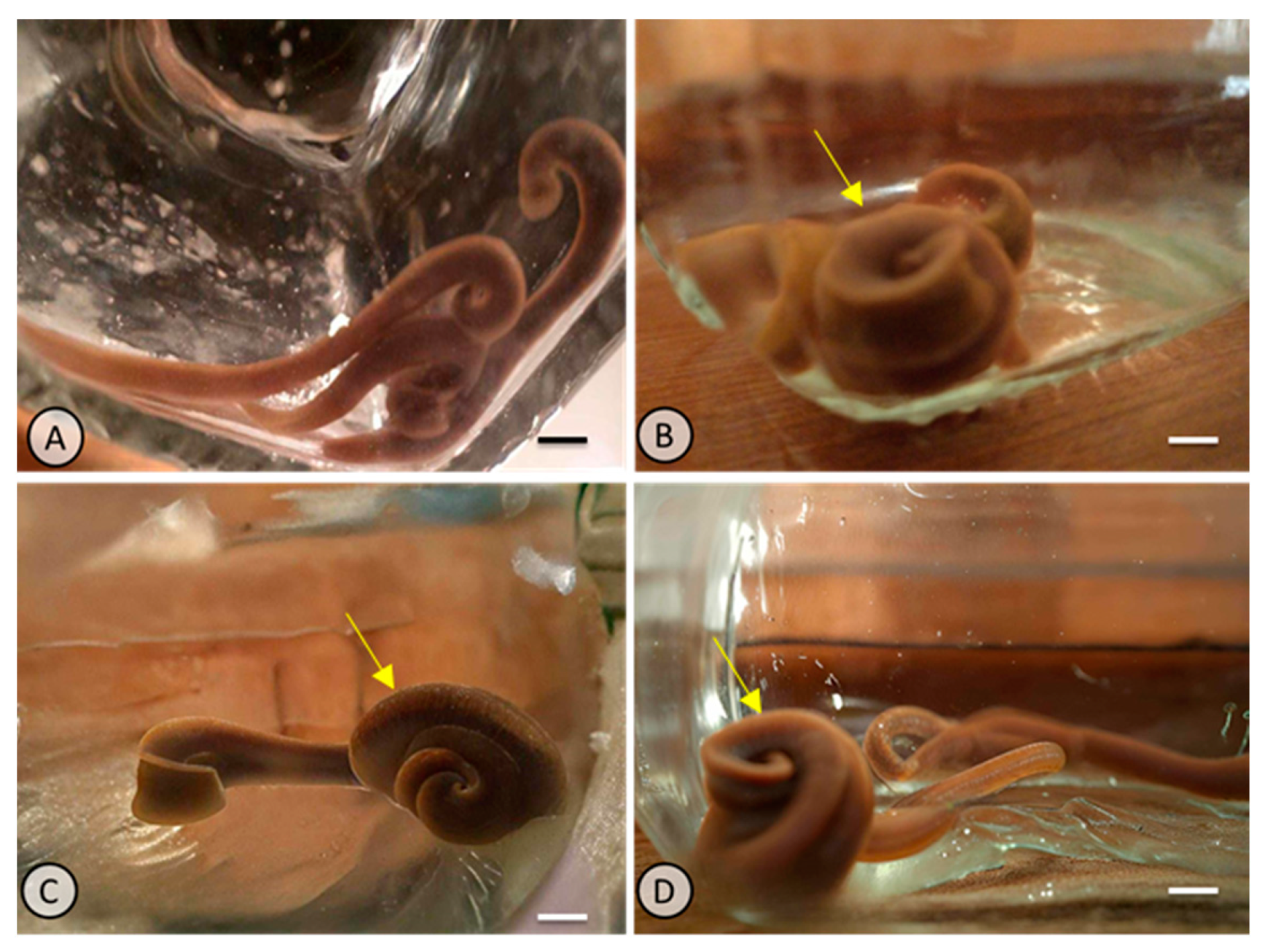
2.1. Experimental Exposure to PE-MPs
2.2. Measurement of Biochemical Parameters
2.3. Behavioral Assessment
2.4. Histological Staining
2.5. Statistical Analysis
3. Results
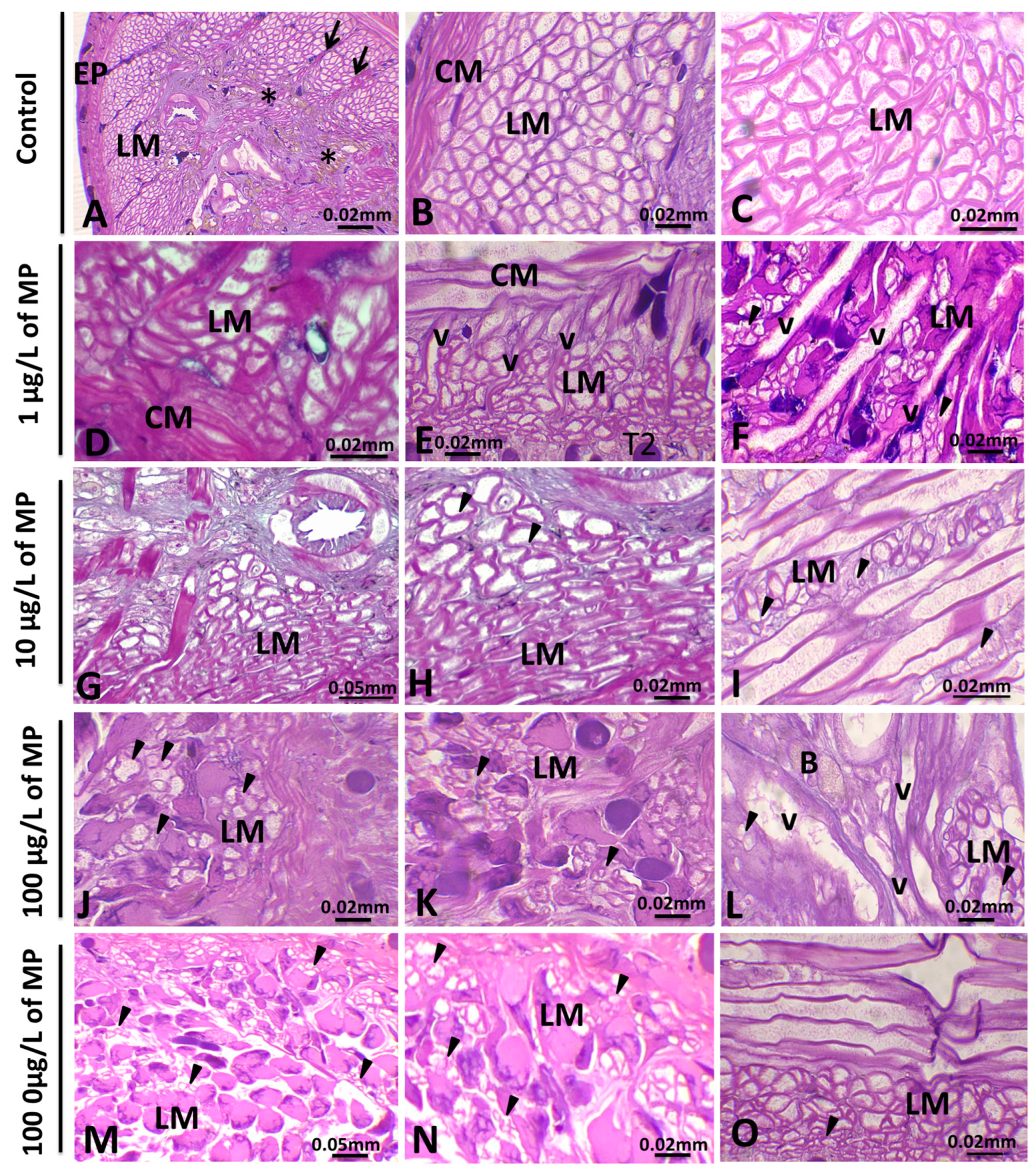
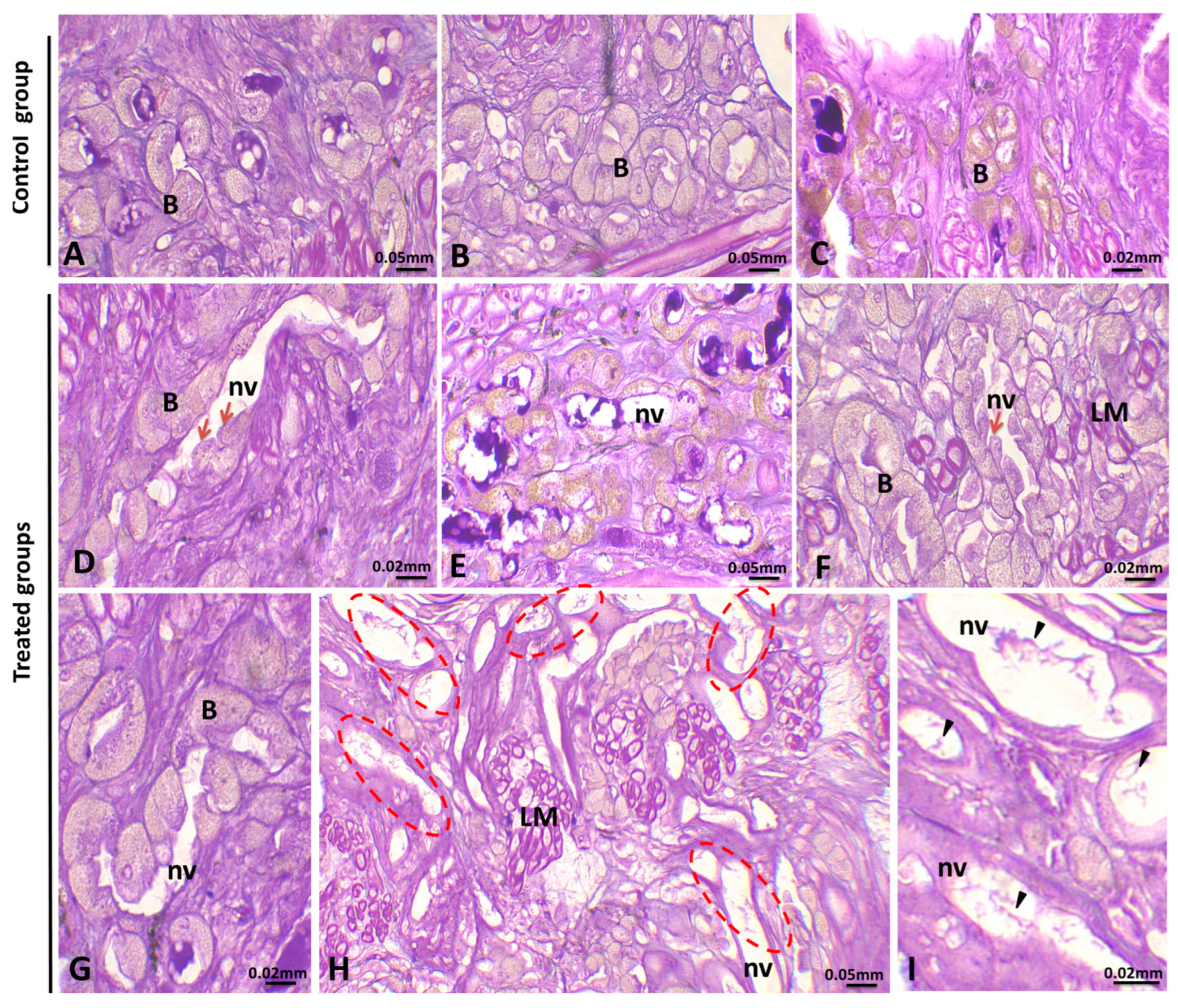
3.1. Histopathological Analysis
3.1.1. Alterations in the Body Wall
3.1.2. Alterations in Muscle Cells
3.1.3. Alterations in the Botryoidal Tissue
3.1.4. Alteration in the Ovary
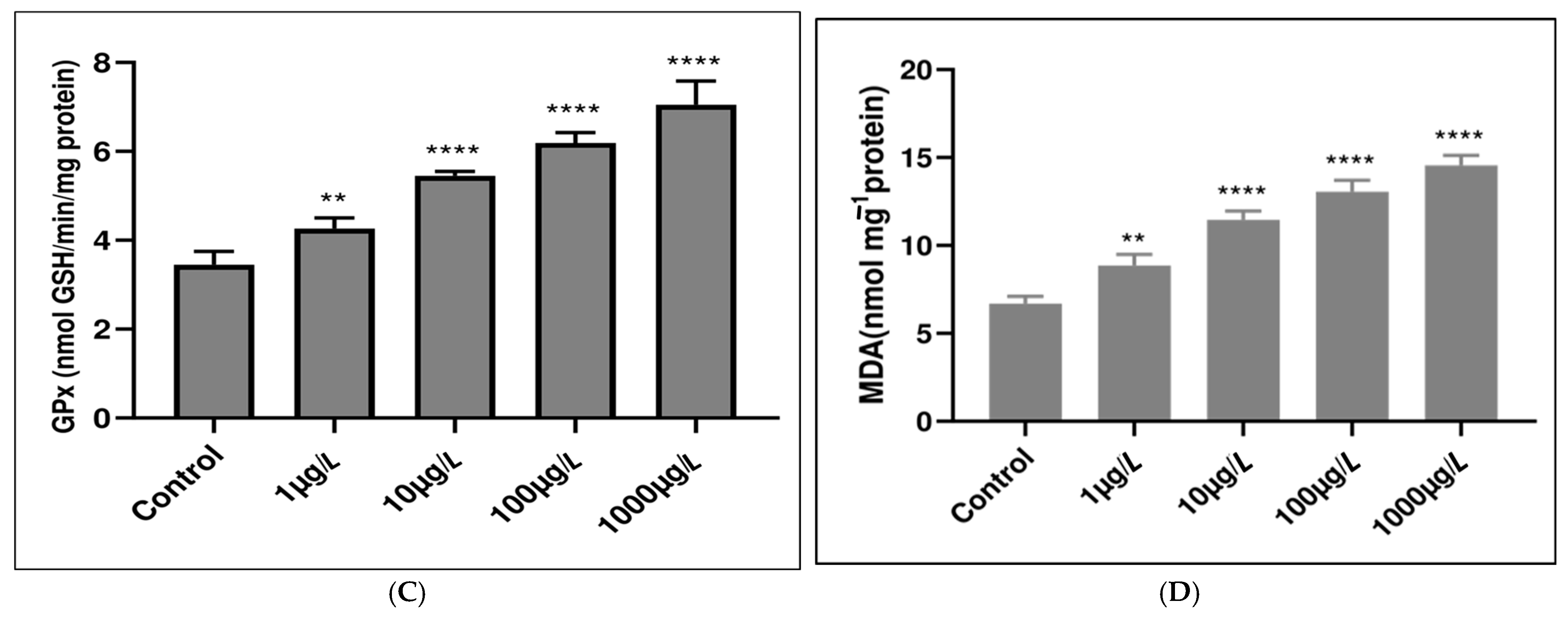
3.2. Enzymatic Antioxidant Responses
3.3. Nonenzymatic Antioxidant Responses
3.4. Behavioral Alteration
4. Discussion
4.1. Microplastic-Induced Tissue Damage
4.2. Histological Analysis of the Body Wall and Botryoidal Tissue of Untreated and PE-MPs-Exposed Leeches
4.3. Histological Analysis of Muscle Cells and Its Possible Relationship with Behavioral Alterations
4.4. Histological Analysis of the Female Gonad
4.5. Microplastic Impact on Antioxidant System Functionality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PE-MPs | Polyethylene microplastics |
| MPs | Microplastics |
| MDA | Malondialdehyde |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| GPx | Glutathione peroxidase |
References
- Sudhakar, M.; Trishul, A.; Doble, M.; Kumar, K.S.; Jahan, S.S.; Inbakandan, D.; Viduthalai, R.; Umadevi, V.; Murthy, P.S.; Venkatesan, R. Biofouling and biodegradation of polyolefins in ocean waters. Polym. Degrad. Stab. 2007, 92, 1743–1752. [Google Scholar] [CrossRef]
- Dowarah, K.; Patchaiyappan, A.; Thirunavukkarasu, C.; Jayakumar, S.; Devipriya, S.P. Quantification of microplastics using Nile Red in two bivalve species Perna viridis and Meretrix meretrix from three estuaries in Pondicherry, India and microplastic uptake by local communities through bivalve diet. Mar. Pollut. Bull. 2020, 153, 110982. [Google Scholar] [CrossRef]
- Saud, S.; Yang, A.; Jiang, Z.; Ning, D.; Fahad, S. New insights into the environmental behavior and ecological toxicity of microplastics. J. Hazard. Mater. Adv. 2023, 10, 100298. [Google Scholar] [CrossRef]
- Ma, M.; Xu, D.; Zhao, J.; Gao, B. Disposable face masks release microparticles to the aqueous environment after simulating sunlight aging: Microplastics or non-microplastics? J. Hazard. Mater. 2023, 443, 130146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Ding, G.; Shi, H.; Cong, Y.; Li, Z.; Wang, J. Polystyrene microplastics alleviate adverse effects of benzo[a]pyrene on tissues and cells of the marine mussel, Mytilus galloprovincialis. Aquat. Toxicol. 2023, 256, 106430. [Google Scholar] [CrossRef]
- Yin, Z.; Zhao, Y. Microplastics pollution in freshwater sediments: The pollution status assessment and sustainable management measures. Chemosphere 2023, 314, 137727. [Google Scholar] [CrossRef]
- Sun, J.; Yang, S.; Zhou, G.-J.; Zhang, K.; Lu, Y.; Jin, Q.; Lam, P.K.S.; Leung, K.M.Y.; He, Y. Release of microplastics from discarded surgical masks and their adverse impacts on the marine copepod Tigriopus japonicus. Environ. Sci. Technol. Lett. 2021, 8, 1065–1070. [Google Scholar] [CrossRef]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity, and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Kleppel, G.S. Policy: Classify plastic waste as hazardous. Science 2013, 339, 1022–1023. [Google Scholar] [CrossRef]
- Gouin, T.; Roche, N.; Vasseur, P.; Lagarde, F. Microplastics in the marine environment: Impacts of microplastics on aquatic organisms. Environ. Toxicol. Chem. 2015, 34, 2021–2032. [Google Scholar] [CrossRef]
- Jemec, A.; Štrus, J.; Fink, P. Microplastic pollution and its effects on aquatic organisms. Environ. Sci. Pollut. Res. 2016, 23, 24712–24723. [Google Scholar] [CrossRef]
- Rist, S.; Lusher, A.L.; Hurley, R.R.; Thompson, R.C. Microplastics in aquatic organisms: A review of the impacts on health and behavior. Environ. Pollut. 2018, 239, 624–637. [Google Scholar] [CrossRef]
- Wong, K.; Lee, K.; Kuok Ho, D.T.; Yap, P.S. Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions. Sci. Total Environ. 2020, 719, 137512. [Google Scholar] [CrossRef]
- Zitouni, N.; Bousserrhine, N.; Belbekhouche, S.; Missawi, O.; Alphonse, V.; Boughatass, I.; Banni, M. First report on the presence of small microplastics (≤3 μm) in tissue of the commercial fish Serranus scriba (Linnaeus, 1758) from Tunisian coasts and associated cellular alterations. Environ. Pollut. 2020, 263, 114576. [Google Scholar] [CrossRef]
- Zitouni, N.; Bousserrhine, N.; Missawi, O.; Boughattas, I.; Chèvre, N.; Santos, R.; Belbekhouche, S.; Alphonse, V.; Tisserand, F.; Balmassiere, L.; et al. Uptake, tissue distribution and toxicological effects of environmental microplastics in early juvenile fish Dicentrarchus labrax. J. Hazard. Mater. 2021, 403, 124055. [Google Scholar] [CrossRef] [PubMed]
- Blettler, M.C.; Abrial, E.; Khan, F.R.; Sivri, N.; Espinola, L.A. Freshwater plastic pollution: Recognizing research biases and identifying knowledge gaps. Water Res. 2018, 143, 416–424. [Google Scholar] [CrossRef]
- Erkes-Medrano, D.; Thompson, R.; Aldridge, D. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritization of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- De Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Smith, J.A.; Brown, K.P.; Taylor, L.M. Microplastic pollution in freshwater systems: A review of contamination sources, accumulation, and impacts on aquatic biota. Environ. Pollut. 2022, 295, 118679. [Google Scholar] [CrossRef]
- Johnson, R.K.; Green, D.L.; Martinez, P. Assessing microplastic distribution and ecological risks in riverine environments. Sci. Total Environ. 2023, 857, 159545. [Google Scholar] [CrossRef]
- Moore, C.J.; Lattin, G.L.; Zellers, A. Quantity and type of plastic debris flowing from two urban rivers to coastal waters and beaches of Southern California. Rev. Gestão Costeira Integr. J. Integr. Coast. Zone Manag. 2011, 11, 65–73. [Google Scholar] [CrossRef]
- Panno, S.V.; Hackley, K.C.; Hwang, H.H.; Greenberg, S.E.; Krapac, I.G.; Landsberger, S.; O’Kelly, D.J. Microplastic contamination in karst groundwater systems. Groundwater 2019, 57, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Goeppert, N.; Goldscheider, N. Experimental field evidence for transport of microplastic tracers over large distances in an alluvial aquifer. J. Hazard. Mater. 2021, 408, 124844. [Google Scholar] [CrossRef]
- Toumi, H.; Abidli, S.; Bejaoui, M.; Lahbib, Y. First evaluation of microplastics in surface and deep sediments of the southern Mediterranean Sea: The case of Tunisia. Mar. Pollut. Bull. 2019, 136, 265–273. [Google Scholar] [CrossRef]
- Cha, J.; Lee, J.-Y.; Chia, R.W. Microplastics contamination and characteristics of agricultural groundwater in Haean Basin of Korea. Sci. Total Environ. 2023, 864, 161027. [Google Scholar] [CrossRef]
- Shu, X.; Xu, L.; Yang, M.; Qin, Z.; Zhang, Q.; Zhang, L. Spatial distribution characteristics and migration of microplastics in surface water, groundwater and sediment in karst areas: The case of Yulong River in Guilin, Southwest China. Sci. Total Environ. 2023, 868, 161578. [Google Scholar] [CrossRef]
- Abidli, S.; Lahbib, Y.; Trigui El Menif, N. Microplastics in commercial molluscs from Tunisia: Assessment of the contamination and effect of digestive processes. Environ. Sci. Pollut. Res. 2017, 24, 13499–13507. [Google Scholar] [CrossRef]
- Toumi, H.; Abidli, S.; Bejaoui, M. Microplastics in freshwater environment: The first evaluation in sediments from seven water streams surrounding the lagoon of Bizerte (Northern Tunisia). Environ. Sci. Pollut. Res. 2019, 26, 14673–14682. [Google Scholar] [CrossRef]
- Fischer, E.K.; Scholz-Böttcher, B.M. Microplastics in sediments of the River Thames Basin, UK—Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2016, 113, 1–6. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Kühn, S.; Van Oyen, A.; Booth, A.M.; Meijboom, A.; Van Franeker, J.A. Marine microplastic: Preparation of relevant test materials for laboratory assessment of ecosystem impacts. Chemosphere 2018, 213, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Abidli, S.; Lahbib, Y.; El Menif, N.T. Microplastics in commercial molluscs from the lagoon of Bizerte (Northern Tunisia). Mar. Pollut. Bull. 2019, 142, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2011, 45, 2843–2849. [Google Scholar] [CrossRef]
- Abidli, S.; Akkari, N.; Lahbib, Y.; El Menif, N.T. First evaluation of microplastics in two commercial fish species from the lagoons of Bizerte and Ghar El Melh (Northern Tunisia). Reg. Stud. Mar. Sci. 2021, 41, 101581. [Google Scholar] [CrossRef]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Scrimgeour, G.J.; Wicklum, D.; Pruss, S.D. Selection of an aquatic indicator species to monitor organic contaminants in trophically simple lotic food webs. Arch. Environ. Contam. Toxicol. 1998, 35, 565–572. [Google Scholar] [CrossRef]
- Koperski, P. Testing the suitability of leeches (Hirudinea, Clitellata) for biological assessment of lowland streams. Pol. J. Ecol. 2005, 53, 65–80. [Google Scholar]
- Mihaljević, Z.; Ternjej, I.; Stanković, I.; Ivković, M.; Želježić, D.; Mladinić, M.; Kopjar, N. Assessment of genotoxic potency of sulfate-rich surface waters on medicinal leech and human leukocytes using different versions of the Comet assay. Ecotoxicol. Environ. Saf. 2011, 74, 1416–1426. [Google Scholar] [CrossRef]
- Khaled, I.; Ferjani, H.; Ben Ahmed, R.; Harrath, A.H. Effect of oil-related environmental pollutants on gonads of the freshwater leech Limnatis nilotica (Annelida, Hirudinea). Invertebr. Reprod. Dev. 2016, 60, 263–270. [Google Scholar] [CrossRef]
- Macova, S.; Harustiakova, D.; Kolarova, J.; Machova, J.; Zlabek, V.; Vykusova, B.; Randak, T.; Velisek, J.; Poleszczuk, G.; Hajslova, J. Leeches as sensor-bioindicators of river contamination by PCBs. Sensors 2009, 9, 1807–1820. [Google Scholar] [CrossRef]
- Khaled, I.; Ferjani, H.; Sirotkin, A.V.; Alwasel, S.; Harrath, A.H. Impact of oil-related environmental pollutants on the ovary structure in the freshwater leech Erpobdella johanssoni (Johansson, 1927) (Clitellata: Hirudinea). Eur. Zool. J. 2017, 84, 286–293. [Google Scholar] [CrossRef]
- Khaled, I.; Saidi, I.; Ferjani, H.; Ahmed, R.B.; Alrezaki, A.; Guesmi, F.; Bouzenna, H.; Harrath, A.H. BTEX induces histopathological alterations, oxidative stress response and DNA damage in the testis of the freshwater leech Erpobdella johanssoni (Johansson, 1927). J. King Saud Univ. Sci. 2022, 34, 102196. [Google Scholar] [CrossRef]
- Ben Ahmed, R.; Tekaya, S.; Małota, K.; Świątek, P. An ultrastructural study of the ovary cord organization and oogenesis in Erpobdella johanssoni (Annelida, Clitellata: Hirudinida). Micron 2013, 44, 275–286. [Google Scholar] [CrossRef]
- Eriksen, M.; Cowger, W.; Erdle, L.M.; Coffin, S.; Villarrubia-Gómez, P.; Moore, C.J.; Carpenter, E.J.; Day, R.H.; Thiel, M.; Wilcox, C. A growing plastic smog, now estimated to be over 170 trillion plastic particles afloat in the world’s oceans—Urgent solutions required. PLoS ONE 2023, 18, e0281596. [Google Scholar] [CrossRef] [PubMed]
- Eckhart, L.; Lippens, S.; Tschachler, E.; Declercq, W. Cell death by cornification. Biochimica Biophysica Acta (BBA) Mol. Cell Res. 2013, 1833, 3471–3480. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Gül, M.U.; Çakıcı, Ö. Comparison of body wall histologic structure of two medicinal leeches Hirudo sulukii and Hirudo verbana (Hirudinida: Hirudinidae). Cell Tissue Res. 2022, 387, 75–84. [Google Scholar] [CrossRef]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(nano)plastics: A threat to human health? Curr. Opin. Environ. Sci. Health 2019, 1, 17–23. [Google Scholar] [CrossRef]
- Abidli, S.; Pinheiro, M.; Lahbib, Y.; Neuparth, T.; Santos, M.M.; Trigui El Menif, N. Effects of environmentally relevant levels of polyethylene microplastic on Mytilus galloprovincialis (Mollusca: Bivalvia): Filtration rate and oxidative stress. Environ. Sci. Pollut. Res. 2021, 28, 26643–26652. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H.; Wyss, S.R.; Scherz, B.; Skbaril, F. Heterogeneity of Erythrocyte Catalase II. Eur. J. Biochem. 1974, 48, 137–145. [Google Scholar] [CrossRef]
- Beyer, J.W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar]
- Gutteridge, J.M.C.; Halliwell, B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem. Sci. 1990, 15, 129–135. [Google Scholar] [CrossRef]
- Akinsanya, B.; Isibor, P.O.; Kuton, M.P.; Saliu, J.K.; Dada, E.O. Aspidogastrea africanus Infections, comparative assessment of BTEX and heavy metals bioaccumulation, and histopathological alterations as biomarker response in Chrysichthys nigrodigitatus (Lacépède, 1803) of Lekki Lagoon, Nigeria. Sci. Afr. 2019, 3, e00060. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Y.; Wang, D.; Teng, M.; Yan, J.; Yan, S.; Meng, Z.; Li, R.; Zhou, Z.; Zhu, W. The effects of hexaconazole and epoxiconazole enantiomers on metabolic profile following exposure to zebrafish (Danio rerio) as well as the histopathological changes. Chemosphere 2019, 226, 520–533. [Google Scholar] [CrossRef]
- Zhang, H.; Hong, X.; Yan, S.; Zha, J.; Qin, J. Environmentally relevant concentrations of bifenthrin induce changes in behaviour, biomarkers, histological characteristics, and the transcriptome in Corbicula fluminea. Sci. Total Environ. 2020, 728, 138821. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Rahemo, Z.I. Studies on the histology of the body wall of two species of leeches, Erpobdella octoculata and Haemopis sanguisuga. Adv. J. Biol. Sci. Res. 2013, 1, 1–7. [Google Scholar]
- Khaled, I.; Ahmed, R.B.; Saidi, I.; Pacioglu, O.; Harrath, A. Cadmium exposure induced oxidative stress and histopathological disruption in the body wall of the freshwater leech Limnatis nilotica (Savigny, 1822). Invertebr. Surv. J. 2023, 44–54. [Google Scholar] [CrossRef]
- Anilkumar, U.; Jadhav, A.S. Light microscopy and ultrastructure of body wall in leech Haemadipsa zeylanica. J. Microsc. Ultrastruct. 2023, 11, 81–86. [Google Scholar] [CrossRef]
- Sayers, C.; Coleman, J.; Shain, D. Cell dynamics during cocoon secretion in the aquatic leech, Theromyzon tessulatum (Annelida: Clitellata: Glossiphoniidae). Tissue Cell 2009, 41, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Gorgees, N.; Rashan, L. Histomorphological and histochemical studies on the epidermis of the lumbricid worm, Dendrobaena atheca Cernosvitov. Z. Mikrosk.-anat. Forsch. 1982, 96, 1078–1088. [Google Scholar]
- Morris, G. The cocoon-producing cells of Eisenia foetida (Annelida, Oligochaeta): A histochemical and ultrastructural study. J. Morphol. 1983, 177, 41–50. [Google Scholar] [CrossRef]
- Baranzini, N.; Pulze, L.; Bon, C.; Izzo, L.; Pragliola, S.; Venditto, V.; Grimaldi, A. Hirudo verbana as a freshwater invertebrate model to assess the effects of polypropylene micro and nanoplastics dispersion in freshwater. Fish Shellfish Immunol. 2022, 127, 492–507. [Google Scholar] [CrossRef]
- Khaled, I.; Saidi, I.; Ben Ahmed, R.; Amari, R.; Aldahmash, W.; Pacioglu, O.; Hfaiedh, N.; Harrath, A.H. Cadmium exposure induces testicular oxidative damage and histopathological changes in the freshwater leech Limnatis nilotica (Savigny, 1822): The protective role of salicylic acid. Afr. J. Aquat. Sci. 2023, 48, 189–198. [Google Scholar] [CrossRef]
- Kutlu, M.; Tanatmış, M.; İşcan, A.; Ertorun, N.; Çalım, M. Effect of copper on the body wall structures of the medicinal leech Hirudo verbana Carena, 1820. Fresenius Environ. Bull. 2010, 19, 1186–1190. [Google Scholar]
- Frolov, A.K.; Litvinenko, R.A. Basic morphofunctional features of pharmaceutic leech (Hirudo verbana Carena, 1820) tissues in various forms of response after hirudotherapeutic procedures. Ann. Parasitol. 2015, 61, 27–35. [Google Scholar]
- Girardello, R.; Drago, F.; de Eguileor, M.; Valvassori, R.; Vizioli, J.; Tettamanti, G.; Grimaldi, A. Cytokine impregnated biomatrix: A new tool to study multi-wall carbon nanotubes effects on invertebrate immune cells. J. Nanomed. Nanotechnol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Girardello, R.; Tasselli, S.; Baranzini, N.; Valvassori, R.; de Eguileor, M.; Grimaldi, A. Effects of carbon nanotube environmental dispersion on an aquatic invertebrate, Hirudo medicinalis. PLoS ONE 2015, 10, e0144361. [Google Scholar] [CrossRef]
- Prasad, S. Life of Invertebrates; Vikas Publishing House: New Delhi, India, 1980. [Google Scholar]
- Bergter, A.; Hunnekuhl, V.S.; Schniederjans, M.; Paululat, A. Evolutionary aspects of pattern formation during clitellate muscle development. Evol. Dev. 2007, 9, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Kristan Jr, W.B.; Skalak, R.; Wilson, R.J.; Skierczynski, B.A.; Murray, J.A.; Eisenhart, F.J.; Cacciatore, T.W.; Chiel, H.J.; Beer, R.D. Biomechanics of hydroskeletons: Studies of crawling in the medicinal leech. In Biomechanics and Neural Control of Posture and Movement; Springer: New York, NY, USA, 2000; pp. 206–220. [Google Scholar]
- Petrauskienė, L. Water and sediment toxicity assessment by use of behavioural responses of medicinal leeches. Environ. Int. 2003, 28, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Jason, E. Effects of copper sulfate exposure on the nervous system of the Hirudo verbana leech. Emerg. Investig. 2023, 6, 1–9. [Google Scholar]
- Zink, L.; Wiseman, G.P. Single and combined effects of cadmium, microplastics, and their mixture on whole-body serotonin and feeding behaviour following chronic exposure and subsequent recovery in the freshwater leech, Nephelopsis obscura. Aquat. Toxicol. 2023, 259, 106538. [Google Scholar] [CrossRef]
- Zink, L.; Wiseman, G.P. Effects of microplastics on the freshwater leech Nephelopsis obscura: Implications for environmental health assessments. Environ. Toxicol. Chem. 2021, 40, 1534–1545. [Google Scholar]
- Ben Ahmed, R.; Tekaya, S.; Ben Ahmed, S.; Tekaya, S.; Gorzel, M.; Harrath, A.H. Erpobdellid leeches (Annelida, Clitellata, Hirudinida) from Tunisia: New records with the description of a new Trocheta species. Zootaxa 2013, 3682, 431–441. [Google Scholar]
- Qiang, L.; Cheng, J. Exposure to polystyrene microplastics impairs gonads of zebrafish (Danio rerio). Chemosphere 2021, 263, 128161. [Google Scholar] [CrossRef]
- Laily, A.N.; Fadjar, M.; Kilawati, Y. Effect of Microplastic Exposures to The Male Gonad Histology of Catfish (Clarias gariepinus). J. Aquacult. Fish Health 2023, 12, 94–104. [Google Scholar] [CrossRef]
- Vaschenko, M.A.; Hsieh, H.-L.; Radashevsky, V.I. Gonadal state of the oyster Crassostrea angulata cultivated in Taiwan. J. Shellfish Res. 2013, 32, 471–482. [Google Scholar] [CrossRef]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 0116. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Lewis, C. Marine microplastics spell big problems for future generations. Proc. Natl. Acad. Sci. USA 2016, 113, 2331–2333. [Google Scholar] [CrossRef]
- Prokić, M.D.; Petrović, T.G.; Gavrić, J.P.; Despotović, S.G.; Gavrilović, B.R.; Radovanović, T.B.; Faggio, C.; Saičić, Z.S. Comparative assessment of the antioxidative defense system in subadult and adult anurans: A lesson from the Bufotes viridis toad. Zoology 2018, 130, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Abidli, S.; Lahbib, Y.; Santos, M.M.; Trigui El Menif, N. Microplastic ingestion by commercial fish from the Mediterranean Sea around Tunisia. Mar. Pollut. Bull. 2021, 164, 112097. [Google Scholar]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Pagano, M.; Alampi, R.; Vazzana, I.; Felice, M.R. Cytotoxicity, haemolymphatic parameters, and oxidative stress following exposure to sub-lethal concentrations of quaternium-15 in Mytilus galloprovincialis. Aquat. Toxicol. 2016, 180, 258–265. [Google Scholar] [CrossRef]
- Chetoui, I.; Bejaoui, S.; Fouzai, C.; Trabelsi, W.; Nechi, S. Effect of lead graded doses in Mactra corallina gills: Antioxidants status, cholinergic function and histopathological studies. J. Drug Des. Med. Chem. 2019, 5, 1–9. [Google Scholar]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Guéraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef]
- Vlahogianni, T.; Dassenakis, M.; Scoullos, M.J.; Valavanidis, A. Integrated use of biomarkers (superoxide dismutase, catalase and lipid peroxidation) in mussels Mytilus galloprovincialis for assessing heavy metals’ pollution in coastal areas from the Saronikos Gulf of Greece. Mar. Pollut. Bull. 2007, 54, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, S.; Michán, C.; Telahigue, K.; Nechi, S.; El Cafsi, M.; Soudani, N.; Blasco, J.; Costa, P.M.; Alhama, J. Metal body burden and tissue oxidative status in the bivalve Venerupis decussata from Tunisian coastal lagoons. Mar. Environ. Res. 2020, 159, 105000. [Google Scholar] [CrossRef] [PubMed]
- Varó, I.; Perini, A.; Torreblanca, A.; Garcia, Y.; Bergami, E.; Vannuccini, M.L.; Corsi, I. Time-dependent effects of polystyrene nanoparticles in brine shrimp Artemia franciscana at physiological, biochemical and molecular levels. Sci. Total Environ. 2019, 675, 570–580. [Google Scholar] [CrossRef]
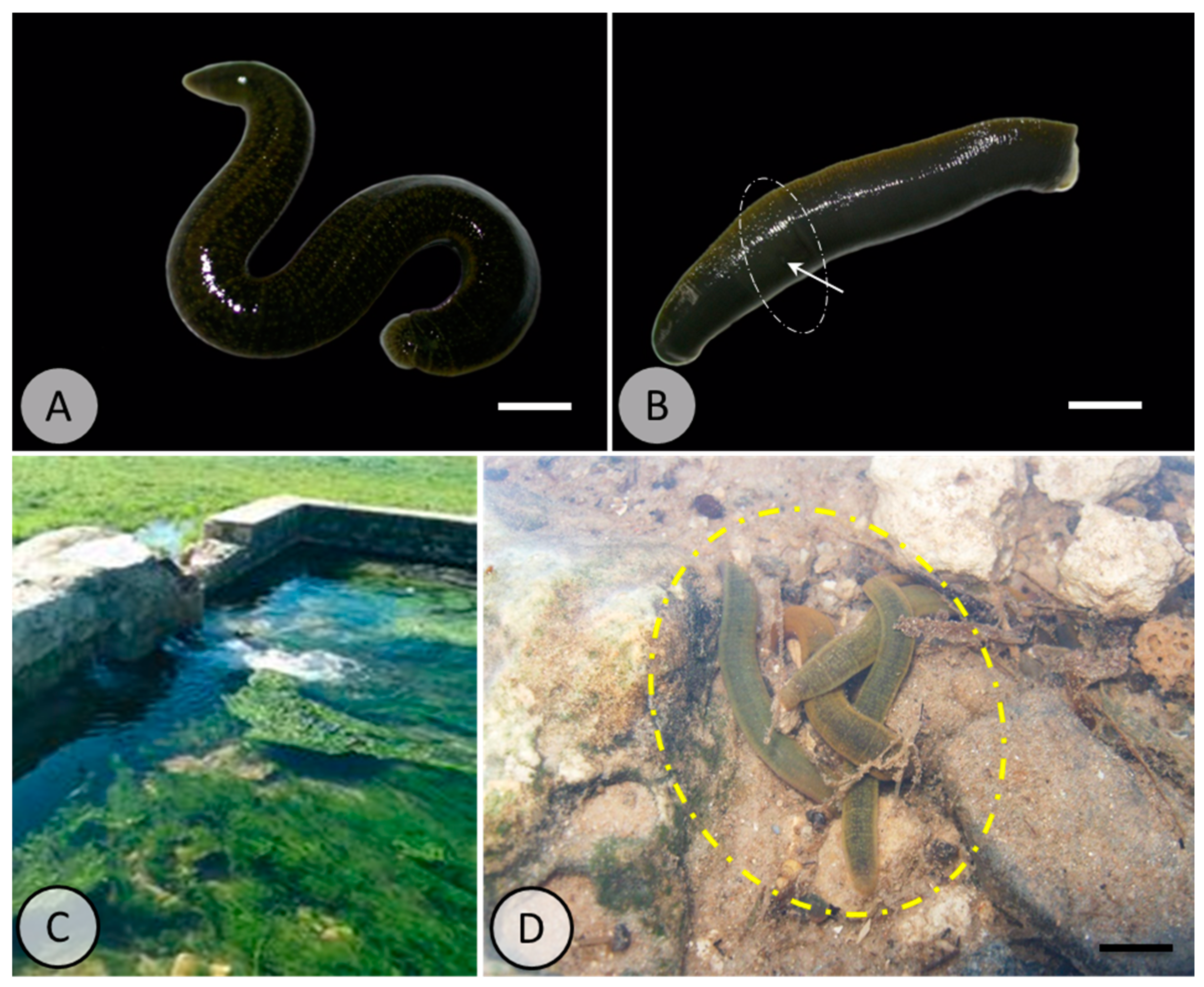



| Study Location | Matrix Type | Average Concentration | Size Range (μm) | Dominant Polymer Types | Reference |
|---|---|---|---|---|---|
| Seine River, France | Water | 100.6 ± 99.9 fibers/m3 | 50–5000 | PET | [29] |
| Teltow Canal, Germany | Water | 7.86 ± 7.26 MPs/L | 450–5000 | PE, PP | [29] |
| Carpathian Basin, Hungary | Water | 3.52–32.05 particles/m3 | 100–2000 | PP, PE, PS | [30] |
| Lake Bolsena, Italy | Water | 0.82–4.42 MPs/m3 | >300 | Not specified | [29] |
| River Thames, UK | Sediment | 33.2 ± 16.1 particles/100 g | 1000–5000 | PP, PET | [31] |
| Jedara Stream, Tunisia | Sediment | 6920 ± 395.98 items/kg | 200–5000 | PP, PE | [28] |
| Khima Stream, Tunisia | Sediment | 2340 ± 227.15 items/kg | 200–5000 | PP, PE | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Ahmed, R.; Khaled, I.; El Ayari, T.; Saidi, I.; Harrath, A.H. Assessing the Effect of Polyethylene Microplastics in the Freshwater Leech Erpobdella johanssoni (Annelida, Hirudinida) Through Integrated Biomarkers and Histopathological Analysis. Animals 2025, 15, 1417. https://doi.org/10.3390/ani15101417
Ben Ahmed R, Khaled I, El Ayari T, Saidi I, Harrath AH. Assessing the Effect of Polyethylene Microplastics in the Freshwater Leech Erpobdella johanssoni (Annelida, Hirudinida) Through Integrated Biomarkers and Histopathological Analysis. Animals. 2025; 15(10):1417. https://doi.org/10.3390/ani15101417
Chicago/Turabian StyleBen Ahmed, Raja, Ichrak Khaled, Tahani El Ayari, Issam Saidi, and Abdel Halim Harrath. 2025. "Assessing the Effect of Polyethylene Microplastics in the Freshwater Leech Erpobdella johanssoni (Annelida, Hirudinida) Through Integrated Biomarkers and Histopathological Analysis" Animals 15, no. 10: 1417. https://doi.org/10.3390/ani15101417
APA StyleBen Ahmed, R., Khaled, I., El Ayari, T., Saidi, I., & Harrath, A. H. (2025). Assessing the Effect of Polyethylene Microplastics in the Freshwater Leech Erpobdella johanssoni (Annelida, Hirudinida) Through Integrated Biomarkers and Histopathological Analysis. Animals, 15(10), 1417. https://doi.org/10.3390/ani15101417







