Simple Summary
Bile acids are synthesized from cholesterol in the liver and play a crucial role in the metabolism of dietary lipids as emulsifiers. Exogenous bile acids supplementation can promote the growth of fish reared at optimal water temperatures by increasing feed intake and digestion, enhancing antioxidant capacity, and improving gut health. However, whether they also have positive effects on fish reared at high water temperatures remains unclear. In this study, we investigated the effects of dietary bile acids on growth, glucose metabolism, and intestinal health in spotted seabass reared at high temperatures (33 °C). The results demonstrated that dietary bile acids promoted fish growth, improved glucose metabolism, and maintained intestinal health in spotted seabass.
Abstract
An 8-week feeding trial was performed to investigate the effects of dietary bile acids on growth, glucose metabolism, and intestinal health in spotted seabass (Lateolabrax maculatus) reared at high temperatures (33 °C). The fish (20.09 ± 1.12 g) were fed diets supplemented with bile acids: 0 (Con), 400 (BA400), 800 (BA800), and 1200 (BA1200) mg/kg, respectively. The results showed that the growth was promoted in fish at the BA800 treatment compared with the control (p < 0.05). Increased enzyme activities and transcripts of gluconeogenesis in the liver were observed, whereas decreased enzyme activities and transcripts of glycolysis, as well as glycogen content, were shown in the BA800 treatment (p < 0.05). The transcripts of bile acid receptors fxr in the liver were up-regulated in the BA800 treatment (p < 0.05). A bile acid supplementation of 800 mg/kg improved the morphological structure in the intestine. Meanwhile, intestinal antioxidant physiology and activities of lipase and trypsin were enhanced in the BA800 treatment. The transcripts of genes and immunofluorescence intensity related to pro-inflammation cytokines (il-1β, il-8, and tnf-α) were inhibited, while those of genes related to anti-inflammation (il-10 and tgf-β) were induced in the BA800 treatment. Furthermore, transcripts of genes related to the NF-κB pathway in the intestine (nfκb, ikkα, ikkβ, and ikbα1) were down-regulated in the BA800 treatment. This study demonstrates that a dietary bile acid supplementation of 800 mg/kg could promote growth, improve glucose metabolism in the liver, and enhance intestinal health by increasing digestive enzyme activity and antioxidant capacity and inhibiting inflammatory response in L. maculatus.
1. Introduction
Spotted seabass (Lateolabrax maculatus) has become a major fish cultured in southern China due to its high nutritional and economic value [1]. Its total production reached approximately 218,000 tons in 2022 [2]. The optimal water temperature range for L. maculatus is from 16 °C to 28 °C [3]. In the main farming areas for L. maculatus, however, water temperature often reached 33 °C or higher along the southeast coast of China in summer. Water temperature is one of the most pervasive and influential environmental factors in intensive aquaculture, which directly impacts almost all aspects of fish physiology [4,5]. A high water temperature may lead to an excessive accumulation of reactive oxygen species (ROS) [6,7], causing energy metabolism disorders in organisms [8,9], ultimately resulting in various adverse effects on growth, antioxidant capacity, immunity response, and survival in fish [10,11,12]. Our recent studies demonstrated that a high water temperature of 33 °C may lead to a decrease in growth [1], antioxidant capacity, immune response, and the survival of L. maculatus, causing hepatic metabolic disorders [13] and intestinal mucosal damage [14] in L. maculatus. Thus, the negative effects of high temperatures on fish have become one of the main obstacles to the development of the aquaculture industry [15].
Bile acid is synthesized by cholesterol in the liver and is mainly stored in the gallbladder as bile salts [16]. In general, bile acids primarily enhance the efficiency of dietary lipid digestion in fish by acting as emulsifiers and also play a crucial role in activating lipases [17,18] and improving the absorption and transportation of lipid and lipid-soluble nutrients in fish [19]. Meanwhile, as a signaling molecule, bile acids may be involved in regulating the metabolism of lipids and glucose in organisms [20]. Furthermore, further research indicated that dietary supplementation of bile acids can also promote antioxidant capacity [21], improve anti-inflammatory properties [22], enhance immune response [23], and maintain intestinal health [24] in fish.
Given the importance of bile acids in the physiological metabolism of the body, their multiple biological properties for fish are also increasingly recognized [25,26,27]. Surprisingly, a previous study in turbot (Scophthalmus maximus) has found that high water temperature inhibited the expression of the cyp27a1 gene in bile acid synthesis compared with the optimal water temperature [28]. In agreement with this, the data of bile acid metabolomics from our study indicated that the synthesis of endogenous bile acid in L. maculatus reared at a high water temperature (33 °C) was significantly lower (p < 0.05) than that of fish reared at the optimal temperature (27 °C) (See Figure S1). However, it remains largely unclear whether bile acid supplementation can mitigate the negative effects of high water temperature on fish. We tested the hypothesis that the supplementation of appropriate levels of exogenous bile acids could be beneficial in mitigating the negative effects on growth and physiological homeostasis caused by high temperatures on fish. Thus, the main objective of this study was to evaluate the effects of dietary supplementation of bile acids on growth performance, glucose metabolism, and intestinal health in L. maculatus reared under high water-temperature conditions (33 °C).
2. Materials and Methods
2.1. Ethics Statement
All the animal trials in this study were conducted according to the guidelines of the Ethics Committee of Care and Use for Laboratory Animals of Jimei University, Xiamen, Fujian, China (No. JMU202303004, Approval date: 10 March 2023).
2.2. Preparation of Experimental Diets
Four isonitrogenous (43.0% crude protein) and isolipidic (12% crude lipid) diets were prepared, four diets of which were supplemented with different levels of bile acids mixture (0, 400, 800, and 1200 mg/kg). These treatments were defined as Con, BA400, BA800, and BA1200, respectively. The bile acid mixture (including 75.2% hyodeoxycholic acid (HDCA), 17.4% chenodeoxycholic acid (CDCA), and 4.2% hyodeoxycholicacid (HCA)) was observed from Longchang Animal Health Co., Ltd. (Dezhou, Shandong, China). In this study, fishmeal, soybean meal, and poultry by-product meal were used as the main protein sources, and fish oil and soybean oil were used as lipid sources. The formulation and proximate composition of five diets for juvenile L. maculatus are shown in Table 1. The diets were prepared as described in our previous study [1]. Prior to diet preparation, all ingredients were crushed and sieved below 60-mesh. After mixing all the dry ingredients, the oil mixture was added, followed by the addition of 30% (w/w) deionized distilled water to form a dough. The feed at ~2.5 mm diameter was made using a cold-extruded pellet producer. The feeds were dried at 50 °C for 10 h, then stored at −20 °C until use.

Table 1.
Formulation and proximate composition of the experimental diets (g/kg dry-matter basis).
2.3. Experimental Design and Feeding Trial
One thousand juvenile L. maculatus were obtained from the commercial fish breeding farm (Zhangzhou, Fujian, China) and transferred to the aquaculture research facilities of Jimei University. The fish were housed in a 1000 L semi-static system with a pre-set temperature of 33 °C. They were fed the same control diets twice daily for 2 weeks to acclimate to the experimental environment. After acclimation, 240 fish (20.09 ± 1.12 g) were randomly grouped into twelve 150 L tanks (20 fish per tank) connected to a recirculating aquaculture system with a pre-set temperature of 33 °C. Three replicates were allocated to each dietary bile acid treatment. During the feeding trial for 8 weeks, fish were fed the bile acid-supplemented diet at ~3% of their weight twice daily at 8:00 and 17:00. Uneaten food and feces were removed after 30 min of feeding. The dechlorinated tap water was renewed, and feed intake was recorded daily. The conditions of the culture were as follows: water temperature at 33 ± 0.5 °C, 12 h light–12 h dark photoperiod, total ammonia–nitrogen < 0.35 mg/L, dissolved oxygen ≥ 6.2 mg/L, and pH 7.0~7.4.
2.4. Sample Collection
At the end of the feeding experiment, the experimental fish were starved for 24 h to allow emptying of the intestine. All fish from each tank were anesthetized with eugenol (0.1 mL/L) for ~1 min to record the body length and weight and for subsequent sample collection. Two fish were pooled as one composite sample randomly selected from each tank and stored at −20 °C to determine the proximate composition of whole body (n = 3). The livers of 2 fish were pooled as one composite sample per tank (n = 3) was collected for measuring the enzyme activity related to glucose metabolism. The livers of 2 fish were pooled as one composite sample per tank was collected for measuring the transcripts of genes related to bile acid receptors and glucose metabolism (n = 3). The midgut of 2 fish were pooled as one composite sample per tank was collected for measuring the antioxidant capacity and digestive enzyme activity (n = 3). The midgut of 2 fish were pooled as one composite sample per tank was collected for measuring the transcripts of genes related to inflammatory cytokines and NF-κB pathway (n = 3). The midgut of 2 fish per tank was fixed in a 4% paraformaldehyde solution (Biosharp, Beijing, China) for histological examinations and immunofluorescence detection (n = 6).
2.5. Proximate Composition of Diets and Whole Fish
The proximate compositions of diets and whole-body were measured according to the standard procedures [29]. Crude protein content was measured using a Kjeldahl System (2300-Auto-analyzer, FOSS, Hillerød, Denmark). Crude lipid content was analyzed following Soxhlet’s extraction method. For the determination of moisture content, samples were dried to a constant weight at 105 °C. Ash content was measured by combusting using a muffle furnace at 550 °C for 8 h.
2.6. Glucose Metabolism of Liver
The liver weighing 0.1 g from each replicate was weighed, homogenized at 4 °C using a 9-fold volume-to-weight ratio of phosphate-buffered saline (PBS, pH = 7.4), and centrifuged at 2500 rpm for 10 min. The supernatants were collected and allocated for the subsequent assays. Enzyme activities of hexokinase (HK, No. A077-3-1), pyruvate kinase (PK, No. A076-1-1), phosphofructokinase (PFK, No. A129-1-1), phosphoenolpyruvate carboxykinase (PEPCK, No. A131-1-1), and phosphoenolpyruvate carboxylase (PEPC, No. A130-1-1) in the liver samples were determined following the protocols of commercial assay kits from Jiancheng Bioengineering Institute (Nanjing, China). Results were obtained based on the wet weight of the samples.
The liver glycogen content was measured in strict accordance with the instructions provided by Nanjing Jiancheng Bioengineering Institute (No. A043-1-1). In brief, 95 mg of liver samples were hydrolyzed in a boiling water bath for 20 min with an acidic solution (1:3 weight to volume ratio). The resulting hydrolysate was diluted with deionized water to prepare the glycogen assay solution. A 0.1 mL aliquot of this solution was mixed with 0.9 mL deionized water, and a chromogenic agent was heated for 5 min. OD values were then measured at 620 nm after cooling.
2.7. Intestinal Antioxidant Capacity and Digestive Enzyme Activity
The mid-intestine weighing 0.1 g from each replicate was weighed, homogenized at 4 °C using a 9-fold volume-to-weight ratio of phosphate-buffered saline (PBS, pH = 7.4), and centrifuged at 2500 rpm for 10 min. The activities of three digestive enzymes (lipase, trypsin, and amylase) as well as total antioxidant capacity (T-AOC, No. A015-2-1), superoxide dismutase (SOD, No. A001-3-2) activity, and malondialdehyde (MDA, No. A003-1-2) content in the intestine of experimental fish were determined according to the methods described in the commercial assay kits (Jiancheng Bioengineering Institute, Nanjing, China).
2.8. Histological Analyses and Immunofluorescence Detection in the Intestine
Histological analyses of the mid-intestine samples were performed following the method with minor modifications [30]. In brief, paraformaldehyde-fixed intestines were cut off with a length of ~0.6 cm, followed by dehydration in graded alcohols and clearance in xylene. Afterward, the muscle samples were embedded in paraffin wax, sectioned at 6 μm thickness, and stained with hematoxylin and eosin (H&E). Six randomly chosen fields of the intestine from each treatment were observed under a microscope (Leica DM5500B, Heidelberg, Baden-Württemberg, Germany). The villus length (VL), villus width (VW), and thicknesses of muscularis (MT) were determined using the Image-Pro Plus 0.53 software connected to the microscope (n = 6).
The procedures of immunofluorescence staining for inflammatory cytokines (IL-1β, IL-8, TNF-α) were carried out consistently with H&E staining prior to embedding. Subsequently, antigen retrieval was performed, followed by encircling sealing serum, addition of primary and secondary antibodies, staining of cell nuclei with DAPI staining solution, tissue staining with autofluorescence quencher B solution, and sealing with anti-fluorescence quenching sealer before being placed under an orthogonal fluorescence microscope (Nikon, NIKON ECLIPSE C1, Tokyo, Tokyo Metropolis, Japan) for observation [31].
2.9. RNA Extraction and Quantitative Real-Time PCR (qPCR) Assay
The total RNA extraction, RNA quantity and quality, synthesis cDNA, and qPCR procedures were conducted following the previously described method with modifications [30]. Extraction of total RNA from the liver and intestine was performed according to the instructions of FastPure Cell/Tissue Total RNA Isolation Kit (Vazyme Biotechnology Co., Ltd., Nanjing, China). The concentration and purity of extracted total RNA were quantified using a spectrophotometer (NanoDrop Technologies, Waltham, MA, USA) at a wavelength of 260/280 nm, and then the integrity of the RNA was assessed through 1.5% agarose gel electrophoresis. For each sample, 3 μg (0.15 μg/μL) RNA was reverse-transcribed into cDNA for quantitative real-time PCR (RT-qPCR) following the protocol of commercial kit (R211-01, Vazyme, Nanjing, Jiangsu, China). The transcriptional expression of genes in the liver and intestine was quantified by real-time quantitative PCR (QuantStudio™ 6 Flex, AppliedBiosystems, Waltham, MA, USA). In brief, the RT-qPCR program was run at 95 °C for 30 s, followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s. After the PCR reaction, melting curve analysis was performed to confirm the specificity of the genes.
In this study, the sequences of the primers were designed using Primer 5.0 software and synthesized by Genewiz Co., Ltd., Suzhou, China (Table 2). The relative expression of target genes was normalized to β-actin and 18s using the 2−ΔΔCt method [32]. In addition, the amplification efficiency of all primers in this study was verified between 90 and 110%.

Table 2.
Sequences of the PCR primers of L. maculatus used in this study.
2.10. Statistical Analyses
All data were analyzed following one-way analysis of variance (ANOVA) using SPSS 22.0 statistical software. Prior to statistical analysis, Kolmogorov−Smirnov and Levene’s tests were used to test the assumptions of normality and homogeneity of variance, respectively. Multiple comparisons were performed using Tukey’s test to analyze the differences between the experimental groups. The level of significance was set at p < 0.05. All results are presented as mean ± standard error of the mean (SE).
3. Results
3.1. Growth Performance and Proximate Composition Analyses
After an 8-week feeding trial, the final body weight (FBW) and weight gain (WG) of L. maculatus were significantly increased in the BA800 treatment compared to the control fish (Table 3) (p < 0.05). Meanwhile, the feed intake (FI) was elevated in the BA400, BA800, and BA1200 treatments (p < 0.05). However, no significant differences in the feed conversion rate (FCR), abdominal fat ratio (AFR), or survival of L. maculatus were observed among all treatments.

Table 3.
The growth, feed utilization, and morphometric parameters of L. maculatus fed different levels of bile acids for 8 weeks.
The results of proximate composition analyses in the whole fish were reported in Table 4. In comparison to the control treatment, an increased content of crude lipid was observed in the BA800 treatment (p < 0.05), while an opposite result was shown in crude ash content (p < 0.05). Furthermore, there were no significant differences in the moisture and crude protein content of L. maculatus among all treatments (p > 0.05).

Table 4.
The proximate composition (%, wet weight) in the whole fish of L. maculatus fed different levels of bile acids for 8 weeks.
3.2. Glucose Metabolism of Liver
As shown in Figure 1, compared to the control treatment, glycogen content and activities of phosphoenolpyruvate carboxykinase (PEPCK) and phosphoenolpyruvate carboxylase (PEPC) in the liver of L. maculatus were significantly reduced (p < 0.05) in the BA800 treatment, whereas there was no difference among the other treatments (p > 0.05). Activities of hexokinase (HK) in the liver of fish were increased (p < 0.05) in the BA800 treatment. Meanwhile, activities of pyruvate kinase (PK) and phosphofructokinase (PFK) in the liver of fish were increased (p < 0.05) in the BA400 and BA800 treatments but decreased at the BA1200 treatment. The maximum values of these three parameters were obtained in the BA800, BA800, and BA400 treatments, respectively.
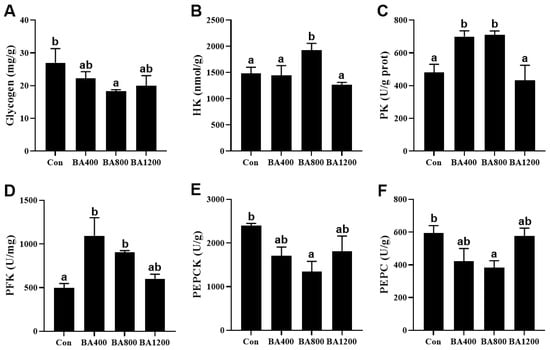
Figure 1.
Effects of dietary bile acid on the glycogen content (panel (A)) and enzyme activities of hexokinase (panel (B), HK), pyruvate kinase (panel (C), PK), phosphofructokinase (panel (D), PFK), phosphoenolpyruvate carboxykinase (panel (E), PEPCK), phosphoenolpyruvate carboxylase (panel (F), PEPC) in the liver of L. maculatus. Bars with different letters are different at p < 0.05.
The transcripts of the farnesoid X receptor (fxr) related to bile acid receptors in the liver from the BA800 treatment were significantly up-regulated (p < 0.05) compared to the control treatment, whereas the transcripts of trans-membrane G protein-coupled receptor-5 (tgr5) were not affected by the addition of dietary bile acids (p > 0.05) (Figure 2A). Transcripts of glycogen synthase (gys) and phosphorylase glycogen L (pygl) in the liver displayed an initial down-regulation followed by an up-regulation with the increase in dietary bile acid levels and were significantly lower in the BA800 treatment compared to the other treatments (p < 0.05) (Figure 2B). Meanwhile, the transcripts of glucose-6-phosphatase (g6pase) in the liver were inhibited by dietary bile acid, whereas transcripts of pepck and fructose 1,6-bisphosphatase (fbp) were only inhibited in the BA800 treatment (p < 0.05), with no alteration among the other treatments (p > 0.05) (Figure 2C). Furthermore, transcripts of pfk, hk, and pk in the liver were significantly up-regulated in the BA800 treatment compared to the control treatment (p < 0.05), while no differences were found among the other treatments (p > 0.05) (Figure 2C).
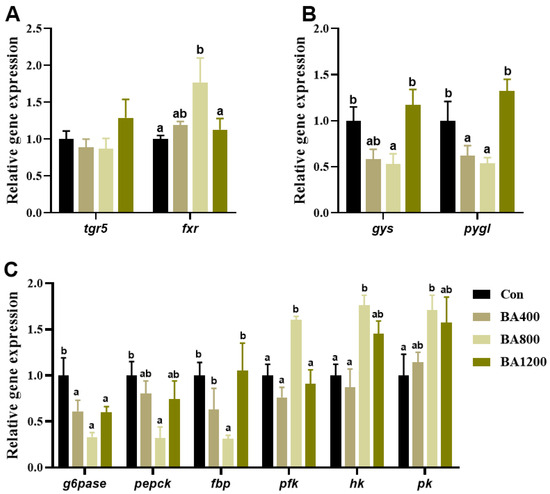
Figure 2.
Effects of dietary bile acids on the transcriptional expression of genes related to bile acid receptors (panel (A)), glycogen synthesis (gys), glycogen catabolism (pygl) (panel (B)), and glycolysis (g6pase, pepck, and fbp) and gluconeogenesis (pfk, hk, and pk) (panel (C)) in the liver of L. maculatus. Bars with different letters are different at p < 0.05.
3.3. Histological and Morphological Analyses in the Intestine
The histological analysis showed that control fish showed a typical inflammatory response in the intestine, including irregular arrangement of intestinal mucosa epithelium (Figure 3A). However, the damage to the morphology of the intestinal villus was significantly improved with the supplementation of dietary bile acids. The number and length of villus exhibited an increasing trend with increasing the levels of dietary bile acid supplementation, with both being significantly higher in the BA800 treatment compared to control fish (Figure 3B,C) (p < 0.05). Meanwhile, the width of the villus exhibited an opposite trend, and a significant decrease in villus width was observed in the BA400 and BA800 treatments compared to control fish (Figure 3D) (p < 0.05). In addition, the muscular thickness of the intestine was not altered by dietary bile acid supplementation.
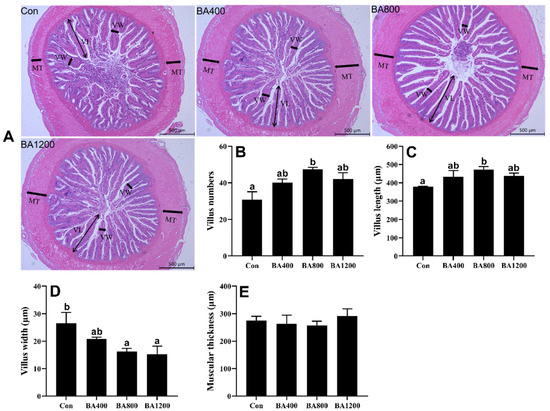
Figure 3.
Representative images of intestinal morphology (bar: 500 μm) in L. maculatus fed different levels of bile acids for 8 weeks (panel (A)) and morphological alterations (panels (B–E)). VL: villus length; VW: villus width; MT: muscular thickness (n = 3). Bars with different letters are different at p < 0.05.
3.4. Antioxidant Capacity in the Intestine
The antioxidant capacity in the intestine of L. maculatus was elevated by increasing the levels of dietary bile acids supplementation. Compared to the control treatment, total antioxidant capacity (T-AOC) was increased in the BA800 and BA1200 treatments (Figure 4A), while the activity of superoxide dismutase (SOD) was significantly induced by the BA400 and BA800 treatments (Figure 4B) (p < 0.05). Meanwhile, with the elevation of bile acid levels, MDA content in the BA400 and BA800 treatments exhibited a significant reduction compared to the control (Figure 4C) (p < 0.05).
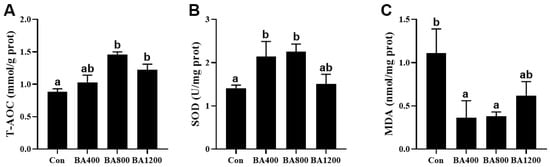
Figure 4.
Effects of dietary bile acids on the total antioxidant capacity (T-AOC, panel (A)) and activity of superoxide dismutase (SOD, panel (B)) and malondialdehyde (MDA, panel (C)) in the intestine of L. maculatus. Bars with different letters are different at p < 0.05.
3.5. Digestive Enzyme Activities in the Intestine
The activity of digestive enzymes in the intestine of L. maculatus was altered by increasing the levels of dietary bile acid supplementation. The activities of lipase and trypsin exhibited an initial increase followed by a subsequent decrease, with their highest value observed in the BA800 treatment (Figure 5A) (p < 0.05). The activity of amylase was no different among the dietary bile acid supplementation treatments (Figure 5C) (p > 0.05).
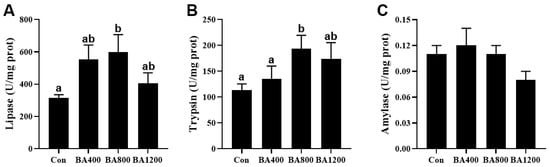
Figure 5.
Effects of dietary bile acids on the activities of lipase (panel (A)), trypsin (panel (B)), and amylase (panel (C)) in the intestine of L. maculatus. Bars with different letters are different at p < 0.05.
3.6. Immunofluorescence and Transcriptional Expression of Genes Related to Inflammatory Cytokines
The immunofluorescence and transcriptional expression of genes related to inflammatory cytokines in the intestine are shown in Figure 6 and Figure 7. The fluorescence intensities of intestinal pro-inflammatory cytokines interleukin-1β (IL-1β), interleukin-8 (IL-8), and tumor necrosis factor-α (TNF-α) in the BA800 treatment were significantly lower than in the other treatments (Figure 6) (p < 0.05). Similarly, transcripts of il-1β, il-8, and tnf-α were down-regulated as bile acid levels increased up to the BA800 diet and then up-regulated (Figure 7A) (p < 0.05). Meanwhile, transcripts of intestinal anti-inflammatory cytokines il-10 and transforming growth factor-β (tgf-β) were significantly up-regulated as bile acid levels increased up to the BA800 diet, followed by down-regulation (Figure 7B) (p < 0.05). However, the transcriptional expression of il-4 was no different among the dietary bile acid supplementation treatments (p > 0.05).
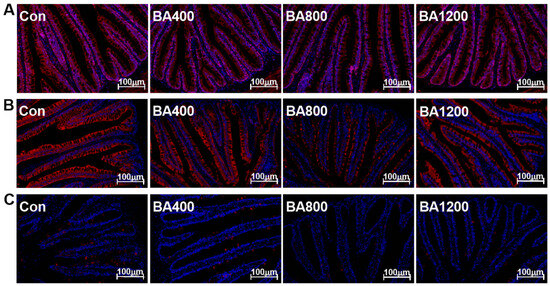
Figure 6.
Representative immunofluorescence images of inflammatory cytokines in the intestine of L. maculatus fed different levels of bile acids for 60 d (bar: 100 μm). (A) IL-1β, interleukin-1β; (B) IL-8, interleukin-8; (C) TNF-α, tumor necrosis factor-α (n = 3). The nucleus is stained blue, and the target inflammatory cytokines are stained red in the image above.
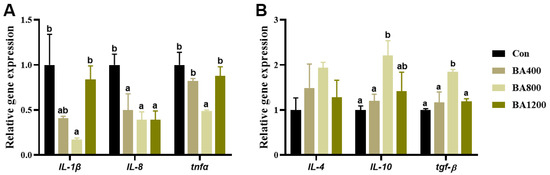
Figure 7.
Effects of dietary bile acids on the transcriptional expression of genes related to pro-inflammatory (panel (A)) and anti-inflammatory cytokines (panel (B)) in the intestine of L. maculatus. Bars with different letters are different at p < 0.05.
3.7. Transcriptional Expression of Genes Related to NF-κB Pathway
As shown in Figure 8, transcripts of nuclear factor κB (nf-κb), an inhibitor of κB kinase-α (ikkα), and NF-κB inhibitory protein α1 (ikbα1) in the intestine of L. maculatus were significantly down-regulated as bile acid levels increased up to BA800 diet, followed by up-regulation (Figure 8) (p < 0.05). For the inhibitor of κB kinase-α (ikkα), compared to the control, there was no difference in the transcripts across all dietary bile acid supplementation treatments, but the transcript of ikkα in the BA800 treatment was lower than that in the BA1200 treatment.
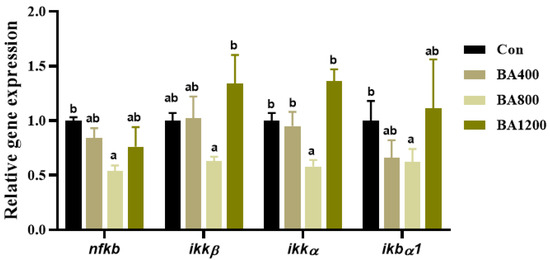
Figure 8.
Effects of dietary bile acids on the transcriptional expression of genes related to nuclear factor κB (NF-κB) pathway in the intestine of L. maculatus. Bars with different letters are different at p < 0.05.
4. Discussion
As indicated by many previous studies [14,33,34], three biological replicates were set up for each treatment in this study, and the samples from two fish were mixed together as one replicate to reduce variability between individual samples. Due to limitations in sample size and difficulties in obtaining certain samples (i.e., serum), such experimental methods are often used in research on aquatic animals [30]. In this study, the FBW and WG of L. maculatus were promoted by supplementing 800 mg/kg of bile acids in the diet, suggesting that the appropriate level of exogenous bile acid supplementation in the diet can promote the growth of L. maculatus. Similarly, the promoting effect of appropriate supplementation levels of dietary bile acids on the growth of fish has been widely reported in tilapia (Oreochromis niloticus) [35], largemouth bass (Micropterus salmoides) [36], European eel (Anguilla anguilla) [37], grass carp (Ctenopharyngodon idella) [27], turbot (Scophthalmus maximus) [38]. Meanwhile, the FI of L. maculatus increased with the increase in dietary bile acid levels, which seemed to be attributed to the promotion of growth. Previous studies have also indicated that the promotion of fish growth by bile acid is often positively correlated with increased FI [39], which is an undisputed result. Bile acids play a crucial role in regulating lipid metabolism by effectively enhancing the emulsification and transportation of lipids [40], as evidenced by the slight increase in abdominal fat ratio and significant accumulation in crude lipids in the whole fish with dietary supplementation of bile acids up to 800 mg/kg in this study. Furthermore, increased activity of intestinal lipase in fish fed with the diet supplemented with 800 mg/kg of bile acids also further demonstrates the beneficial effect of bile acids on lipid absorption. More similar results on the promotion of lipid digestion and absorption by the supplementation of exogenous bile acids have been obtained in large yellow croaker [33], largemouth bass [41], tilapia (Oreochromis niloticus) [26], and rainbow trout (Oncorhynchus mykiss) [42].
The liver is an important tissue that regulates glucose metabolism in organisms [43]. TGR5 and FXR are the two most important receptors for bile acids in animals, playing a crucial role in the molecular pathways regulating glucose metabolism involving bile acids [44,45]. It has been reported that FXR, as a nuclear transcription factor, regulates glucose and lipid metabolism balance through pyruvate dehydrogenase kinase 4 [46]. In this study, the up-regulated transcript of fxr was found in the liver of fish fed with a diet supplemented with 800 mg/kg of bile acids. Meanwhile, the same dietary bile acids level down-regulated transcripts of the genes related to gluconeogenesis (g6pase, pepck, and fbp) and up-regulated the genes related to glycolysis (pfk, hk, and pk). These findings are consistent with the effect of 800 mg/kg dietary bile acids on the activity of enzymes related to glucose metabolism in the liver. A previous study has also shown that bile acids and FXR inhibit the activities of PEPCK, G6Pase, and FBPase, all of which are enzymes involved in the hepatic gluconeogenesis pathway [47]. In addition, in this study, the reduction of hepatic glycogen content at the level of 800 mg/kg dietary bile acids further strengthens our evidence, suggesting that dietary bile acid supplementation promotes the conversion of glycogen to glucose for energy supply, thereby reducing the accumulation of glycogen in the liver of L. maculatus reared at high water temperature. Overall, these previous studies, together with our own results, confirm that the supplementing exogenous bile acids may inhibit hepatic gluconeogenesis and promote hepatic glycolysis by activating the bile acid receptor FXR, regulating glucose metabolism in fish [44].
Intestinal health is crucial for maintaining the normal growth and survival of fish [48]. The intestine in fish serves as the primary site for the digestion of food and absorption of nutrients, relying on the microvilli at the edges of the single-layer columnar epithelial cells [30]. Once intestinal mucosa is impaired, it can lead to the malabsorption of nutrients, increased membrane permeability, and inflammatory infiltration, thereby causing a potential risk to the growth and health of fish [49]. In the present study, the control fish exhibited a typical intestinal inflammation accompanied by a short and irregular arrangement of intestinal mucosa epithelium. However, dietary bile acid supplementation of 800 mg/kg increased the number and length of villus and decreased the villus width. Meanwhile, in this study, dietary bile acid supplementation of 800 mg/kg down-regulated the transcripts and fluorescence intensities of pro-inflammatory cytokines (il-1β, il-8, and tnf-α) and up-regulated the transcripts of anti-inflammatory cytokines (il-10 and tgf-β) in the intestine. These findings are consistent with a previous study showing that dietary bile acid supplementation inhibits the transcripts of il-1β, il-8, and tnf-α in the intestine by activating intestinal bile acid reporter TGR5 in grass carp [27]. NF-κB is one of the key nuclear transcription factors involved in inflammatory responses in organisms, regulating the transcription of many genes related to inflammation [50]. Previous studies have demonstrated that bile acids inhibit the nuclear translocation of nfκb to down-regulate the transcription of nfκb-dependent pro-inflammatory cytokines (i.e., il-1β, il-8, and tnf-α), thereby achieving the goal of inhibiting intestinal inflammation [27]. In the present study, dietary bile acid supplementation of 800 mg/kg down-regulated the transcripts of nfκb and its downstream regulation of key genes (ikkα, ikkβ, and ikbα1). Overall, synthesizing our previous research, it is clear that thermal stress-induced intestinal inflammation can cause damage to the intestinal mucosa in L. maculatus [14]. Nevertheless, optimal exogenous bile acid supplementation in diets has been shown to effectively mitigate this negative impact, protect intestinal barrier integrity, and promote intestinal health in L. maculatus.
Intestinal digestive enzymes also play a crucial role in the digestion and absorption of nutrients [51]. In this study, the increased activity of lipase can be recognized as an indicator that lipid utilization is promoted, which has been discussed in the preceding text. Meanwhile, dietary bile acid supplementation of 800 mg/kg increased trypsin activity in the intestine of L. maculatus and may imply an overall promotional effect of bile acids on the digestion and absorption of nutrients in feed rather than just the utilization of lipids. A similar result has also been reported that trypsin activity was induced when dietary bile acid was given at 130 mg/kg in thinlip mullet [39].
Antioxidant capacity is a crucial index for evaluating the health and oxidative stress status of fish [52]. Generally, oxidative stress occurs due to an imbalance between the generation and elimination of ROS in organisms [53]. The accumulation of undetoxified ROS can lead to lipid peroxidative damage to the cell membrane [30]. The increase in MDA content can reflect the degree of lipid peroxidation and cellular damage [54]. Bile acids may exert antioxidant effects by scavenging free radicals and inhibiting oxidative stress, helping to protect cells from oxidative damage in fish [21]. Previous studies have indicated that the dietary supplementation of bile acids reduces MDA content and increases the activities of SOD and T-AOC in largemouth bass (Micropterus salmoides) [21], large yellow croaker [25], striped catfish (Pangasianodon hypophthalmus) [16], and tongue sole (Cynoglossus semilaevis) [55]. In agreement with these, our results showed that the MDA content in the intestine was reduced when dietary bile acids were given as high as 800 mg/kg, whereas the T-AOC level and the activity of SOD were induced in the same level of bile acid supplementation in the diets, indicating that optimal exogenous bile acid supplementation helps prevent lipid peroxidation and maintain normal oxidative/antioxidant physiological homeostasis in fish via the rapid clearance of excessive ROS [21].
5. Conclusions
In summary, this study demonstrates that dietary supplementation with bile acids at 800 mg/kg improves growth and increases intestinal digestive enzyme activity and antioxidant capacity, thereby protecting barrier integrity in the intestine of L. maculatus. Furthermore, bile acids may promote glucose metabolism in the liver by activating the FXR receptor, and they may alleviate intestinal inflammatory responses by inhibiting the transcripts of the NF-κB pathway and inflammatory cytokines.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani14091299/s1, Figure S1: Effect of dietary bile acid levels on the content of the total bile acid in of L. maculatus reared at two temperatures for 8 weeks. References [56,57] are cited in the supplementary materials.
Author Contributions
Y.L.: data curation, writing—original draft preparation. X.L. (Xiao Li): data curation, writing—original draft preparation, funding acquisition. J.L.: methodology, formal analysis. K.S.: conceptualization, resources. X.L. (Xueshan Li): resources, visualization. L.W.: writing—reviewing and editing, validation. C.Z.: funding acquisition, supervision, writing—reviewing and editing. K.L.: conceptualization, project administration, writing—reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Agriculture Research System of China (CARS-47), the Special Fund Project for Promoting High-quality Development of Marine and Fishery Industry of Fujian Province (FJHYF-L-2023-6), the Natural Science Foundation of Fujian Province of China (grant number: 2023J01766) and the Open Research Fund of Key Laboratory of Healthy Mariculture for the East China Sea, Ministry of Agriculture and Rural Affairs (grant number: 2023ESHML03).
Institutional Review Board Statement
All the animal trials in this study were conducted according to the guidelines of the Ethics Committee of Care and Use for Laboratory Animals of Jimei University, China (No. JMU202303004, Approval date: 10 March 2023).
Informed Consent Statement
Written informed consent has been obtained from the owner of the animals involved in this study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
There are no conflicts of interest in the submission of “Effects of dietary supplementation of bile acids on growth, glucose metabolism and intestinal health of spotted seabass (Lateolabrax maculatus)”, and this manuscript is approved by all authors for publication.
References
- Cai, L.; Wang, L.; Song, K.; Lu, K.; Zhang, C.; Rahimnejad, S. Evaluation of protein requirement of spotted seabass (Lateolabrax maculatus) under two temperatures, and the liver transcriptome response to thermal stress. Aquaculture 2020, 516, 734615. [Google Scholar] [CrossRef]
- Ministry of Agriculture of the People’s Republic of China. China fishery statistical yearbook. In Department of Fishery of the Ministry of Agriculture; China Agricultural Press: Beijing, China, 2023; pp. 50–51. [Google Scholar]
- Wen, H.S.; Li, J.F.; Zhang, M.Z.; Li, Y.; Qi, X.; Zhang, K.Q. Physiology and Breeding Technology of Seabass in Mariculture; China Agricultural Press: Beijing, China, 2019; pp. 183–186. [Google Scholar]
- Buentello, J.A.; Gatlin, D.M.; Neill, W.H. Effects of water temperature and dissolved oxygen on daily feed consumption, feed utilization and growth of channel catfish (Ictalurus punctatus). Aquaculture 2000, 182, 339–352. [Google Scholar] [CrossRef]
- Zak, M.A.; Manzon, R.G. Expression and activity of lipid and oxidative metabolism enzymes following elevated temperature exposure and thyroid hormone manipulation in juvenile lake whitefish (Coregonus clupeaformis). Gen. Comp. Endocrinol. 2019, 275, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Z.; Quan, J.; Lu, J.; Zhao, G.; Sun, J. Dietary nanoselenium supplementation for heat-stressed rainbow trout: Effects on organizational structure, lipid changes, and biochemical parameters as well as heat-shock-protein- and selenoprotein-related gene expression. Fish Physiol. Biochem. 2022, 48, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Kameda, M.; Shoji, Y.; Hayashi, S.; Yamaguchi, T.; Sato, M. Effect of severe environmental thermal stress on redox state in salmon. Redox Biol. 2014, 2, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Bacchetta, C.; Cazenave, J. Effect of thermal stress on metabolic and oxidative stress biomarkers of Hoplosternum littorale (Teleostei, Callichthyidae). Ecol. Indic. 2017, 79, 361–370. [Google Scholar] [CrossRef]
- Zhao, H.; Ke, H.; Zhang, L.; Zhao, Z.; Lai, J.; Zhou, J.; Huang, Z.; Li, H.; Du, J.; Li, Q. Integrated analysis about the effects of heat stress on physiological responses and energy metabolism in Gymnocypris chilianensis. Sci. Total Environ. 2022, 806, 151252. [Google Scholar] [CrossRef]
- Kim, B.S.; Jung, S.J.; Choi, Y.J.; Kim, N.N.; Choi, C.Y.; Kim, J.W. Effects of different light wavelengths from LEDs on oxidative stress and apoptosis in olive flounder (Paralichthys olivaceus) at high water temperatures. Fish Shellfish Immun. 2016, 55, 460–468. [Google Scholar] [CrossRef]
- Matthews, K.R.; Berg, N.H. Rainbow trout responses to water temperature and dissolved oxygen stress in two southern California stream pools. J. Fish Biol. 1997, 50, 50–67. [Google Scholar] [CrossRef]
- Vinagre, C.; Madeira, D.; Narciso, L.; Cabral, H.N.; Diniz, M. Effect of temperature on oxidative stress in fish: Lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol. Indic. 2012, 23, 274–279. [Google Scholar] [CrossRef]
- Yang, X.; Weng, Q.; Li, X.; Lu, K.; Wang, L.; Song, K.; Zhang, C.; Rahimnejad, S. High water temperature raised the requirements of methionine for spotted seabass (Lateolabrax maculatus). Fish Physiol. Biochem. 2022, 50, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, X.; Song, K.; Li, X.; Zhang, C.; Lu, K.; Wang, L. Effects of dietary zinc levels on growth, intestinal health, antioxidant and non-specific immunity of spotted seabass (Lateolabrax maculatus) reared at two temperatures. Aquaculture 2023, 577, 739959. [Google Scholar] [CrossRef]
- Song, M.; Zhao, J.; Wen, H.; Li, Y.; Li, J.; Li, L.; Tao, Y.; Soengas, J.L. The impact of acute thermal stress on the metabolome of the black rockfish (Sebastes schlegelii). PLoS ONE 2019, 14, e217133. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.H.; Verdegem, M.; Soliman, A.A.; Zaki, M.; Khalil, R.H.; Nour, A.M.; Khaled, A.A.; El Basuini, M.F.; Khalil, H.S. Effect of dietary bile acids: Growth performance, immune response, genes expression of fatty acid metabolism, intestinal, and liver morphology of striped catfish (Pangasianodon hypophthalmus). Aquac. Rep. 2023, 29, 101510. [Google Scholar] [CrossRef]
- Zhou, J.S.; Chen, H.J.; Ji, H.; Shi, X.C.; Li, X.X.; Chen, L.Q.; Du, Z.Y.; Yu, H.B. Effect of dietary bile acids on growth, body composition, lipid metabolism and microbiota in grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2018, 24, 802–813. [Google Scholar] [CrossRef]
- Romano, N.; Kumar, V.; Yang, G.; Kajbaf, K.; Rubio, M.B.; Overturf, K.; Brezas, A.; Hardy, R. Bile acid metabolism in fish: Disturbances caused by fishmeal alternatives and some mitigating effects from dietary bile inclusions. Rev. Aquac. 2020, 12, 1792–1817. [Google Scholar] [CrossRef]
- Li, X.; Shi, M.; Chen, L.; Zhang, S.; Chi, S.; Dong, X.; Deng, J.; Tan, B.; Xie, S. Effects of bile acids supplemented into low fishmeal diet on growth, molting, and intestinal health of Pacific white shrimp, Litopenaeus vannamei. Aquac. Rep. 2023, 29, 101491. [Google Scholar] [CrossRef]
- Wang, L.; Sagada, G.; Wang, C.; Liu, R.; Li, Q.; Zhang, C.; Yan, Y. Exogenous bile acids regulate energy metabolism and improve the health condition of farmed fish. Aquaculture 2023, 562, 738852. [Google Scholar] [CrossRef]
- Yin, P.; Xie, S.; Zhuang, Z.; He, X.; Tang, X.; Tian, L.; Liu, Y.; Niu, J. Dietary supplementation of bile acid attenuate adverse effects of high-fat diet on growth performance, antioxidant ability, lipid accumulation and intestinal health in juvenile largemouth bass (Micropterus salmoides). Aquaculture 2021, 531, 735864. [Google Scholar] [CrossRef]
- Liu, C.; Zhan, S.; Li, N.; Tu, T.; Lin, J.; Li, M.; Chen, M.; Zeng, Z.; Zhuang, X. Bile acid alterations associated with indolent course of inflammatory bowel disease. Scand. J. Gastroenterol. 2023, 58, 988–997. [Google Scholar] [CrossRef]
- Sun, R.; Xu, C.; Feng, B.; Gao, X.; Liu, Z. Critical roles of bile acids in regulating intestinal mucosal immune responses. Ther. Adv. Gastroenter. 2021, 14, 1088201074. [Google Scholar] [CrossRef]
- Lin, S.; Wang, S.; Wang, P.; Tang, C.; Wang, Z.; Chen, L.; Luo, G.; Chen, H.; Liu, Y.; Feng, B.; et al. Bile acids and their receptors in regulation of gut health and diseases. Prog. Lipid Res. 2023, 89, 101210. [Google Scholar] [CrossRef]
- Ding, T.; Xu, N.; Liu, Y.; Du, J.; Xiang, X.; Xu, D.; Liu, Q.; Yin, Z.; Li, J.; Mai, K.; et al. Effect of dietary bile acid (BA) on the growth performance, body composition, antioxidant responses and expression of lipid metabolism-related genes of juvenile large yellow croaker (Larimichthys crocea) fed high-lipid diets. Aquaculture 2020, 518, 734768. [Google Scholar] [CrossRef]
- Jiang, M.; Wen, H.; Gou, G.W.; Liu, T.L.; Lu, X.; Deng, D.F. Preliminary study to evaluate the effects of dietary bile acids on growth performance and lipid metabolism of juvenile genetically improved farmed tilapia (Oreochromis niloticus) fed plant ingredient-based diets. Aquac. Nutr. 2018, 24, 1175–1183. [Google Scholar] [CrossRef]
- Peng, X.R.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhou, X.Q. Supplementation exogenous bile acid improved growth and intestinal immune function associated with NF-kappaB and TOR signalling pathways in on-growing grass carp (Ctenopharyngodon idella): Enhancement the effect of protein-sparing by dietary lipid. Fish Shellfish Immunol. 2019, 92, 552–569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ma, A.; Yang, S.; Huang, Z. Integrated metabolome and transcriptome analyses revealing the effects of thermal stress on lipid metabolism in juvenile turbot Scophthalmus maximus. J. Therm. Biol. 2021, 99, 102937. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysi; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Liu, H.; Li, X.; Lei, H.; Li, D.; Chen, H.; Schlenk, D.; Yan, B.; Yongju, L.; Xie, L. Dietary seleno-L-methionine alters the microbial communities and causes damage in the gastrointestinal tract of Japanese medaka Oryzias latipes. Environ. Sci. Technol. 2021, 55, 16515–16525. [Google Scholar] [CrossRef]
- Im, K.; Mareninov, S.; Diaz, M.F.P.; Wang, W.H. An introduction to performing immunofluorescence staining. In Biobanking: Methods and Protocols; Springer: New York, NY, USA, 2019; pp. 299–311. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Cao, X.; Wang, J.; Gong, Y.; Wang, X.; Lai, W.; Bu, X.; Zheng, J.; Mai, K.; et al. Effects of supplemental mixed bile acids on growth performance, body composition, digestive enzyme activities, skin color, and flesh quality of juvenile large yellow croaker (Larimichthys crocea) in soybean oil based diet. Front. Mar. Sci. 2023, 10, 1149887. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, X.; Yin, Y.; Wang, K.; Sun, Y.; Ye, J. Dietary high β-conglycinin reduces the growth through enhancing hepatic lipid peroxidation and impairing intestinal barrier function of orange-spotted grouper (Clostridium autoethanogenum). Front. Mar. Sci. 2023, 10, 1237387. [Google Scholar] [CrossRef]
- Bhusare, S.; Sardar, P.; Sahu, N.P.; Shamna, N.; Kumar, P.; Paul, M.; Jana, P.; Raghuvaran, N.; Bhavatharaniya, U. Bile acid improves growth, lipid utilization and antioxidative status of genetically improved farmed tilapia (Oreochromis niloticus) fed with varying protein-lipid diets reared in inland saline water. Anim. Feed Sci. Technol. 2023, 303, 115677. [Google Scholar] [CrossRef]
- Xia, R.; Zhang, Q.; Xia, D.; Hao, Q.; Ding, Q.; Ran, C.; Yang, Y.; Cao, A.; Zhang, Z.; Zhou, Z. The direct and gut microbiota-mediated effects of dietary bile acids on the improvement of gut barriers in largemouth bass (Micropterus salmoides). Anim. Nutr. 2023, 14, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhao, P.; Zhai, S. Dietary bile acids supplementation mainly regulates the amino acid metabolic pathways without decreasing bile acids levels in the liver of farmed European eel (Anguilla anguilla) juveniles. Aquac. Rep. 2022, 26, 101283. [Google Scholar] [CrossRef]
- Gu, M.; Bai, N.; Kortner, T.M. Taurocholate supplementation attenuates the changes in growth performance, feed utilization, lipid digestion, liver abnormality and sterol metabolism in turbot (Scophthalmus maximus) fed high level of plant protein. Aquaculture 2017, 468, 597–604. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Abdel-Latif, H.M.R.; Basuini, M.F.E.; El-Nokrashy, A.M.; Khaled, A.A.; Kord, M.; Soliman, A.A.; Zaki, M.; Nour, A.; Labib, E.M.H.; et al. Effects of exogenous bile acids (BAs) on growth, lipid profile, digestive enzymes, and immune responses of thinlip mullet, Liza ramada. Sci. Rep. 2023, 13, 22875. [Google Scholar] [CrossRef] [PubMed]
- Romański, K.W. The role and mechanism of action of bile acids within the digestive system—Bile acids in the liver and bile. Adv. Clin. Exp. Med. 2007, 16, 793–799. [Google Scholar]
- Guo, J.L.; Kuang, W.M.; Zhong, Y.F.; Zhou, Y.L.; Chen, Y.J.; Lin, S.M. Effects of supplemental dietary bile acids on growth, liver function and immunity of juvenile largemouth bass (Micropterus salmoides) fed high-starch diet. Fish Shellfish Immunol. 2020, 97, 602–607. [Google Scholar] [CrossRef]
- Adhami, B.; Amirkolaie, A.K.; Oraji, H.; Kenari, R.E. Growth performance, nutrient digestibility and lipase activity in juvenile rainbow trout (Oncorhynchus mykiss) fed fat powder in diet containing emulsifiers (cholic acid and Tween-80). Aquac. Nutr. 2017, 23, 1153–1159. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Cao, Y.; An, X.; Chen, J.; Yang, L. Punicalagin protects against diabetic liver injury by upregulating mitophagy and antioxidant enzyme activities. Nutrients 2022, 14, 2782. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, K.; Xu, Y.; Xu, Y.; Li, Y.; Xu, J.; Zhu, Y.; Adorini, L.; Lee, Y.K.; Kasumov, T.; Yin, L.; et al. Reversal of metabolic disorders by pharmacological activation of bile acid receptors TGR5 and FXR. Mol. Metab. 2018, 9, 131–140. [Google Scholar] [CrossRef]
- Makishima, M.; Lu, T.T.; Xie, W.; Whitfield, G.K.; Domoto, H.; Evans, R.M.; Haussler, M.R.; Mangelsdorf, D.J. Vitamin D receptor as an intestinal bile acid sensor. Science 2002, 296, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Fan, W.; Tang, T.; Wan, H.; Zhao, S.; Tan, Y.; Oware, K.A.; Tan, J.; Li, J.; Qu, S.; et al. Farnesoid X receptor deficiency induces hepatic lipid and glucose metabolism disorder via regulation of pyruvate dehydrogenase kinase 4. Oxid. Med. Cell. Longev. 2022, 2022, 3589519–3589525. [Google Scholar] [CrossRef]
- Yamagata, K.; Daitoku, H.; Shimamoto, Y.; Matsuzaki, H.; Hirota, K.; Ishida, J.; Fukamizu, A. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and foxo1. J. Biol. Chem. 2004, 279, 23158–23165. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, N.; Ahmadifar, E.; Soltani, M.; Tayefi-Nasrabadi, H.; Mousavi, S.; Naiel, M.A.E. Brown seaweed (Padina australis) extract can promote performance, innate immune responses, digestive enzyme activities, intestinal gene expression and resistance against aeromonas hydrophila in common carp (Cyprinus carpio). Animals 2022, 12, 3389. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Chen, Y.P.; Yang, M.X.; Zhang, L.L.; Lu, Z.X.; Zhou, Y.M.; Wang, T. Bacillus amyloliquefaciens supplementation alleviates immunological stress and intestinal damage in lipopolysaccharide-challenged broilers. Anim. Feed Sci. Tech. 2015, 208, 119–131. [Google Scholar] [CrossRef]
- Ze, Y.; Sheng, L.; Zhao, X.; Hong, J.; Ze, X.; Yu, X.; Pan, X.; Lin, A.; Zhao, Y.; Zhang, C.; et al. TiO2 nanoparticles induced hippocampal neuroinflammation in mice. PLoS ONE 2014, 9, e92230. [Google Scholar] [CrossRef] [PubMed]
- Kai, Y. Intestinal villus structure contributes to even shedding of epithelial cells. Biophys. J. 2021, 120, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wan, M.; Wang, Z.; Zhang, H.; Zhu, S.; Cao, X.; Xu, N.; Zheng, J.; Bu, X.; Xu, W.; et al. Effects of tributyrin supplementation on growth performance, intestinal digestive enzyme activity, antioxidant capacity, and inflammation-related gene expression of large yellow croaker (Larimichthys crocea) Fed with a High Level of Clostridium autoethanogenum Protein. Aquac. Nutr. 2023, 2023, 2687712–2687734. [Google Scholar]
- Torun, A.N.; Kulaksizoglu, S.; Kulaksizoglu, M.; Pamuk, B.O.; Isbilen, E.; Tutuncu, N.B. Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clin. Endocrinol. 2009, 70, 469–474. [Google Scholar] [CrossRef]
- Turk, R.; Juretić, D.; Gereš, D.; Svetina, A.; Turk, N.; Flegar-Meštrić, Z. Influence of oxidative stress and metabolic adaptation on PON1 activity and MDA level in transition dairy cows. Anim. Reprod. Sci. 2008, 108, 98–106. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Hu, Y.; Cheng, J.; Cheng, X.; Cheng, P.; Cui, Z. Dietary bile acid supplementation reveals beneficial effects on intestinal healthy status of tongue sole (Cynoglossus semiliaevis). Fish Shellfish Immunol. 2021, 116, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; An, Z.; Shi, C.; Li, P.; Liu, L. A sensitive and efficient method for simultaneous profiling of bile acids and fatty acids by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2020, 178, 112815. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Shu, T.; Liu, G.; Mei, H.; Zhu, X.; Huang, X.; Zhang, L.; Jiang, Z. Quantitative profiling of 19 bile acids in rat plasma, liver, bile and different intestinal section contents to investigate bile acid homeostasis and the application of temporal variation of endogenous bile acids. J. Steroid Biochem. Mol. Biol. 2017, 172, 69–78. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).