Artificial Induction of Meiotic Gynogenesis in Koi Carp Using Blunt Snout Bream Sperm and Identification of Gynogenetic Offspring
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Induction of Gynogenetic Offspring
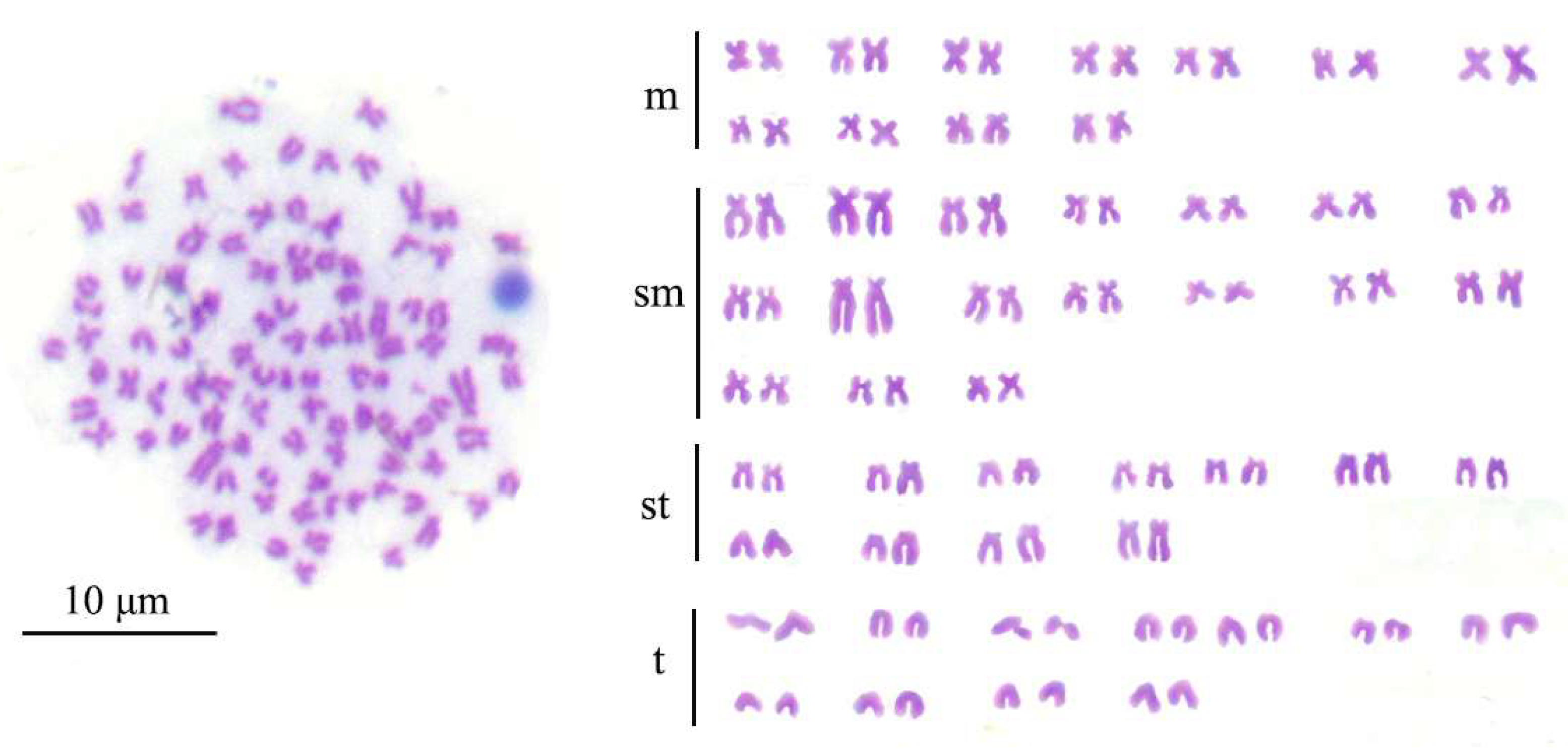
2.3. Karyotyping of Induced Offspring (IO)
2.4. Measurement of DNA Content
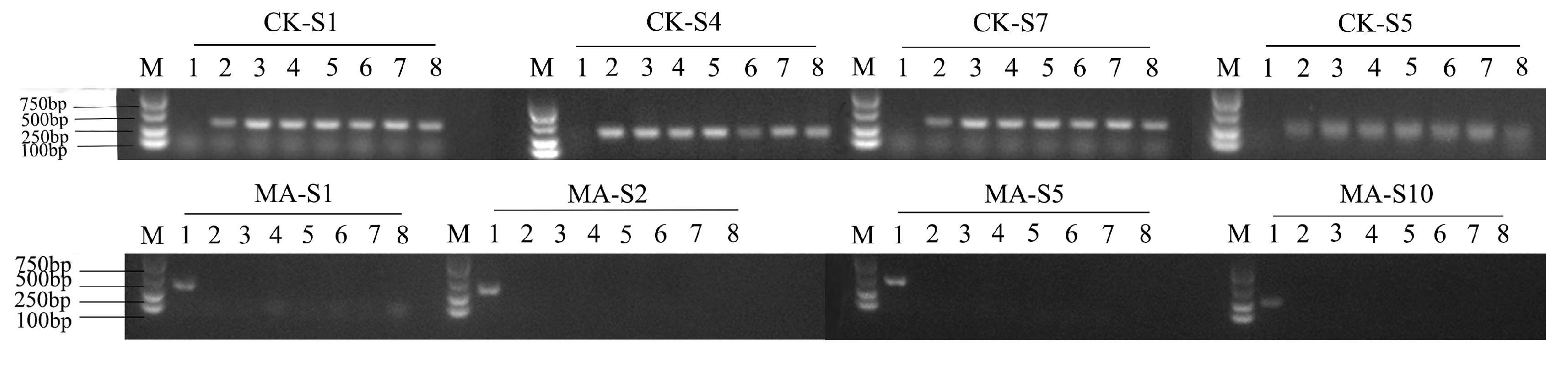
2.5. Microsatellite DNA Analysis
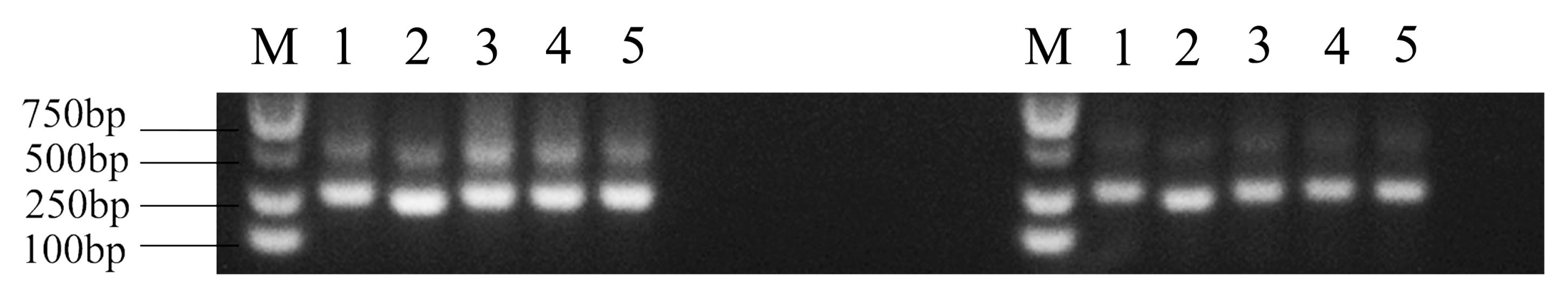
2.6. Amplification of 5S rDNA
2.7. Sex Identification of IO
3. Results
3.1. Number of Surviving Offsprings
3.2. Chromosome Numbers and Karyotypes of IO
3.3. DNA Content of IO
3.4. Microsatellite DNA Analysis of IO
3.5. Molecular Organization of 5S rDNA
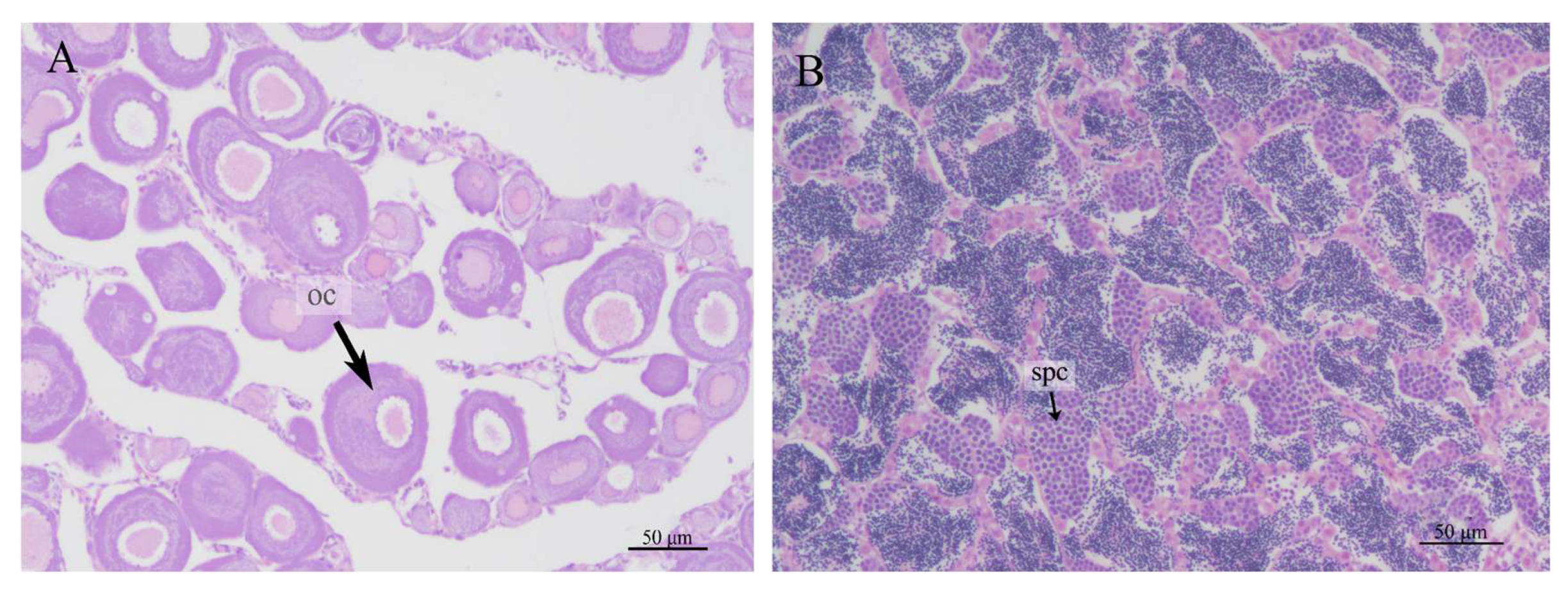
3.6. Sex Ratio in IO
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, M.; Shi, X.; Guo, J.; Lin, K.; Zhu, W.; Fu, J.; Wang, L. Deep spatiotemporal transcriptome analysis provides new insights into early development of koi carp (Cyprinus carpio var. koi). Aquaculture 2023, 575, 739767. [Google Scholar] [CrossRef]
- Chen, R.; Lou, B.; Xu, D.; Zhan, W.; Yutaka, T.; Yang, F.; Liu, F. Induction of meiotic gynogenesis in yellow drum (Nibea albiflora, Sciaenidae) using heterologous sperm and evidence for female homogametic sex determination. Aquaculture 2017, 479, 667–674. [Google Scholar] [CrossRef]
- Li, B.; Luo, S. Cytological observation on the inhibition of the first cleavage by heat shock method to achieve gynogenesis of grass carp. Acta Hydrobiol. Sin. 2003, 27, 155–160. [Google Scholar] [CrossRef]
- Campos-Ramos, R.; Harvey, S.; McAndrew, B.; Penman, D. An investigation of sex determination in the Mozambique tilapia, Oreochromis mossambicus, using synaptonemal complex analysis, FISH, sex reversal and gynogenesis. Aquaculture 2003, 221, 125–140. [Google Scholar] [CrossRef]
- Galbreath, P.; Jean, W.; Anderson, V.; Thorgaard, G. Freshwater performance of all-female diploid and triploid Atlantic salmon. Aquaculture 1994, 128, 41–49. [Google Scholar] [CrossRef]
- Lincoln, R.; Scott, A. Production of all-female triploid rainbow trout. Aquaculture 1998, 30, 375–380. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Z.; Wang, L.; Lu, Y.; Bi, W.; Zhou, D.; Wang, L.; Peng, Z.; You, F. Comparative study on growth performance and morphological characteristics of the meio- and mito-gynogenesis olive flounder (Paralichthys olivaceus). Aquaculture 2021, 535, 736387. [Google Scholar] [CrossRef]
- Li, T.; Jia, Y.; Gao, J.; Zhao, J.; Gu, Z.; Yang, R. Optimization of shock conditions to induce gynogenesis in topmouth culter (Culter alburnus). Aquaculture 2018, 490, 344–349. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Tang, C.; Tao, M.; Zhang, C.; Zhou, Q.; Luo, K.; Wu, C.; Hu, F.; Wang, Y.; et al. The Research Advances in Distant Hybridization and Gynogenesis in Fish. Rev. Aquac. 2024, 17, e12972. [Google Scholar] [CrossRef]
- Marek, J.; Joanna, N.; Joanna, L.; Dariusz, K. The induction of meiotic gynogenesis in Northern pike (Esoxlucius) using the heterologous European perch (Perca fluviatilis) sperm. Fish. Aquat. Life 2023, 31, 186–197. [Google Scholar] [CrossRef]
- Komen, J.; Boer, P.; Richter, C. Male Sex Reversal in Gynogenetic XX Females of Common Carp (Cyprinus carpio L.) by a Recessive Mutation in a Sex-Determining Gene. J. Hered. 1992, 83, 431–434. [Google Scholar] [CrossRef]
- Wang, S.; Ye, X.; Wang, Y.; Chen, Y.; Lin, B.; Yi, Z.; Mao, Z.; Hu, F.; Zhao, R.; Wang, J.; et al. A new type of homodiploid fish derived from the interspecific hybridization of female common carp × male blunt snout bream. Sci. Rep. 2017, 7, 4189. [Google Scholar] [CrossRef] [PubMed]
- Vale, L.; Dieguez, R.; Sanchez, L.; Martine, P.; Vinas, A. A sex-associated sequence identified by RAPD screening in gynogenetic individuals of turbot (Scophthalmus maximus). Mol. Biol. Rep. 2014, 41, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, L.; Yin, H.; Zhu, W.; Fu, J.; Dong, Z. Integrated analysis of long non-coding RNA and mRNA expression in different colored skin of koi carp. BMC Genom. 2019, 20, 515. [Google Scholar] [CrossRef]
- Mao, Z. Establishment of Improved Gynogenetic Grass Carp Population and Research on Its Genetic Characteristics. Ph.D. Thesis, Hunan Normal University, Changsha, China, 2020. [Google Scholar] [CrossRef]
- Gold, R. A fast and easy method for chromosome karyotyping in adult teleosts. Progress. Fish-Cult. 2011, 36, 169–171. [Google Scholar] [CrossRef]
- Levan, A.; Fredga, K.; Sandberg, A. Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, L.; Zhou, Z.; Gui, J. Studies on Microsatellite Markers of Four Artificially Gynogenetic Families in Ornamental Carp. Zool. Res. 2002, 23, 97–105. [Google Scholar] [CrossRef]
- Xiao, J.; Zou, T.; Chen, L.; Liu, S.; Xiao, J. Microsatellite analysis of different ploidy offspring of artificial gynogenesis in Cyprinus carpio. J. Fish Biol. 2011, 78, 150–165. [Google Scholar] [CrossRef]
- Mcconnell, S.; Leamon, J.; Skibinski, D.; Mair, G. Microsatellite markers from the Indian major carp species, Catla catla. Mol. Ecol. Notes 2010, 1, 115–116. [Google Scholar] [CrossRef]
- Xiong, X.; Robinson, A.; Zhou, J.; Chen, Y.; Wang, W.; Wang, X.; Gao, Z. Genetic parameter estimates for intermuscular bone in blunt snout bream (Megalobrama amblycephala) based on a microsatellite-based pedigree. Aquaculture 2018, 502, 371–377. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, X.; Luo, W.; Gao, Z.; Qian, X.; Wang, W. Microsatellite marker analysis of artificial gynogenetic Megalobrama amblycephala. J. Huazhong Agric. Univ. 2012, 31, 7. [Google Scholar] [CrossRef]
- Luo, W.; Deng, W.; Yi, S.; Wang, W.; Gao, Z. Characterization of 20 polymorphic microsatellites for Blunt snout bream (Megalobrama amblycephala) from EST sequence, Conservation Genetics. Resources 2013, 5, 499–501. [Google Scholar] [CrossRef]
- Quillet, E.; Aubard, G.; Queau, I. Mutation in a sex-determining gene in rainbow trout: Detection and genetic analysis. J. Hered. 2002, 93, 91–99. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Zhu, S.; He, W.; Zhou, L.; Zhao, R.; Luo, K.; Tang, C.; Zhang, C.; Liu, S. Localization of 5S rDNA analysis of homologous pairing in tetraploid hybrids of red crucian carp (♀) × common carp (♂). J. World Aquac. Soc. 2021, 53, 714–723. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Luo, K.; Zhang, M.; Qin, Q.; Huo, Y.; Song, J.; Tao, M.; Zhang, C.; Liu, S. The formation of the goldfish-like fish derived from hybridization of female koi carp × male blunt snout bream. Front. Genet. 2018, 9, 437. [Google Scholar] [CrossRef]
- Hu, F.; Wu, C.; Zhou, Y.; Cao, L.; Xiao, J.; Wang, S.; Wu, Y.; Ren, L.; Liu, Q.; Li, W.; et al. Production of androgenetic, triploid and tetraploid hybrids from the interspecific hybridization of female Japanese crucian carp and male blunt snout bream. Aquaculture 2018, 49150–49158. [Google Scholar] [CrossRef]
- Ijiri, K.; Egami, N. Hertwig effect caused by UV-irradiation of sperm of Oryzias latipes (teleost) and its photoreactivation. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1980, 69, 241–248. [Google Scholar] [CrossRef]
- Refstie, T.; Vassvik, V.; Gjedrem, T. Induction of polyploidy in salmonids by cytochalasin B. Aquaculture 1977, 10, 65–74. [Google Scholar] [CrossRef]
- Joanna, N.; Dariusz, K.; Tomasz, L.; Katarzyna, T.; Roman, K. Comparison of temperature shock timing to induced artificial mitotic gynogenesis and androgenesis in common tench. Aquac. Int. 2015, 23, 45–53. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Li, Z.; Qiu, B.; Li, J.; Geng, G.; Hu, B.; Liao, A.; Cai, Y.; Wen, M.; et al. Improvement and application of genetic resources of grass carp (Ctenopharyngodon idella). Reprod. Breed. 2024, 4, 126–133. [Google Scholar] [CrossRef]
- Ocalewicz, K.; Gurgul, A.; Polonis, M.; Dobosz, S. Preliminary identification of candidate genes related to survival of gynogenelic rainbow trout (Oncorhynchus mykiss) based on comparative transcriptome analysis. Animals 2020, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Qi, D.; Cao, Y.; Dong, X.; Zhang, Y.; Huo, H. Studies on chromosome ploidy of Pyrus germplasm collection by flow cytometry. Acta Hortic. 2021, 1303, 79–84. [Google Scholar] [CrossRef]
- Feng, X.; Chen, H.; Xiao, B.; Wu, Q.; Zhang, J.; Zhang, N.; Li, P.; Wang, L.; Yin, J.; Sui, Z. Ploidy Identification by Flow Cytometry and Application of the Method to Characterize Seasonal Ploidy Variation of Wild Populations of the Red Alga Gracilariopsis lemaneiformis. Mar. Biotechnol. 2022, 24, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, Y.; Wang, Z.; Cai, M.; Weng, C. Genetic analysis of yellow drum Nibea albiflora meiotic gynogens. J. Fish. China 2020, 27, 1285–1294. [Google Scholar]
- MacNamara, R.; Mankiewicz, J.L.; Salger, S.A.; Stuart, K.; Borski, R.J.; Godwin, J.; Drawbridge, M. Temperature regulates sex determination and growth in the paralichthid flatfish California halibut. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2024, 341, 811–821. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, J.; Luo, Y.; Tan, H.; Huang, X.; Wang, S.; Qin, Q.; Zhang, C.; Tao, M.; Dabrowski, K.; et al. Two new types of homodiploid fish and polyploid hybrids derived from the distant hybridization of female koi carp and male bighead carp. Mar. Biotechnol. 2021, 23, 628–640. [Google Scholar] [CrossRef]
- Müller-Belecke, A.; Hörstgen-Schwark, G. Sex determination in tilapia (Oreochromis niloticus) sex ratios in homozygous gynogenetic progeny and their offspring. Aquaculture 1995, 137, 57–65. [Google Scholar] [CrossRef]
- Streisinger, G.; Walker, C.; Dower, N.; Knauber, D.; Singer, F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 1981, 291, 293–296. [Google Scholar] [CrossRef]



| Primer Name | Sequence Features | Annealing Temperature/°C | GenBank Number |
|---|---|---|---|
| CK-S1 | F: TCCAAGTCAGTTTAATCACCG | 60 | XM_042754805.1 |
| R: GGGAAGCGTTGAGAACAAGC | |||
| CK-S2 | F: GAATCCTCCATCATGCAAAC | 57 | — |
| R: GCACAAACTCCACATTGTGCC | |||
| CK-S3 | F: GCTCCAGATTGCACATTATAG | 64 | XM_042765836.1 |
| R: CTACACACACGCACAGCCTTTC | |||
| CK-S4 | F: AGACCACCGCAGTAACAA | 53 | XM_042716969.1 |
| R: GACTCACTCAGCACCAGA | |||
| CK-S5 | F: GTACAGCGTGACAGCATT | 53 | XM_042749979.1 |
| R: AAGTTCATCGGTGTCCTC | |||
| CK-S6 | F: ATCATTTGTATTCGTGCTTG | 53 | XM_042745909.1 |
| R: GATCCACTGGGTCCTTTT | |||
| CK-S7 | F: CACGACGTTGTAAAACGACTTGT | 54 | XM_042767288.1 |
| R: ATTGGTGCAGAGCATCAGTG | |||
| CK-S8 | F: CACGACGTTGTAAAACGACGGGG | 57 | — |
| R: CGGCGACTTGATCCTCTTTA | |||
| CK-S9 | F: CACGACGTTGTAAAACGACACTA | 56 | — |
| R: CTTGTACCTGCACAGTCTCATC | |||
| CK-S10 | F: CACGACGTTGTAAAACGACCCAA | 61 | XM_042723695.1 |
| R: AACAAGCATGTAGGCACTA | |||
| MA-S1 | F: TGGAGTTAGTGTCCGCTTGT | 56 | XM_051888809.1 |
| R: AGGATACGGGTGAGTTCG | |||
| MA-S2 | F: TTCGGTTCTGCCTTCACTCT | 65 | XM_048170375.1 |
| R: AAGACGCATGCTCAACAAC | |||
| MA-S3 | F: GTCCAGACTGTCATCAGGAG | 60 | XM_048208277.1 |
| R: GAGGTGTACACTGAGTCACGC | |||
| MA-S4 | F: TCAGCTGAGGGATGGATGGA | 55 | XM_048163853.1 |
| R: AAGGGAGGCTCAGTGTTTCG | |||
| MA-S5 | F: GAGCTCCTCAGAAGGGCTTC | 57 | XM_048163362.1 |
| R: CTTTGGGTTCCGTCGACTGA | |||
| MA-S6 | F: GATAGTGAGCACGAGCAGGA | 57 | XM_048211096.1 |
| R: CCCAGCATGCTTTGTGTAGG | |||
| MA-S7 | F: GACTGGAGTCGTCAGGCTTC | 60.5 | — |
| R: TGCCCCACATTGTTAGACTG | |||
| MA-S8 | F: GGGGAAATAAAGGGAGAAAGTG | 60.5 | XM_048195326.1 |
| R: TTTCTCCTGATCCGTTGACC | |||
| MA-S9 | F: AAACAGGCTCGCCAATTTC | 55.9 | XM_048202478.1 |
| R: TCACCCACACACTCTTATTCTCT | |||
| MA-S10 | F: AGGCGAAAGAAACACTGTGT | 56 | XM_048178383.1 |
| R: GGTGTTCGTGCGATGTTGTA |
| Fish | Average DNA Content | Actual Ratio | Theoretical Ratio | ||
|---|---|---|---|---|---|
| Ratio to CK | Ratio to MA | ||||
| CK | 37.59 ± 1.188 | \ | \ | \ | |
| MA | 28.12 ± 0.796 | \ | \ | \ | |
| IO | WR | 36.39 ± 0.576 | 0.97 | 1.3 | 1 |
| WW | 36.52 ± 0.935 | ||||
| RW | 36.57 ± 0.577 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Shi, X.; Guo, J.; Lin, K.; Luo, M.; Dong, Z. Artificial Induction of Meiotic Gynogenesis in Koi Carp Using Blunt Snout Bream Sperm and Identification of Gynogenetic Offspring. Animals 2025, 15, 1411. https://doi.org/10.3390/ani15101411
Chen X, Shi X, Guo J, Lin K, Luo M, Dong Z. Artificial Induction of Meiotic Gynogenesis in Koi Carp Using Blunt Snout Bream Sperm and Identification of Gynogenetic Offspring. Animals. 2025; 15(10):1411. https://doi.org/10.3390/ani15101411
Chicago/Turabian StyleChen, Xiaoyu, Xiulan Shi, Jun Guo, Kai Lin, Mingkun Luo, and Zaijie Dong. 2025. "Artificial Induction of Meiotic Gynogenesis in Koi Carp Using Blunt Snout Bream Sperm and Identification of Gynogenetic Offspring" Animals 15, no. 10: 1411. https://doi.org/10.3390/ani15101411
APA StyleChen, X., Shi, X., Guo, J., Lin, K., Luo, M., & Dong, Z. (2025). Artificial Induction of Meiotic Gynogenesis in Koi Carp Using Blunt Snout Bream Sperm and Identification of Gynogenetic Offspring. Animals, 15(10), 1411. https://doi.org/10.3390/ani15101411






