Frontal Sinus Trephination and Repeated Irrigation in a Cat with Chronic Rhinosinusitis: A Case Report
Simple Summary
Abstract
1. Introduction
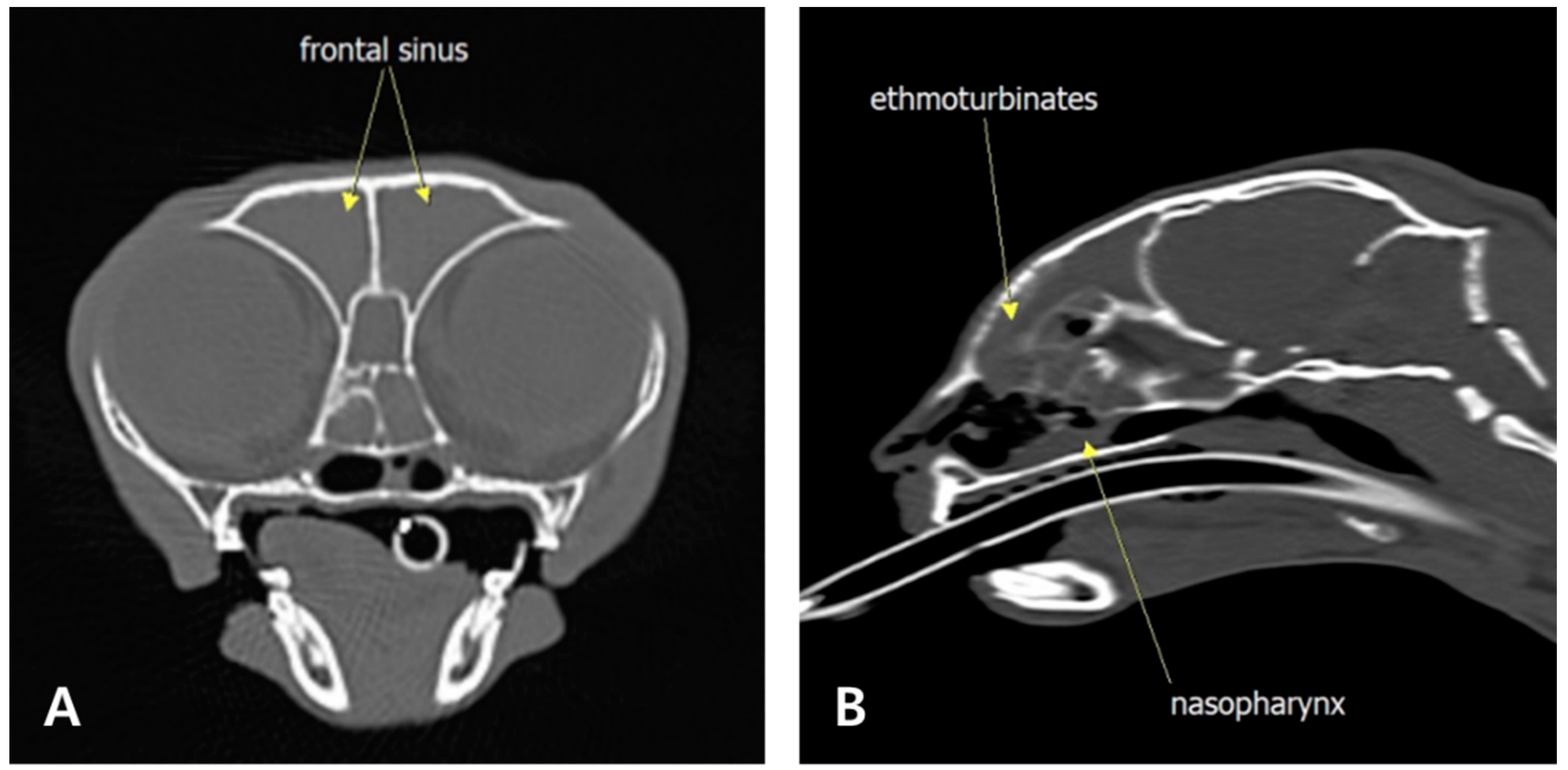
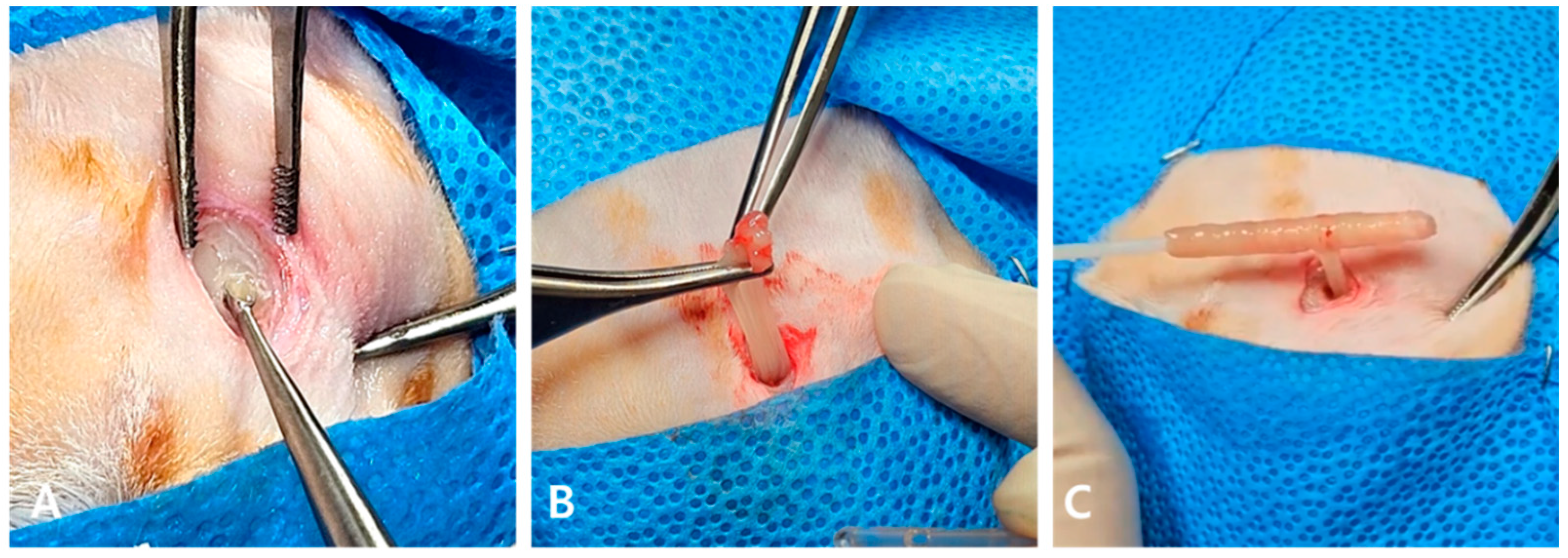
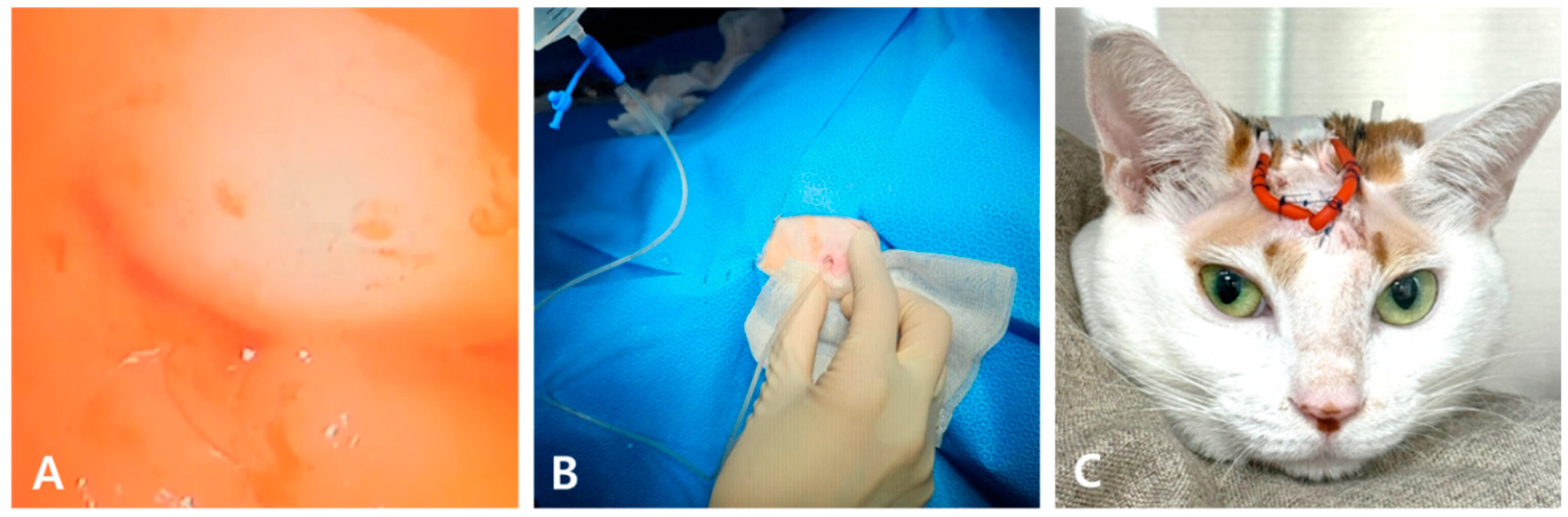
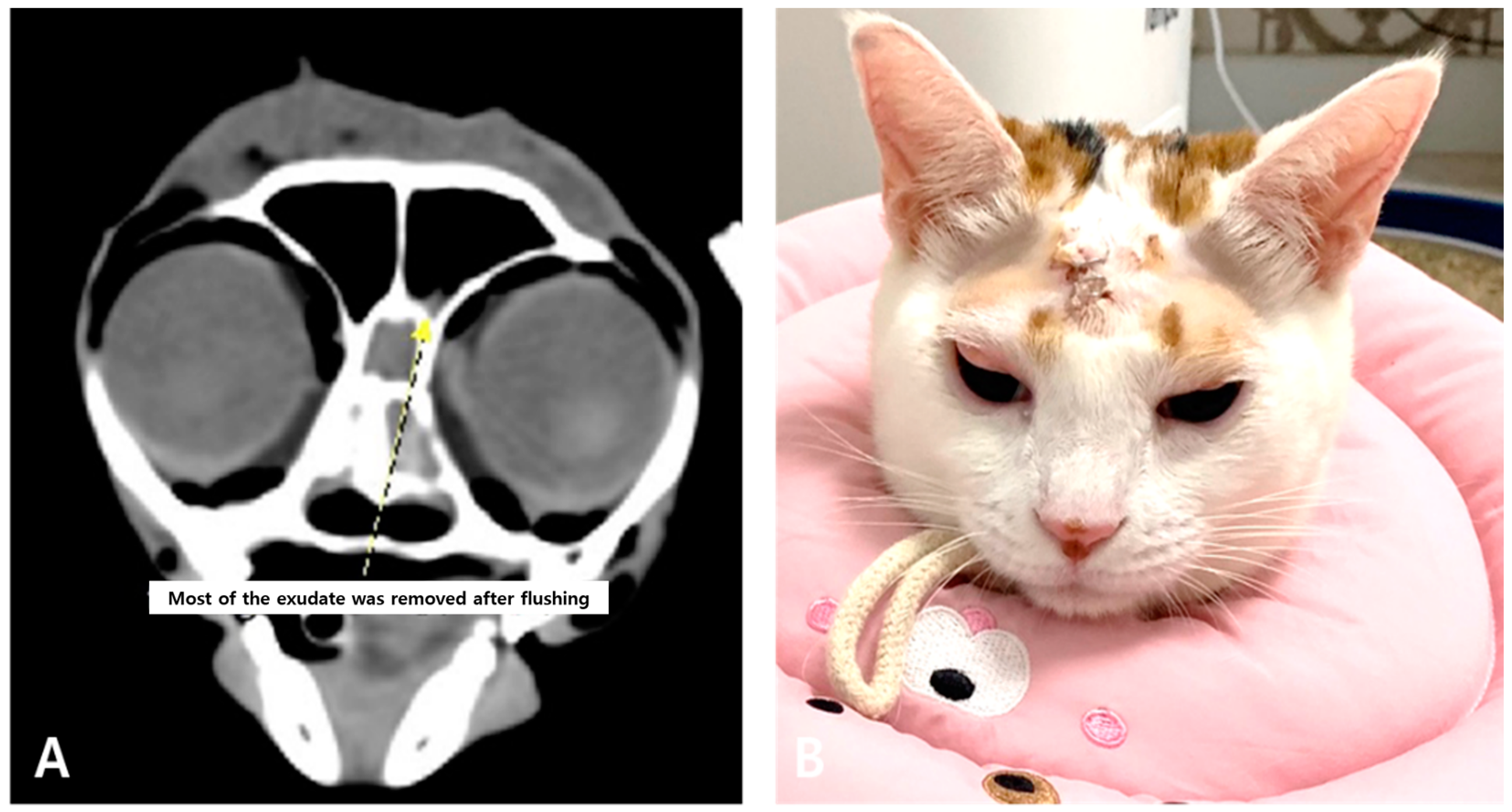
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBC | Complete blood count |
| CT | Computed tomography |
| FCR | Feline chronic rhinitis |
| FCRS | Feline chronic rhinosinusitis |
References
- Michiels, L.; Day, M.J.; Snaps, F.; Hansen, P.; Clercx, C. A retrospective study of non-specific rhinitis in 22 cats and the value of nasal cytology and histopathology. J. Feline Med. Surg. 2003, 5, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Reed, N. Chronic rhinitis in the cat: An update. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Demko, J.L.; Cohn, L.A. Chronic nasal discharge in cats: 75 cases (1993–2004). J. Am. Vet. Med. Assoc. 2007, 230, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.R.; Foley, J.E.; De Cock, H.E.; Clarke, H.E.; Maggs, D.J. Assessment of infectious organisms associated with chronic rhinosinusitis in cats. J. Am. Vet. Med. Assoc. 2005, 227, 579–585. [Google Scholar] [CrossRef]
- Johnson, L.R.; Maggs, D.J. Feline herpesvirus type-1 transcription is associated with increased nasal cytokine gene transcription in cats. Vet. Microbiol. 2005, 108, 225–233. [Google Scholar] [CrossRef]
- Gaskell, R.; Dawson, S.; Radford, A.; Thiry, E. Feline herpesvirus. Vet. Res. 2007, 38, 337–354. [Google Scholar] [CrossRef]
- Nelson, R.W.; Couto, C.G. Small Animal Internal Medicine, 6th ed.; Elsevier Health Sciences: St. Louis, MO, USA, 2019. [Google Scholar]
- Van Pelt, D.R.; Lappin, M.R. Pathogenesis and treatment of feline rhinitis. Vet. Clin. N. Am. Small Anim. Pract. 1994, 24, 807–823. [Google Scholar] [CrossRef]
- Beauvois, M.; Colombe, P.; Canonne, A.M.; Mortier, J. Cats with idiopathic chronic rhinosinusitis that develop clinical signs before two years of age have more severe nasal conchal lysis, sinus malformation, and more severe inflammation on histological examination. J. Am. Vet. Med. Assoc. 2023, 261, 1481–1487. [Google Scholar] [CrossRef]
- Scherk, M. Snots and snuffles: Rational approach to chronic feline upper respiratory syndromes. J. Feline Med. Surg. 2010, 12, 548–557. [Google Scholar] [CrossRef]
- Henderson, S.M.; Bradley, K.; Day, M.J.; Tasker, S.; Caney, S.M.A.; Hotston Moore, A. Investigation of nasal disease in the cat—A retrospective study of 77 cases. J. Feline Med. Surg. 2004, 6, 245–257. [Google Scholar] [CrossRef]
- Holmberg, D.L.; Fries, C.; Cockshutt, J.; Van Pelt, D. Ventral rhinotomy in the dog and cat. Vet. Surg. 1989, 18, 446–449. [Google Scholar] [CrossRef]
- Anderson, G.I. The treatment of chronic sinusitis in six cats by ethmoid conchal curettage and autogenous fat graft sinus ablation. Vet. Surg. 1987, 16, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Dorn, E.S.; Tress, B.; Suchodolski, J.S.; Nisar, T.; Ravindran, P.; Weber, K.; Hartmann, K.; Schulz, B.S. Bacterial microbiome in the nose of healthy cats and in cats with nasal disease. PLoS ONE 2017, 12, e0180299. [Google Scholar] [CrossRef]
- Schulz, B.S.; Wolf, G.; Hartmann, K. Bacteriological and antibiotic sensitivity test results in 271 cats with respiratory tract infections. Vet. Rec. 2006, 158, 269–270. [Google Scholar] [CrossRef]
- Veir, J.K.; Ruch-Gallie, R.; Spindel, M.E.; Lappin, M.R. Prevalence of selected infectious organisms and comparison of two anatomic sampling sites in shelter cats with upper respiratory tract disease. J. Feline Med. Surg. 2008, 10, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Pakravan, N.; Pritchard, J.C.; Hartmann, F.A.; Young, K.M. Mucoid Pseudomonas aeruginosa infection in a cat with severe chronic rhinosinusitis. Vet. Clin. Pathol. 2019, 48, 300–304. [Google Scholar] [CrossRef]
- Vangrinsven, E.; Duprez, J.N.; Meex, C.; Taminiau, B.; Daube, G.; Billen, F.; Mainil, J.; Clercx, C. Comparison of culture-dependent and -independent bacterial detection results on nasal swabs in dogs with nasal discharge. J. Small Anim. Pract. 2024, 65, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Fawsitt, J.; Russell, O.; Alexander, A.; Davies, E. Clinical remission of feline sino-nasal aspergillosis despite evidence of persistent infection. JFMS Open Rep. 2023, 9, 20551169231201605. [Google Scholar] [CrossRef]
- Tamborini, A.; Robertson, E.; Talbot, J.J.; Barrs, V.R. Sinonasal aspergillosis in a British Shorthair cat in the UK. JFMS Open Rep. 2016, 2, 2055116916653775. [Google Scholar] [CrossRef]
- Brown, J.D.; Woerde, D.J.; Hoffmann, K.L.; MacKillop, E.; Degeling, C.; Mansfield, C.S. Partial resolution of chronic unilateral sinonasal obstructive disease in a cat using a temporary polyvinylchloride stent. JFMS Open Rep. 2020, 6, 2055116920943689. [Google Scholar] [CrossRef]
- Serrano, E.; Klossek, J.M.; Percodani, J.; Yardeni, E.; Dufour, X. Surgical management of paranasal sinus mucoceles: A long-term study of 60 cases. Otolaryngol. Head Neck Surg. 2004, 131, 133–140. [Google Scholar] [CrossRef]
- Trimarchi, M.; Bertazzoni, G.; Bussi, M. Endoscopic treatment of frontal sinus mucoceles with lateral extension. Indian J. Otolaryngol. Head Neck Surg. 2013, 65, 151–156. [Google Scholar] [CrossRef]
- Har-El, G. Endoscopic management of 108 sinus mucoceles. Laryngoscope 2001, 111, 2131–2134. [Google Scholar] [CrossRef]
- Norsworthy, G. Surgical treatment of chronic nasal discharge in 17 cats. Vet. Med. 1993, 88, 526–530, 532–537. [Google Scholar]
- Sorrentino, P.J.; MacArthur, S.L. Use of intranasal povidone-iodine packing in the management of infectious rhinosinusitis in three cats. JFMS Open Rep. 2024, 10, 20551169241275303. [Google Scholar] [CrossRef]

| Result | Reference Range | |
|---|---|---|
| Hematocrit (%) | 48.7 | 30.3–52.3 |
| White Blood Cells (k/μL) | 10.07 | 2.87–17.02 |
| Neutrophils (k/μL) | 4.5 | 2.30–10.29 |
| Lymphocytes (k/μL) | 4.82 | 0.92–6.88 |
| Monocytes (k/μL) | 0.22 | 0.05–0.67 |
| Eosinophils (k/μL) | 0.47 | 0.17–1.57 |
| Platelet (k/μL) | 262 | 151–600 |
| Glucose (mg/dL) | 104 | 74–159 |
| BUN (mg/dL) | 21 | 16–36 |
| Creatinine (mg/dL) | 1.3 | 0.8–2.4 |
| ALT (U/L) | 67 | 12–130 |
| ALP (U/L) | 28 | 14–111 |
| GGT (U/L) | 0 | 0–4 |
| Total protein (g/dL) | 8.2 | 5.7–8.9 |
| Albumin (g/dL) | 3.3 | 2.2–4.0 |
| Globulin (g/dL) | 4.9 | 2.8–5.1 |
| Phosphorus (mg/dL) | 4.7 | 3.1–7.5 |
| Calcium (mg/dL) | 10.1 | 7.8–11.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.; Kwon, H.; Kim, S.; Sohn, J.; Kim, J.-i.; Jung, D.-I. Frontal Sinus Trephination and Repeated Irrigation in a Cat with Chronic Rhinosinusitis: A Case Report. Animals 2025, 15, 1382. https://doi.org/10.3390/ani15101382
Jang H, Kwon H, Kim S, Sohn J, Kim J-i, Jung D-I. Frontal Sinus Trephination and Repeated Irrigation in a Cat with Chronic Rhinosinusitis: A Case Report. Animals. 2025; 15(10):1382. https://doi.org/10.3390/ani15101382
Chicago/Turabian StyleJang, Hyomi, Hyojun Kwon, Sunyoung Kim, Jiheui Sohn, Jong-in Kim, and Dong-In Jung. 2025. "Frontal Sinus Trephination and Repeated Irrigation in a Cat with Chronic Rhinosinusitis: A Case Report" Animals 15, no. 10: 1382. https://doi.org/10.3390/ani15101382
APA StyleJang, H., Kwon, H., Kim, S., Sohn, J., Kim, J.-i., & Jung, D.-I. (2025). Frontal Sinus Trephination and Repeated Irrigation in a Cat with Chronic Rhinosinusitis: A Case Report. Animals, 15(10), 1382. https://doi.org/10.3390/ani15101382







