Immunohistochemical Detection of Iron-Related Proteins in Sertoli Cell-Only Patterns in Canine Testicular Lesions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Histology
2.3. Immunohistochemistry
3. Results
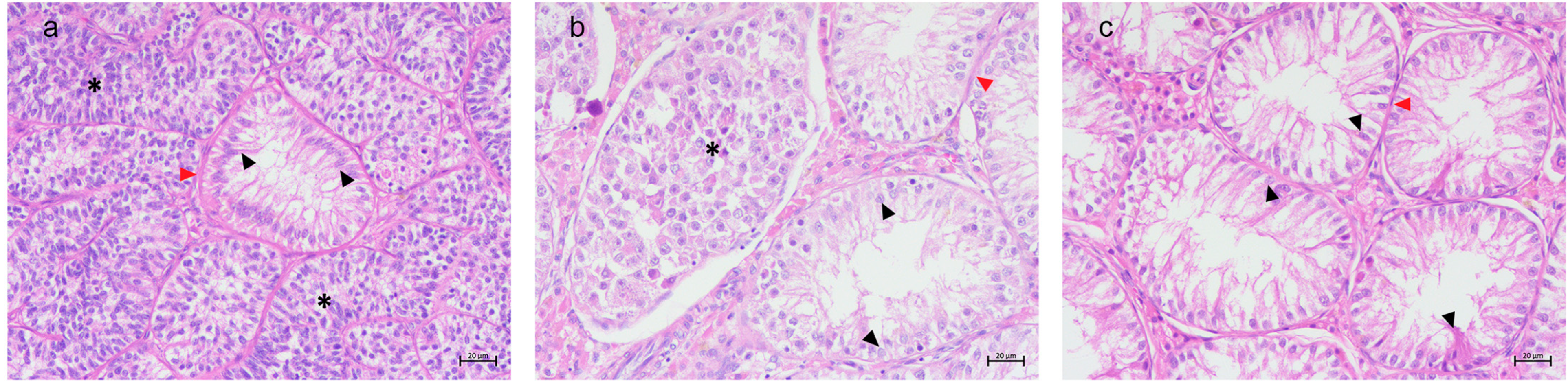
3.1. Histological Results
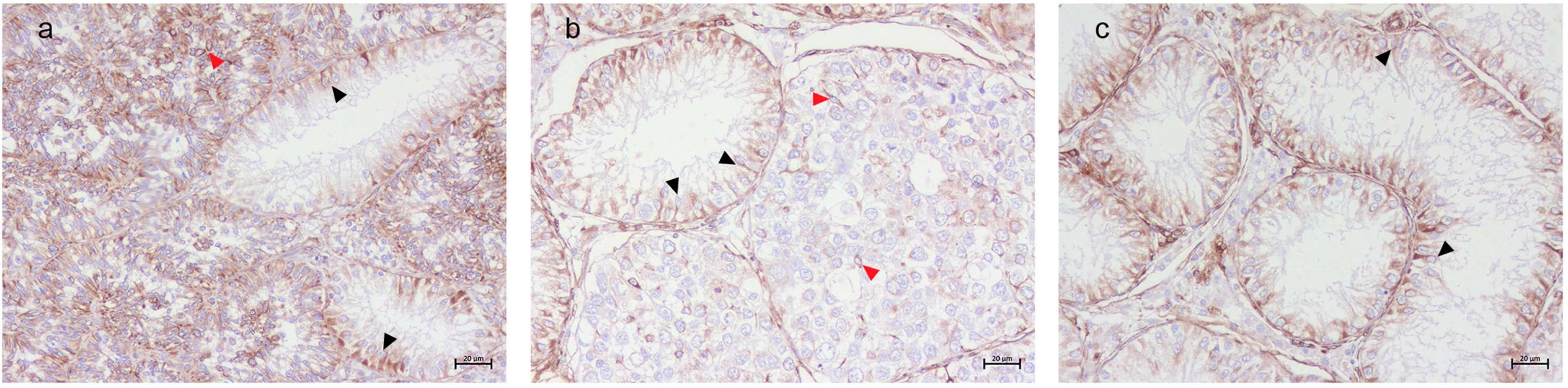
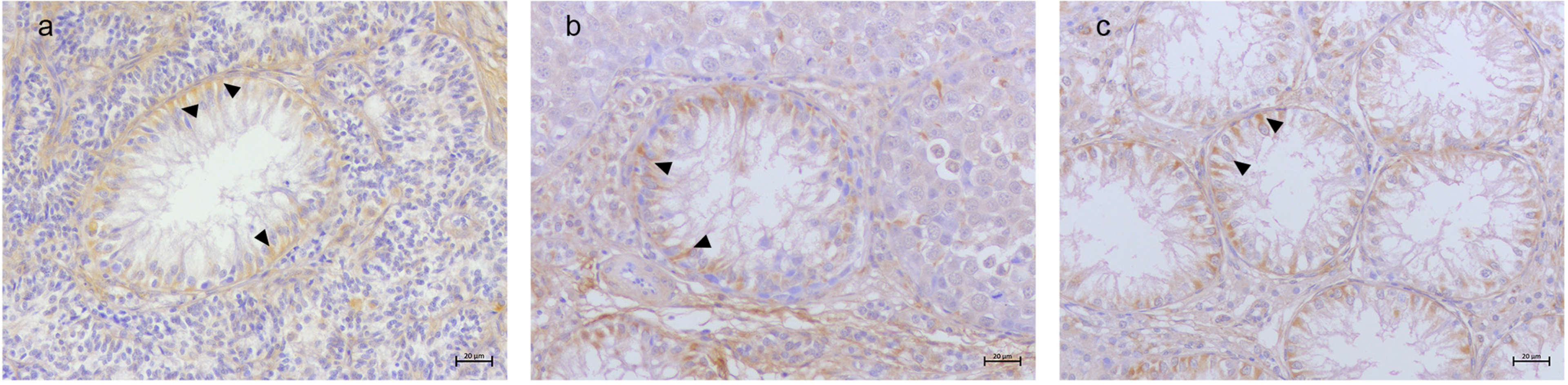
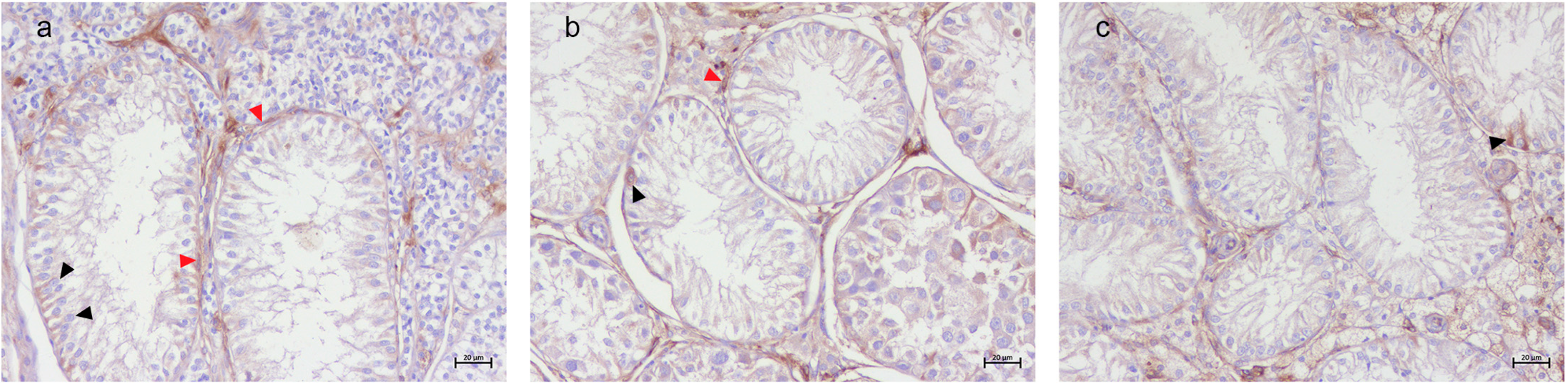
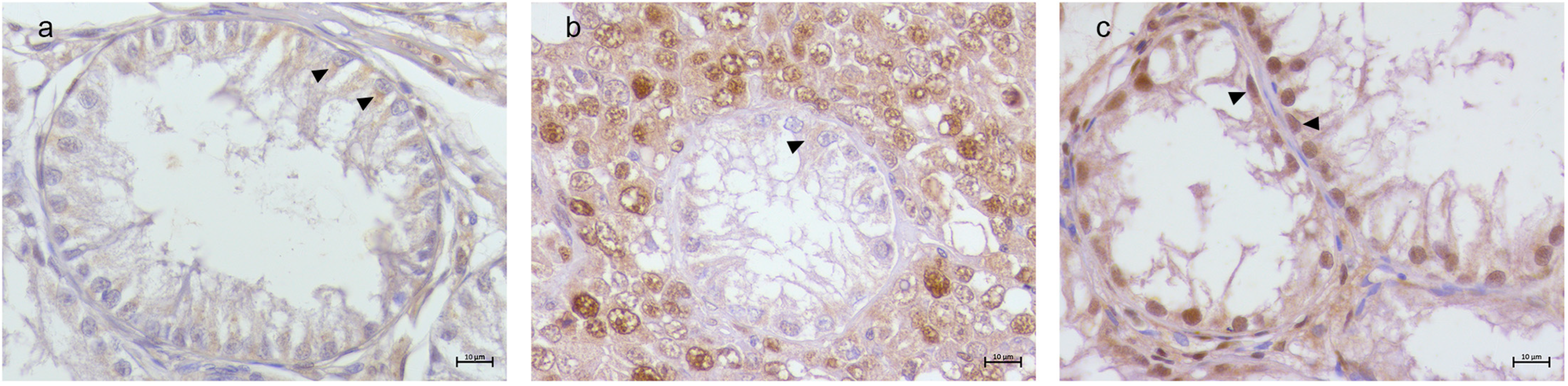
3.2. Immunohistochemical Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Liu, X.; Qu, M.; Li, H. Sertoli Cell-Only Syndrome: Advances, Challenges, and Perspectives in Genetics and Mechanisms. Cell. Mol. Life Sci. CMLS 2023, 80, 67. [Google Scholar] [CrossRef] [PubMed]
- Koc, G.; Ozdemir, A.A.; Girgin, G.; Akbal, C.; Kirac, D.; Avcilar, T.; Guney, A.I. Male Infertility in Sertoli Cell-Only Syndrome: An Investigation of Autosomal Gene Defects. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2019, 26, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, F.; Ceylaner, S.; Erdogan, S.; Topaloglu, A.K.; Yuksel, B. Sertoli Cell Only Syndrome with Ambiguous Genitalia. J. Pediatr. Endocrinol. Metab. JPEM 2016, 29, 849–852. [Google Scholar] [CrossRef]
- Anniballo, R.; Brehm, R.; Steger, K. Recognising the Sertoli-Cell-Only (SCO) Syndrome: A Case Study. Andrologia 2011, 43, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Stouffs, K.; Gheldof, A.; Tournaye, H.; Vandermaelen, D.; Bonduelle, M.; Lissens, W.; Seneca, S. Sertoli Cell-Only Syndrome: Behind the Genetic Scenes. BioMed Res. Int. 2016, 2016, 6191307. [Google Scholar] [CrossRef]
- Maymon, B.B.-S.; Paz, G.; Elliott, D.J.; Hammel, I.; Kleiman, S.E.; Yogev, L.; Hauser, R.; Botchan, A.; Yavetz, H. Maturation Phenotype of Sertoli Cells in Testicular Biopsies of Azoospermic Men. Hum. Reprod. 2000, 15, 1537–1542. [Google Scholar] [CrossRef]
- Maymon, B.B.-S.; Yogev, L.; Paz, G.; Kleiman, S.E.; Schreiber, L.; Botchan, A.; Hauser, R.; Yavetz, H. Sertoli Cell Maturation in Men with Azoospermia of Different Etiologies. Fertil. Steril. 2002, 77, 904–909. [Google Scholar] [CrossRef]
- Kliesch, S.; Behre, H.M.; Hertle, L.; Bergmann, M. Alteration of Sertoli Cell Differentiation in the Presence of Carcinoma in Situ in Human Testes. J. Urol. 1998, 160, 1894–1898. [Google Scholar] [CrossRef]
- Steger, K.; Rey, R.; Louis, F.; Kliesch, S.; Behre, H.M.; Nieschlag, E.; Hoepffner, W.; Bailey, D.; Marks, A.; Bergmann, M. Reversion of the Differentiated Phenotype and Maturation Block in Sertoli Cells in Pathological Human Testis. Hum. Reprod. 1999, 14, 136–143. [Google Scholar] [CrossRef]
- Young, J.; Rey, R.; Couzinet, B.; Chanson, P.; Josso, N.; Schaison, G. Antimüllerian Hormone in Patients with Hypogonadotropic Hypogonadism. J. Clin. Endocrinol. Metab. 1999, 84, 2696–2699. [Google Scholar] [CrossRef]
- Brehm, R.; Marks, A.; Rey, R.; Kliesch, S.; Bergmann, M.; Steger, K. Altered Expression of Connexins 26 and 43 in Sertoli Cells in Seminiferous Tubules Infiltrated with Carcinoma-in-Situ or Seminoma. J. Pathol. 2002, 197, 647–653. [Google Scholar] [CrossRef]
- Dohle, G.R.; Elzanaty, S.; van Casteren, N.J. Testicular Biopsy: Clinical Practice and Interpretation. Asian J. Androl. 2012, 14, 88–93. [Google Scholar] [CrossRef]
- Oliver, R.T.D. The Atrophy Hypothesis and Development of Malignant Germ Cell Cancers of the Testis. In Germ Cell Tumours V; Harnden, P., Joffe, J.K., Jones, W.G., Eds.; Springer: London, UK, 2002; pp. 57–60. [Google Scholar]
- Cerván-Martín, M.; Castilla, J.A.; Palomino-Morales, R.J.; Carmona, F.D. Genetic Landscape of Nonobstructive Azoospermia and New Perspectives for the Clinic. J. Clin. Med. 2020, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Tüttelmann, F.; Werny, F.; Cooper, T.G.; Kliesch, S.; Simoni, M.; Nieschlag, E. Clinical Experience with Azoospermia: Aetiology and Chances for Spermatozoa Detection upon Biopsy. Int. J. Androl. 2011, 34, 291–298. [Google Scholar] [CrossRef]
- Wosnitzer, M.; Goldstein, M.; Hardy, M.P. Review of Azoospermia. Spermatogenesis 2014, 4, e28218. [Google Scholar] [CrossRef]
- Quartuccio, M.; Marino, G.; Garufi, G.; Cristarella, S.; Zanghì, A. Sertoli Cell Tumors Associated with Feminizing Syndrome and Spermatic Cord Torsion in Two Cryptorchid Dogs. J. Vet. Sci. 2012, 13, 207–209. [Google Scholar] [CrossRef]
- Russo, M.; England, G.C.W.; Catone, G.; Marino, G. Imaging of Canine Neoplastic Reproductive Disorders. Animals 2021, 11, 1213. [Google Scholar] [CrossRef]
- Goericke-Pesch, S.; Reifarth, L.; Behrens Mathiesen, C.; Schuler, G.; Umbach, A.-K.; Körber, H. Chronic Immune-Mediated Orchitis Is the Major Cause of Acquired Non-Obstructive Azoospermia in Dogs. Front. Vet. Sci. 2022, 9, 865967. [Google Scholar] [CrossRef]
- Memon, M.A. Common Causes of Male Dog Infertility. Theriogenology 2007, 68, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, S. Two Common Causes of Infertility in the Male Dog. In Proceedings of the 2006 WSAVA Congress Proceedings Online-WSAVA2006-VIN, Prague, Czech Republic, 11–14 October 2006. [Google Scholar]
- Giudice, C.; Banco, B.; Veronesi, M.C.; Ferrari, A.; Di Nardo, A.; Grieco, V. Immunohistochemical Expression of Markers of Immaturity in Sertoli and Seminal Cells in Canine Testicular Atrophy. J. Comp. Pathol. 2014, 150, 208–215. [Google Scholar] [CrossRef]
- Pecile, A.; Groppetti, D.; Pizzi, G.; Banco, B.; Bronzo, V.; Giudice, C.; Grieco, V. Immunohistochemical Insights into a Hidden Pathology: Canine Cryptorchidism. Theriogenology 2021, 176, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, S.; Gunn, I.; Champness, K. Azoospermia in Two Labrador Retrievers. Aust. Vet. J. 1999, 77, 570–573. [Google Scholar] [CrossRef]
- Rehm, S. Spontaneous Testicular Lesions in Purpose-Bred Beagle Dogs. Toxicol. Pathol. 2000, 28, 782–787. [Google Scholar] [CrossRef]
- Goedken, M.J.; Kerlin, R.L.; Morton, D. Spontaneous and Age-Related Testicular Findings in Beagle Dogs. Toxicol. Pathol. 2008, 36, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Olson, P.N.; Schultheiss, P.; Seim, H.B. Clinical and Laboratory Findings Associated with Actual or Suspected Azoospermia in Dogs: 18 Cases (1979–1990). J. Am. Vet. Med. Assoc. 1992, 201, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, E.; Tsutsui, T.; Ogasa, A. Histological Observations of the Reproductive Organs of the Male Dog from Birth to Sexual Maturity. J. Vet. Med. Sci. 1991, 53, 241–248. [Google Scholar] [CrossRef]
- Wise, T.; Lunstra, D.D.; Rohrer, G.A.; Ford, J.J. Relationships of Testicular Iron and Ferritin Concentrations with Testicular Weight and Sperm Production in Boars. J. Anim. Sci. 2003, 81, 503–511. [Google Scholar] [CrossRef]
- Gabrielsen, J.S.; Lamb, D.J.; Lipshultz, L.I. Iron and a Man’s Reproductive Health: The Good, the Bad and the Ugly. Curr. Urol. Rep. 2018, 19, 60. [Google Scholar] [CrossRef]
- Tsao, C.-W.; Liao, Y.-R.; Chang, T.-C.; Liew, Y.-F.; Liu, C.-Y. Effects of Iron Supplementation on Testicular Function and Spermatogenesis of Iron-Deficient Rats. Nutrients 2022, 14, 2063. [Google Scholar] [CrossRef]
- Ferrer, M.; Palomares, R.; Maldonadox-Estrada, J. Role of Trace Minerals in Bull Reproductive Physiology and Semen Quality. Clin. Theriogenol. 2024, 16, 10351. [Google Scholar] [CrossRef]
- Leandri, R.; Power, K.; Buonocore, S.; De Vico, G. Preliminary Evidence of the Possible Roles of the Ferritinophagy-Iron Uptake Axis in Canine Testicular Cancer. Animals 2024, 14, 2619. [Google Scholar] [CrossRef] [PubMed]
- Roemhild, K.; von Maltzahn, F.; Weiskirchen, R.; Knüchel, R.; von Stillfried, S.; Lammers, T. Iron Metabolism: Pathophysiology and Pharmacology. Trends Pharmacol. Sci. 2021, 42, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martínez-Pastor, M.T. The Elemental Role of Iron in DNA Synthesis and Repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef]
- Leichtmann-Bardoogo, Y.; Cohen, L.A.; Weiss, A.; Marohn, B.; Schubert, S.; Meinhardt, A.; Meyron-Holtz, E.G. Compartmentalization and Regulation of Iron Metabolism Proteins Protect Male Germ Cells from Iron Overload. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1519–E1530. [Google Scholar] [CrossRef]
- Sylvester, S.R.; Skinner, M.K.; Griswold, M.D. A Sulfated Glycoprotein Synthesized by Sertoli Cells and by Epididymal Cells Is a Component of the Sperm Membrane. Biol. Reprod. 1984, 31, 1087–1101. [Google Scholar] [CrossRef]
- Vannelli, B.G.; Orlando, C.; Barni, T.; Natali, A.; Serio, M.; Balboni, G.C. Immunostaining of Transferrin and Transferrin Receptor in Human Seminiferous Tubules. Fertil. Steril. 1986, 45, 536–541. [Google Scholar] [CrossRef]
- França, L.R.; Hess, R.A.; Dufour, J.M.; Hofmann, M.C.; Griswold, M.D. The Sertoli Cell: One Hundred Fifty Years of Beauty and Plasticity. Andrology 2016, 4, 189–212. [Google Scholar] [CrossRef]
- Kee, N.; Sivalingam, S.; Boonstra, R.; Wojtowicz, J.M. The Utility of Ki-67 and BrdU as Proliferative Markers of Adult Neurogenesis. J. Neurosci. Methods 2002, 115, 97–105. [Google Scholar] [CrossRef]
- Leandri, R.; Buonocore, S.; Power, K. Transferrin Receptor 2 in Canine Testicular Tumors: An Emerging Key Role in Seminomas. Animals 2025, 15, 264. [Google Scholar] [CrossRef]
- Roberts, K.P.; Griswold, M.D. Characterization of Rat Transferrin Receptor cDNA: The Regulation of Transferrin Receptor mRNA in Testes and in Sertoli Cells in Culture. Mol. Endocrinol. 1990, 4, 531–542. [Google Scholar] [CrossRef]
- Yuan, W.; Sun, Z.; Ji, G.; Hu, H. Emerging Roles of Ferroptosis in Male Reproductive Diseases. Cell Death Discov. 2023, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Vela, D. Iron in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1259, 39–51. [Google Scholar] [CrossRef]
- Lucesoli, F.; Caligiuri, M.; Roberti, M.F.; Perazzo, J.C.; Fraga, C.G. Dose-Dependent Increase of Oxidative Damage in the Testes of Rats Subjected to Acute Iron Overload. Arch. Biochem. Biophys. 1999, 372, 37–43. [Google Scholar] [CrossRef]
- Tvrda, E.; Peer, R.; Sikka, S.C.; Agarwal, A. Iron and Copper in Male Reproduction: A Double-Edged Sword. J. Assist. Reprod. Genet. 2015, 32, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Banco, B.; Giudice, C.; Veronesi, M.C.; Gerosa, E.; Grieco, V. An Immunohistochemical Study of Normal and Neoplastic Canine Sertoli Cells. J. Comp. Pathol. 2010, 143, 239–247. [Google Scholar] [CrossRef]
- Grieco, V.; Banco, B.; Ferrari, A.; Rota, A.; Faustini, M.; Veronesi, M.C. Inhibin-α Immunohistochemical Expression in Mature and Immature Canine Sertoli and Leydig Cells. Reprod. Domest. Anim. Zuchthyg. 2011, 46, 920–923. [Google Scholar] [CrossRef]
- Banco, B.; Veronesi, M.C.; Giudice, C.; Rota, A.; Grieco, V. Immunohistochemical Evaluation of the Expression of Anti-Müllerian Hormone in Mature, Immature and Neoplastic Canine Sertoli Cells. J. Comp. Pathol. 2012, 146, 18–23. [Google Scholar] [CrossRef]
- Rehder, P.; Packeiser, E.-M.; Körber, H.; Goericke-Pesch, S. Chronic Asymptomatic Orchitis in Dogs Alters Sertoli Cell Number and Maturation Status. Front. Vet. Sci. 2025, 12, 1519105. [Google Scholar] [CrossRef]
- Bojarzadeh, H.; Lazzarini, G.; Gatta, A.; Sadeghinezhad, J.; Samieeroudy, L.; Pirone, A.; Miragliotta, V. Three-Dimensional Morphometry of the Testis in Dog Using Design-Unbiased Stereology. Anat. Histol. Embryol. 2024, 53, e12968. [Google Scholar] [CrossRef]
- Candelaria, P.V.; Leoh, L.S.; Penichet, M.L.; Daniels-Wells, T.R. Antibodies Targeting the Transferrin Receptor 1 (TfR1) as Direct Anti-Cancer Agents. Front. Immunol. 2021, 12, 607692. [Google Scholar] [CrossRef]
- Kawabata, H.; Germain, R.S.; Vuong, P.T.; Nakamaki, T.; Said, J.W.; Koeffler, H.P. Transferrin Receptor 2-α Supports Cell Growth Both in Iron-Chelated Cultured Cells and In Vivo. J. Biol. Chem. 2000, 275, 16618–16625. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, H.; Yang, R.; Hirama, T.; Vuong, P.T.; Kawano, S.; Gombart, A.F.; Koeffler, H.P. Molecular Cloning of Transferrin Receptor 2. A New Member of the Transferrin Receptor-like Family. J. Biol. Chem. 1999, 274, 20826–20832. [Google Scholar] [CrossRef]
- Liu, N.Q.; De Marchi, T.; Timmermans, A.M.; Beekhof, R.; Trapman-Jansen, A.M.A.C.; Foekens, R.; Look, M.P.; van Deurzen, C.H.M.; Span, P.N.; Sweep, F.C.G.J.; et al. Ferritin Heavy Chain in Triple Negative Breast Cancer: A Favorable Prognostic Marker That Relates to a Cluster of Differentiation 8 Positive (CD8+) Effector T-Cell Response. Mol. Cell. Proteom. 2014, 13, 1814–1827. [Google Scholar] [CrossRef] [PubMed]
- Orino, K.; Watanabe, K. Molecular, Physiological and Clinical Aspects of the Iron Storage Protein Ferritin. Vet. J. 1997 2008, 178, 191–201. [Google Scholar] [CrossRef]
- Foley, J.F.; Dietrich, D.R.; Swenberg, J.A.; Maronpot, R.R. Detection and Evaluation of Proliferating Cell Nuclear Antigen (PCNA) in Rat Tissue by an Improved Immunohistochemical Procedure. J. Histotechnol. 1991, 14, 237–241. [Google Scholar] [CrossRef]
- Ellison, V.; Stillman, B. Biochemical Characterization of DNA Damage Checkpoint Complexes: Clamp Loader and Clamp Complexes with Specificity for 5′ Recessed DNA. PLoS Biol. 2003, 1, e33. [Google Scholar] [CrossRef]
- Hoogervorst, E.M.; van Steeg, H.; de Vries, A. Nucleotide Excision Repair- and P53-Deficient Mouse Models in Cancer Research. Mutat. Res. Mol. Mech. Mutagen. 2005, 574, 3–21. [Google Scholar] [CrossRef]
- Carella, F.; Figueras, A.; Novoa, B.; De Vico, G. Cytomorphology and PCNA Expression Pattern in Bivalves Mytilus Galloprovincialis and Cerastoderma Edule with Haemic Neoplasia. Dis. Aquat. Org. 2013, 105, 81–87. [Google Scholar] [CrossRef]
- Bouayad, D.; Pederzoli-Ribeil, M.; Mocek, J.; Candalh, C.; Arlet, J.-B.; Hermine, O.; Reuter, N.; Davezac, N.; Witko-Sarsat, V. Nuclear-to-Cytoplasmic Relocalization of the Proliferating Cell Nuclear Antigen (PCNA) during Differentiation Involves a Chromosome Region Maintenance 1 (CRM1)-Dependent Export and Is a Prerequisite for PCNA Antiapoptotic Activity in Mature Neutrophils. J. Biol. Chem. 2012, 287, 33812–33825. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Mocek, J.; Bouayad, D.; Tamassia, N.; Ribeil, J.-A.; Candalh, C.; Davezac, N.; Reuter, N.; Mouthon, L.; Hermine, O.; et al. Proliferating Cell Nuclear Antigen Acts as a Cytoplasmic Platform Controlling Human Neutrophil Survival. J. Exp. Med. 2010, 207, 2631–2645. [Google Scholar] [CrossRef]
- Benjamin, D.R.; Gown, A.M. Aberrant Cytoplasmic Expression of Proliferating Cell Nuclear Antigen in Hodgkin’s Disease. Am. J. Surg. Pathol. 1991, 15, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Cardano, M.; Tribioli, C.; Prosperi, E. Targeting Proliferating Cell Nuclear Antigen (PCNA) as an Effective Strategy to Inhibit Tumor Cell Proliferation. Curr. Cancer Drug Targets 2020, 20, 240–252. [Google Scholar] [CrossRef]
- Sliskovic, I.; Mutus, B. Reversible Inhibition of Caspase-3 Activity by Iron(III): Potential Role in Physiological Control of Apoptosis. FEBS Lett. 2006, 580, 2233–2237. [Google Scholar] [CrossRef][Green Version]
- De Vico, G.; Carella, F. Argomenti di Patologia Comparata dei Molluschi-Aspetti Ecologoci e Sanitari; Loffredo: Rome, Italy, 2012; ISBN 978-88-7564-549-6. [Google Scholar]
- Lowe, S.W.; Lin, A.W. Apoptosis in Cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S. Apoptosis in Cancer: From Pathogenesis to Treatment. J. Exp. Clin. Cancer Res. CR 2011, 30, 87. [Google Scholar] [CrossRef]
- Simões, V.L.; Alves, M.G.; Martins, A.D.; Dias, T.R.; Rato, L.; Socorro, S.; Oliveira, P.F. Regulation of Apoptotic Signaling Pathways by 5α-Dihydrotestosterone and 17β-Estradiol in Immature Rat Sertoli Cells. J. Steroid Biochem. Mol. Biol. 2013, 135, 15–23. [Google Scholar] [CrossRef]
- Aslani, F.; Sebastian, T.; Keidel, M.; Fröhlich, S.; Elsässer, H.-P.; Schuppe, H.-C.; Klug, J.; Mahavadi, P.; Fijak, M.; Bergmann, M.; et al. Resistance to Apoptosis and Autophagy Leads to Enhanced Survival in Sertoli Cells. Mol. Hum. Reprod. 2017, 23, 370–380. [Google Scholar] [CrossRef]
- Grieco, V.; Riccardi, E.; Greppi, G.F.; Teruzzi, F.; Iermanò, V.; Finazzi, M. Canine Testicular Tumours: A Study on 232 Dogs. J. Comp. Pathol. 2008, 138, 86–89. [Google Scholar] [CrossRef]
- Manuali, E.; Forte, C.; Porcellato, I.; Brachelente, C.; Sforna, M.; Pavone, S.; Ranciati, S.; Morgante, R.; Crescio, I.M.; Ru, G.; et al. A Five-Year Cohort Study on Testicular Tumors from a Population-Based Canine Cancer Registry in Central Italy (Umbria). Prev. Vet. Med. 2020, 185, 105201. [Google Scholar] [CrossRef]
- Sacco, A.; Battaglia, A.M.; Botta, C.; Aversa, I.; Mancuso, S.; Costanzo, F.; Biamonte, F. Iron Metabolism in the Tumor Microenvironment—Implications for Anti-Cancer Immune Response. Cells 2021, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Wang, L. Iron Metabolism and the Tumor Microenvironment: A New Perspective on Cancer Intervention and Therapy (Review). Int. J. Mol. Med. 2025, 55, 39. [Google Scholar] [CrossRef] [PubMed]

| Samples | Breed | Age (ys) | Histological Diagnosis |
|---|---|---|---|
| S1 | Mixed Breed | 7 | SCO in SEM |
| S2 | English Setter | 8 | SCO in SEM |
| S3 | English Bulldog | 6 | SCO in SEM |
| S4 | Pitbull | 6 | SCO in SEM |
| S5 | German Sheperd | 8 | SCO in SEM |
| S6 | Beagle | 10 | SCO in SEM |
| S7 | Pitbull | 11 | SCO in SEM |
| S8 | Mixed Breed | 9 | SCO in SEM |
| S9 | German Sheperd | 10 | SCO in SEM |
| S10 | West Highland Terrier | 9 | SCO in SCT |
| S11 | German Sheperd | 7 | SCO in SCT |
| S12 | Beagle | 10 | SCO in SCT |
| S13 | Poodle | 9 | SCO in SCT |
| S14 | German Sheperd | 12 | SCO in SCT |
| S15 | Mixed Breed | 14 | SCO in SCT |
| S16 | English Bulldog | 15 | SCO in SCT |
| S17 | West Highland Terrier | 8 | SCO in SCT |
| S18 | Pitbull | 6 | SCO in SCT |
| S19 | Beagle | 8 | isolated SCO |
| S20 | West Highland Terrier | 7 | isolated SCO |
| S21 | German Sheperd | 7 | isolated SCO |
| S22 | Mixed Breed | 9 | isolated SCO |
| S23 | German Sheperd | 10 | isolated SCO |
| S24 | Mixed Breed | 9 | isolated SCO |
| S25 | German Sheperd | 7 | isolated SCO |
| S26 | Poodle | 10 | isolated SCO |
| S27 | Poodle | 9 | isolated SCO |
| N1 | Mixed Breed | 9 | N.n. testis |
| N2 | German Sheperd | 7 | N.n. testis |
| N3 | English Bulldog | 6 | N.n. testis |
| Antibody | Manufacturer/Clone | Host Species | Dilution |
|---|---|---|---|
| Vimentin | Dako, Carpinteria, CA, USA 3B4 | Mouse | 1:100 |
| TfR1 | ThermoFisher, Carlsbad, CA, USA H68.4 | Mouse | 1:100 |
| TfR2 | Antibodies */Polyclonal | Rabbit | 1:100 |
| FTH1 | Antibodies, Limerick, PA, USA/Polyclonal | Rabbit | 1:100 |
| PCNA | ThermoFisher, Carlsbad, CA, USA PC10 | Mouse | 1:400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leandri, R.; Power, K.; Martano, M.; De Vico, G. Immunohistochemical Detection of Iron-Related Proteins in Sertoli Cell-Only Patterns in Canine Testicular Lesions. Animals 2025, 15, 1377. https://doi.org/10.3390/ani15101377
Leandri R, Power K, Martano M, De Vico G. Immunohistochemical Detection of Iron-Related Proteins in Sertoli Cell-Only Patterns in Canine Testicular Lesions. Animals. 2025; 15(10):1377. https://doi.org/10.3390/ani15101377
Chicago/Turabian StyleLeandri, Rebecca, Karen Power, Manuela Martano, and Gionata De Vico. 2025. "Immunohistochemical Detection of Iron-Related Proteins in Sertoli Cell-Only Patterns in Canine Testicular Lesions" Animals 15, no. 10: 1377. https://doi.org/10.3390/ani15101377
APA StyleLeandri, R., Power, K., Martano, M., & De Vico, G. (2025). Immunohistochemical Detection of Iron-Related Proteins in Sertoli Cell-Only Patterns in Canine Testicular Lesions. Animals, 15(10), 1377. https://doi.org/10.3390/ani15101377






