Heavy Metal Accumulation (Cd, As, Zn, Cu, Cr) in Hair and Bones of Small Mammal Prey of the Sentinel Species Common Genet (Genetta genetta) in an Anthropogenic Environment of Edough Mountain Forest, Northeastern Algeria
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
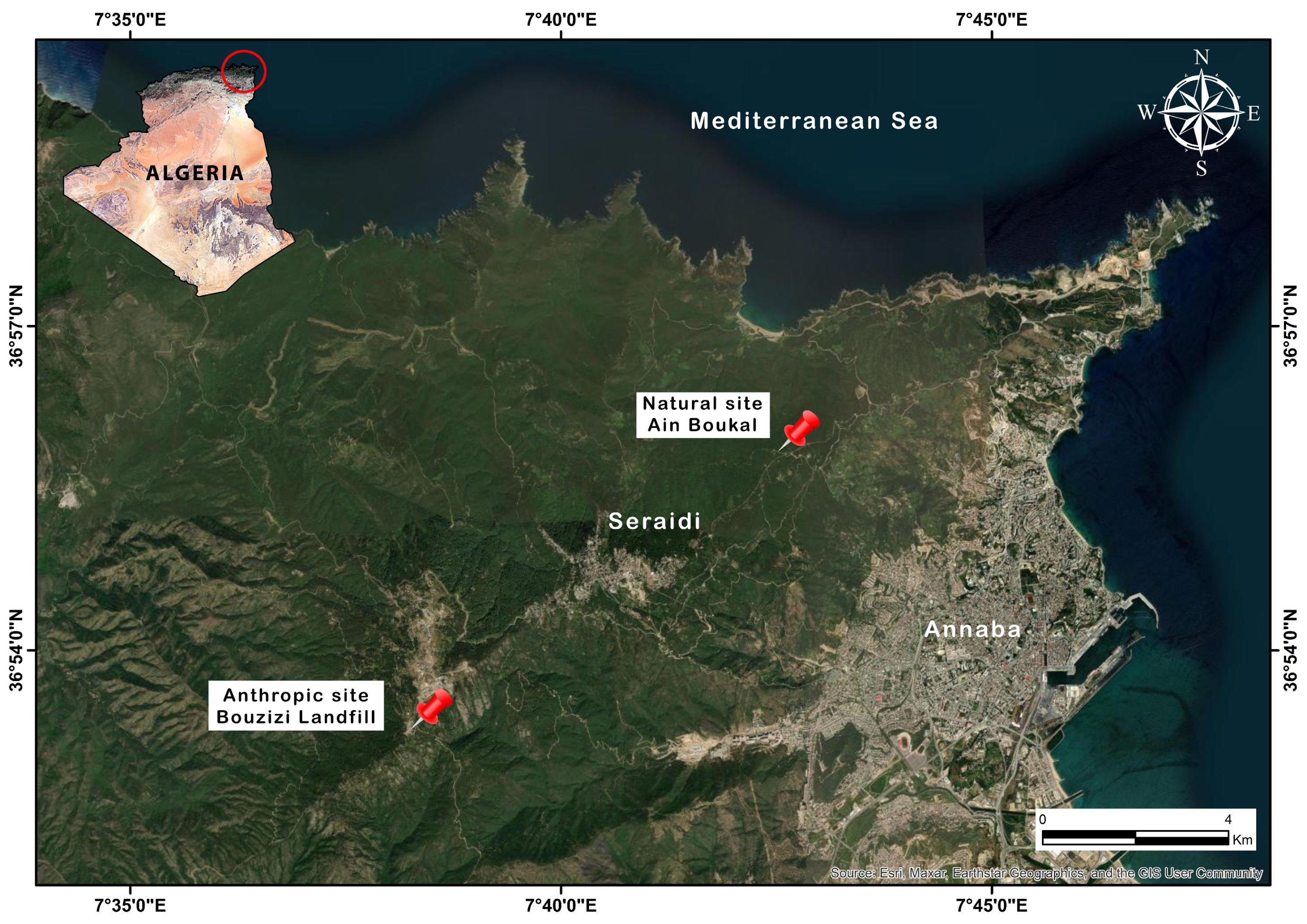
2.1. Study Area
2.2. Sampling Strategy and Sample Collection
2.3. Heavy Metal Analysis
2.4. Data Analysis
3. Results
Heavy Metal Accumulation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Edo, G.I.; Samuel, P.; Oloni, G.O.; Ezekiel, G.O.; Ikpekoro, V.O.; Obasohan, P.; Ongulu, J.; Otunuya, C.F.; Opiti, A.R.; Ajakaye, R.S.; et al. Environmental persistence, bioaccumulation, and ecotoxicology of heavy metals. Chem. Ecol. 2024, 40, 322–349. [Google Scholar] [CrossRef]
- Merian, E.; Clarkson, T.W. Metals and Their Compounds in the Environment, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 1991; p. 1438. [Google Scholar]
- Mishra, S.; Singh, G.; Gupta, A.; Tiwari, R.K. Heavy metal metalloid contamination: Their sources in environment and accumulation in food chain. In Heavy Metal Toxicity: Environmental Concerns, Remediation and Opportunitie; Springer Nature: Singapore, 2023; pp. 19–47. [Google Scholar]
- Angon, P.B.; Shafiul Islam, M.D.; Shreejana, K.C.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef] [PubMed]
- Maľová, J.; Ciberej, J.; Maľa, P.; Zigo, F.; Semjon, B. Heavy metal levels in the tissues of wild living animals from two distinct industrially exploited areas in slovakia. Slovak J. Anim. Sci. 2019, 52, 100–110. [Google Scholar]
- Jota Baptista, C.; Seixas, F.; Gonzalo-Orden, J.M.; Oliveira, P.A. Biomonitoring of heavy metals and metalloids with wild mammals in the Iberian Peninsula: A systematic review. Environ. Rev. 2022, 31, 66–75. [Google Scholar] [CrossRef]
- Kalisinska, E. (Ed.) Mammals and Birds as Bioindicators of Trace Element Contaminations in Terrestrial Environments: An Ecotoxicological Assessment of the Northern Hemisphere; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Zemanova, M.A. Towards more compassionate wildlife research through the 3Rs principles: Moving from invasive to non-invasive methods. Wildl. Biol. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Padrell, M.; Llorente, M.; Amici, F. Invasive Research on Non-Human Primates—Time to Turn the Page. Animals 2021, 11, 2999. [Google Scholar] [CrossRef]
- García-Muñoz, J.; Pérez-López, M.; Soler, F.; Míguez-Santiyán, M.P.; Martínez-Morcillo, S. Trace Metals in the Environment: Non-Invasive Samples for Biomonitoring Heavy Metals in Terrestrial Ecosystems; Chapter book; IntechOpen: Rijeka, Croatia, 2023; pp. 1–23. [Google Scholar]
- Davison, A.; Birks, J.D.; Brookes, R.C.; Braithwaite, T.C.; Messenger, J.E. On the origin of faeces: Morphological versus molecular methods for surveying rare carnivores from their scats. J. Zool. 2002, 257, 141–143. [Google Scholar] [CrossRef]
- Torre, I.; Arrizabalaga, A.; Freixas, L.; Ribas, A.; Flaquer, C.; Díaz, M. Using scats of a generalist carnivore as a tool to monitor small mammal communities in Mediterranean habitats. Basic Appl. Ecol. 2013, 14, 155–164. [Google Scholar] [CrossRef]
- Mazhar, S.; Syed, A.; Khan, B.N.; Yasmin, R. Fecal Matter as a Bio-indicator of Heavy Metal Toxification in Punjab Urial. Lahore Garrison Univ. J. Life Sci. 2020, 1, 99–103. [Google Scholar] [CrossRef]
- Harika, T.L.; Al-Ghanim, K.A.; Riaz, M.N.; Krishnappa, K.; Pandiyan, J.; Govindarajan, M. Fishing Cat Scats as a Biomonitoring Tool for Toxic Heavy Metal Contamination in Aquatic Ecosystems. Toxics 2023, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V. Mammalian Scat as a Bio-indicator of Heavy Metals Contaminationin Western Rajasthan, India. Int. J. Sci. Res. Publ. 2012, 2, 1–7. [Google Scholar]
- Torre, I.; Arrizabalaga, H.; Ribas, A. The diet of the genet (Genetta genetta Linnaeus, 1758) as a source of information on local small mammal communities. Galemys 2015, 27, 71–75. [Google Scholar] [CrossRef]
- Boukheroufa, M.; Sakraoui, F.; Benyakoub, S.; Giraudoux, P.; Raoul, F. Ecologie alimentaire de la Genette commune (Genetta genetta) dans un écosystème forestier du parc national d’El-Kala (nord est algérien). Mésogée 2009, 65, 83–91. [Google Scholar]
- Boukheroufa, M.; Sakraoui, F.; Belbel, F.; Sakraoui, R. Winter diet of the common genet, Genetta genetta (Carnivora, Viverridae), and the African golden wolf, Canis anthus (Carnivora, Canidae), in altitudinal locality of the Edough Forest (northeastern Algeria). Vestn. Zool. 2020, 54, 553–560. [Google Scholar]
- Belbel, F.; Boukheroufa, M.; Benotmane, C.H.; Sakraoui, R.; Henada, L.R.I.; Sakraoui, F. Selection Strategy of Small Mammalian Preys by the Common Genet Genetta genetta between Natural and Anthropized Environments in Edough Forest Massif (Northeastern Algeria). J. Bioresour. Manag. 2022, 9, 4. [Google Scholar]
- Sánchez, C.A.; Nadal, J. Bioaccumulation of metals and effects of landfill pollution in small mammals, Part I. The greater white-toothed shrew, Crocidura russula. Chemosphere 2007, 68, 703–711. [Google Scholar] [CrossRef]
- Sánchez-Chardi, A.; Peñarroja-Matutano, C.; Ribeiro, C.A.; Nadal, J. Bioaccumulation of metals and effects of a landfill in small mammals. Part II. The wood mouse, Apodemus sylvaticus. Chemosphere 2007, 70, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Gaukler, S.M.; Murphy, S.M.; Berryhill, J.T.; Thompson, B.E.; Sutter, B.J.; Hathcock, C.D. Investigating effects of soil chemicals on density of small mammal bioindicators using spatial capture recapture models. PLoS ONE 2020, 15, e0238870. [Google Scholar] [CrossRef]
- Mojtaba, Z.; Tahereh, B.; Fariba, A.; Mohammad, R. Monitoring of heavy metal impacts by small mammals. World J. Adv. Healthc. Res. 2020, 4, 47–53. [Google Scholar]
- Torre, I.; Jaime-González, C.; Díaz, M. Habitat Suitability for Small Mammals in Mediterranean Landscapes: How and Why Shrubs Matter. Sustainability 2022, 14, 1562. [Google Scholar] [CrossRef]
- Vela, E.; Benhouhou, S. Evaluation d’un nouveau point-chaud de biodiversité végétale dans le bassin méditerranéen (Afrique du nord). C. R. Biol. 2007, 330, 589–605. [Google Scholar] [CrossRef]
- Hadiby, R.; Boukheroufa, M.; Adjami, Y.; Djedda, H.; Boussaha, A.; Frih, A.; Benotmane, K.; Sakraoui, F. Part comparée des saproxyliques dans le peuplement de Coléoptères entre milieu naturel et milieu post-incendié du massif forestier de l’Édough (Nord-Est, Algérie). Bull. Soc. Zool. Fr. 2022, 147, 167–175. [Google Scholar]
- Delibes, M.; Rodríguez, A.; Parreño, F.F. Food of the genet (Genette genetta) in northern African. Zool. Soc. Lond. 1989, 218, 321–328. [Google Scholar] [CrossRef]
- Le Jacques, D.; Lodé, T. L’alimentation de la Genette d’Europe Genetta genetta L. 1758, dans un bocage de l’ouest de la France. Mammalia 1994, 58, 383–389. [Google Scholar] [CrossRef]
- Sanchez, M.; Rodrigues, P.; Ortuño, V.; Herrero, J. Feeding habits of the Genetta genetta in an iberian continental wetland. Hystrix 2008, 19, 133–142. [Google Scholar]
- Hamdine, W.; Thevenot, M.; Sellami, M.; De-Smet, K. Le régime alimentaire de la Genette (Genetta genetta Linné, 1758) dans le parc national du Djurdjura, Algérie. Mammalia 1993, 57, 9–19. [Google Scholar]
- Hammer, O.; Harper DA, T.; Ryan, P.D. PAST: Paleontological Statistic soft ware package for education and data analysis. Paleontol. Eletronica 2001, 4, 1–9. [Google Scholar]
- Da Costa, L.S.; De Souza, A.P.; Bovendorp, R.S. Heavy metals in hair of small mammals from the cacao agroforestry and Brazilian Atlantic Forest. Glob. Ecol. Conserv. 2023, 46, e02620. [Google Scholar] [CrossRef]
- Beernaert, J.; Scheirs, J.; Leirs, H.; Blust, R.; Verhagen, R. Non-destructive pollution exposure assessment by means of wood mice hair. Environ. Pollut. 2007, 145, 443–451. [Google Scholar] [CrossRef]
- McLean, C.M.; Koller, C.E.; Rodger, J.C.; MacFarlane, G.R. Mammalian hair as an accumulative bioindicator of metal bioavailability in Australian terrestrial environments. Sci. Total Environ. 2009, 407, 3588–3596. [Google Scholar] [CrossRef] [PubMed]
- Tête, N.; Afonso, E.; Crini, N.; Drouhot, S.; Prudent, A.S.; Scheifler, R. Hair as a noninvasive tool for risk assessment: Do the concentrations of cadmium and lead in the hair of wood mice (Apodemus sylvaticus) reflect internal concentrations? Cotoxicology Environ. Saf. 2014, 108, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Glimcher, M.J. Bone: Nature of the calcium phosphate crystals and cellular, structural, and physical chemical mechanisms in their formation. Rev. Miner. Geochem. 2006, 64, 223–282. [Google Scholar] [CrossRef]
- Martiniaková, M.; Omelka, R.; Jančová, A.; Stawarz, R.; Formicki, G. Concentrations of selected heavy metals in bones and femoral bone structure of bank (Myodes glareolus) and common (Microtus arvalis) voles from different polluted biotopes in Slovakia. Arch. Environ. Contam. Toxicol. 2011, 60, 524–532. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Springer Environmental Pollution: Berlin/Heidelberg, Germany, 2013; Volume 22, p. 493. [Google Scholar]
- Hani, A.; Djarbri, L.; Mania, J. Etude des caractéristiques physico-chimiques du massif cristallophyllien de Séraïdi (nord-est Algérien). Hard Rock Hydrosystenis. AH IAHSPubl 1997, 241, 47–59. [Google Scholar]
- Malmsten, A.; Dalin, A.M.; Pettersson, J.; Persson, S. Concentrations of cadmium, lead, arsenic, and some essential metals in wild boar from Sweden. Eur. J. Wildl. Res. 2021, 67, 1–8. [Google Scholar] [CrossRef]
- Cerar, S.; Serianz, L.; Koren, K.; Prestor, J.; Mali, N. Synoptic Risk Assessment of Groundwater Contamination from Landfills. Energies 2022, 15, 5150. [Google Scholar] [CrossRef]
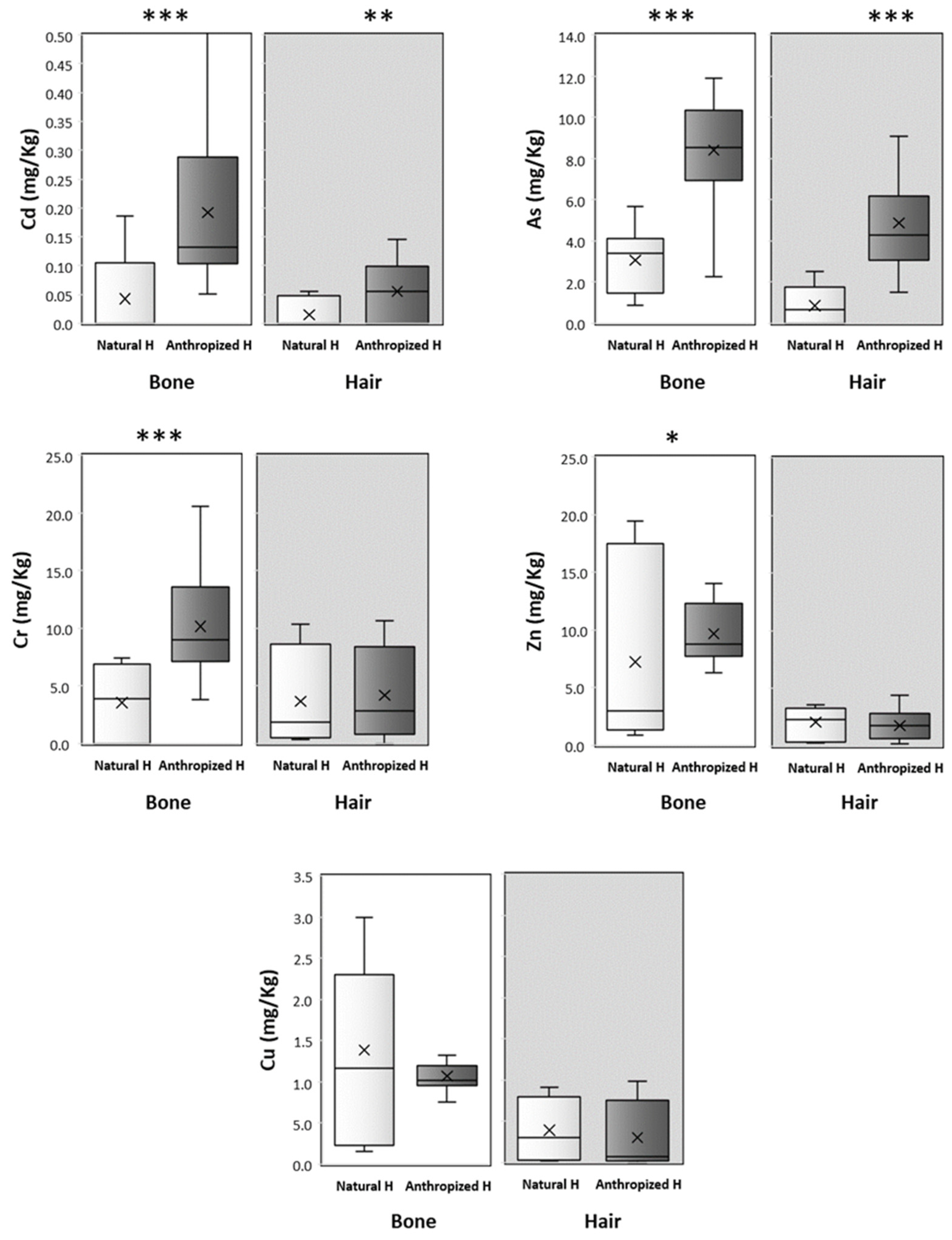
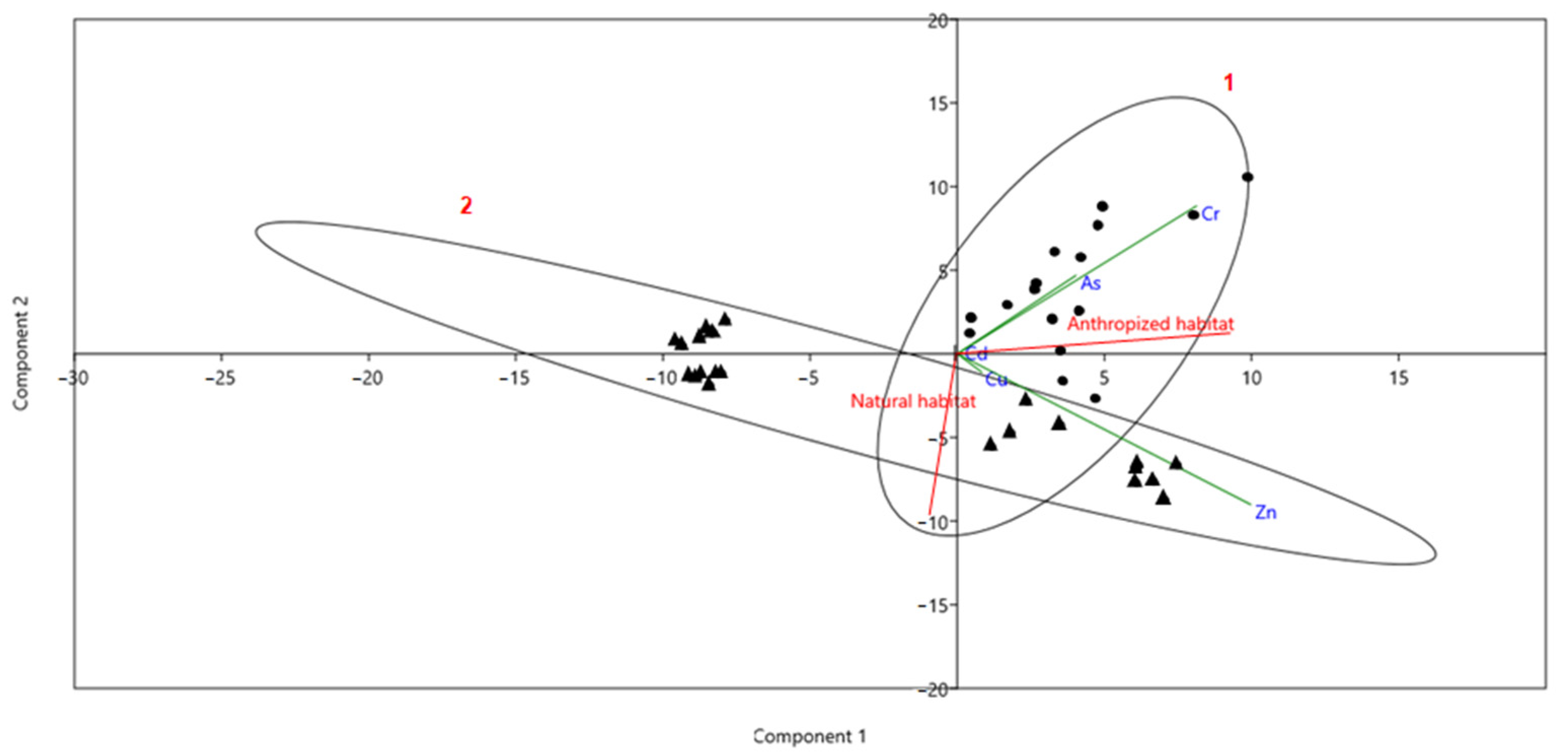
| Cd | As | Cr | Zn | Cu | |||
|---|---|---|---|---|---|---|---|
| Bone | Natural Habitat (n = 19) | Median | 0.000 | 3.391 | 3.873 | 3.024 | 1.170 |
| Max | 0.186 | 5.665 | 7.414 | 19.463 | 3.001 | ||
| Min | 0.000 | 0.902 | 0.000 | 0.904 | 0.158 | ||
| Anthropized Habitat (n = 20) | Median | 0.132 | 8.550 | 8.988 | 8.819 | 1.025 | |
| Max | 0.521 | 11.906 | 20.556 | 14.040 | 1.321 | ||
| Min | 0.051 | 2.251 | 3.827 | 6.330 | 0.757 | ||
| Mann–Whitney (Bone: Natural H/Anthropized H) | P | 0.0002 *** | 0.0001 *** | 0.0001 *** | 0.0538 * | 0.6150 | |
| U | 54 | 17 | 35 | 120 | 168 | ||
| Hair | Natural Habitat (n = 19) | Median | 0.000 | 0.669 | 1.891 | 2.359 | 0.307 |
| Max | 0.056 | 2.505 | 10.341 | 3.610 | 0.915 | ||
| Min | 0.000 | 0.000 | 0.399 | 0.306 | 0.025 | ||
| Anthropized Habitat (n = 20) | Median | 0.056 | 4.309 | 2.879 | 1.768 | 0.077 | |
| Max | 0.146 | 9.063 | 10.700 | 4.469 | 0.990 | ||
| Min | 0.000 | 1.532 | 0.000 | 0.252 | 0.000 | ||
| Mann–Whitney (Hair: Natural H/Anthropized H) | P | 0.0056 ** | 0.0001 *** | 0.4162 | 0.4119 | 0.2414 | |
| U | 125.5 | 12.5 | 159.5 | 159.5 | 169.0 | ||
| Mann–Whitney (Natural Habitat: Bone/Hair) | P | 0.5423 | 0.0001 *** | 0.7427 | 0.794 | 0.0011 *** | |
| U | 162.5 | 42 | 169 | 119.5 | 74.5 | ||
| Mann–Whitney (Anthropized Habitat: Bone/Hair) | P | 0.0001 *** | 0.0001 *** | 0.0003 *** | 0.0001 *** | 0.0001 *** | |
| U | 51 | 59 | 68 | 0 | 13 | ||
| Animals | Approach | Method | Habitat | Metals (mg kg−1) | References |
|---|---|---|---|---|---|
| Rodents: Akodon cursor, Guerlinguetus ingrami, Hylaeamys seuanezi, Oecomys catherinae, Rattus sp., Rhipidomys mastacalis | Non invasive | Hair | Cacao Agroforests | Mn: 0.12–12.41 Cu: 0.2–4.86 | [33] |
| Apodemus sylvaticus | Non invasive | Hair | Forests Near Industrial Sites | Pb: 0.5–10 Cd: 1–12 | [34] |
| Rodents: Rattus rattus and Rattus norvegicus | Non-invasive | Hair | Urban and Forested Anthropized Areas, Industrial Pollution (near an old Pb/Zn smelter) | Cd: 0.02–1.03 Cu: 6.71–19.80 Zn: 103–460 | [35] |
| Pool of small mammalian preys (Rodents: Apodemus, Rattus and Mus) | Non invasive | Hair found in scat | Bouzizi Landfill | Cd: 0–0.146 As: 1.532–9.063 Zn: 0.252–4.469 Cu: 0–0.99 Cr: 0–10.70 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belbel, F.; Boukheroufa, M.; Micle, V.; Sur, I.M.; Sakraoui, F.; Smical, I. Heavy Metal Accumulation (Cd, As, Zn, Cu, Cr) in Hair and Bones of Small Mammal Prey of the Sentinel Species Common Genet (Genetta genetta) in an Anthropogenic Environment of Edough Mountain Forest, Northeastern Algeria. Animals 2025, 15, 114. https://doi.org/10.3390/ani15010114
Belbel F, Boukheroufa M, Micle V, Sur IM, Sakraoui F, Smical I. Heavy Metal Accumulation (Cd, As, Zn, Cu, Cr) in Hair and Bones of Small Mammal Prey of the Sentinel Species Common Genet (Genetta genetta) in an Anthropogenic Environment of Edough Mountain Forest, Northeastern Algeria. Animals. 2025; 15(1):114. https://doi.org/10.3390/ani15010114
Chicago/Turabian StyleBelbel, Fatma, Mehdi Boukheroufa, Valer Micle, Ioana Monica Sur, Feriel Sakraoui, and Irina Smical. 2025. "Heavy Metal Accumulation (Cd, As, Zn, Cu, Cr) in Hair and Bones of Small Mammal Prey of the Sentinel Species Common Genet (Genetta genetta) in an Anthropogenic Environment of Edough Mountain Forest, Northeastern Algeria" Animals 15, no. 1: 114. https://doi.org/10.3390/ani15010114
APA StyleBelbel, F., Boukheroufa, M., Micle, V., Sur, I. M., Sakraoui, F., & Smical, I. (2025). Heavy Metal Accumulation (Cd, As, Zn, Cu, Cr) in Hair and Bones of Small Mammal Prey of the Sentinel Species Common Genet (Genetta genetta) in an Anthropogenic Environment of Edough Mountain Forest, Northeastern Algeria. Animals, 15(1), 114. https://doi.org/10.3390/ani15010114






