Effect of the Combination of Synthetic Anthelmintics with Carvacryl Acetate in Emulsions with and without a Sodium Alginate Matrix on Haemonchus contortus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Acetylation of Carvacrol
2.2. Preparation of Emulsions
2.3. Physicochemical Characterization of Nanoemulsions
2.4. Homogeneity and Morphology
2.5. Particle Size and Zeta Potential
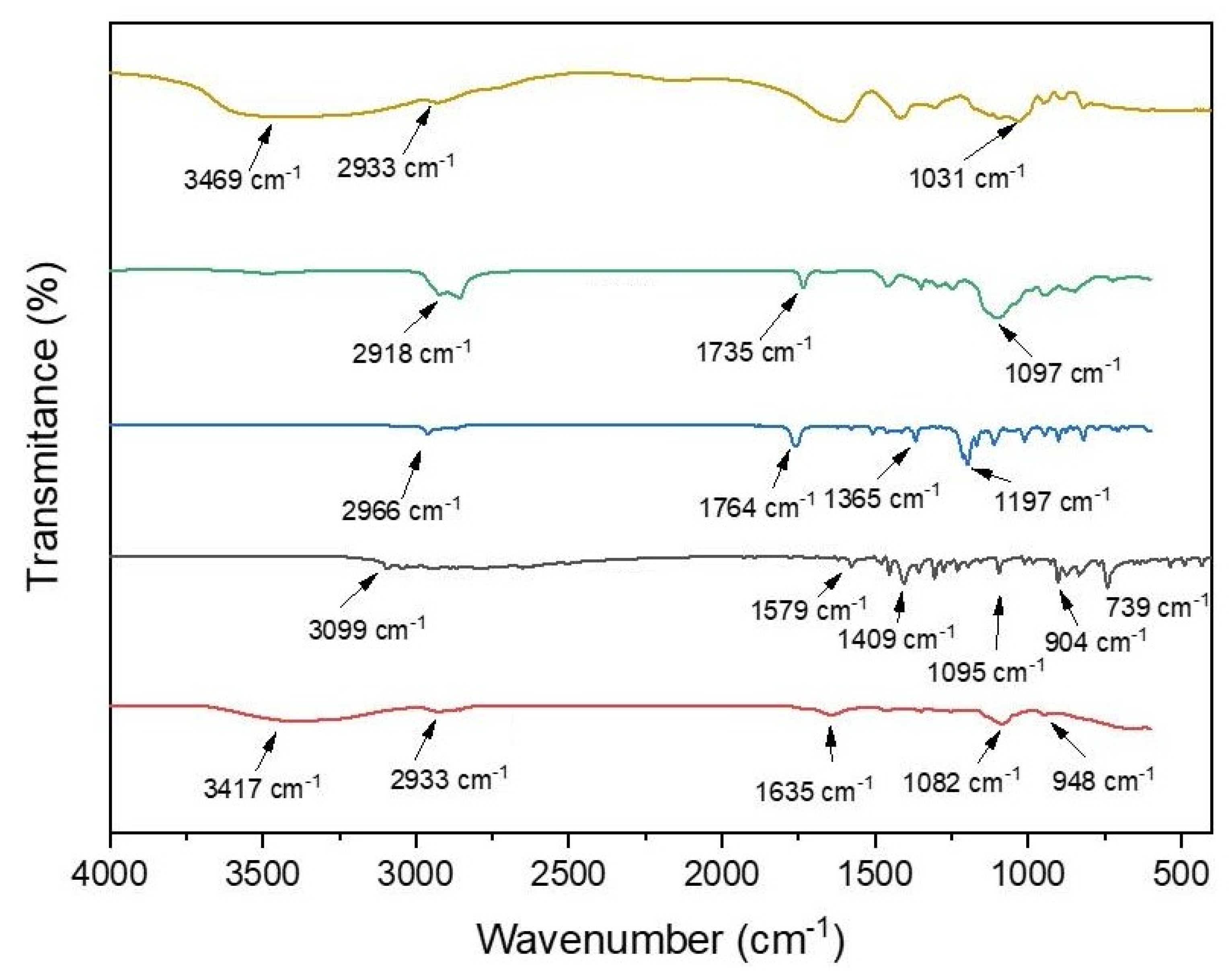
2.6. Infrared Spectroscopy Analysis
2.7. Encapsulation Efficiency
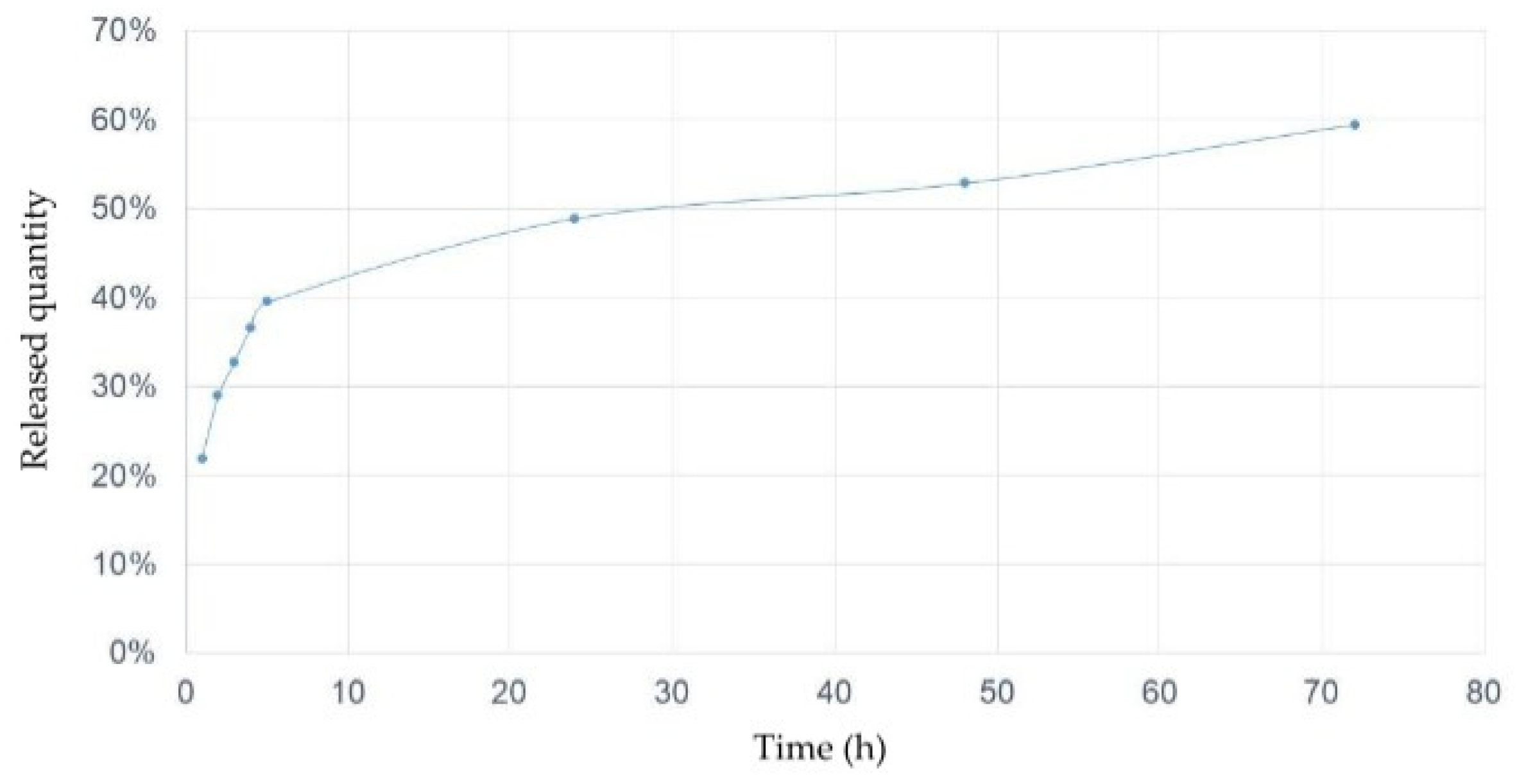
2.8. In Vitro Release Profile
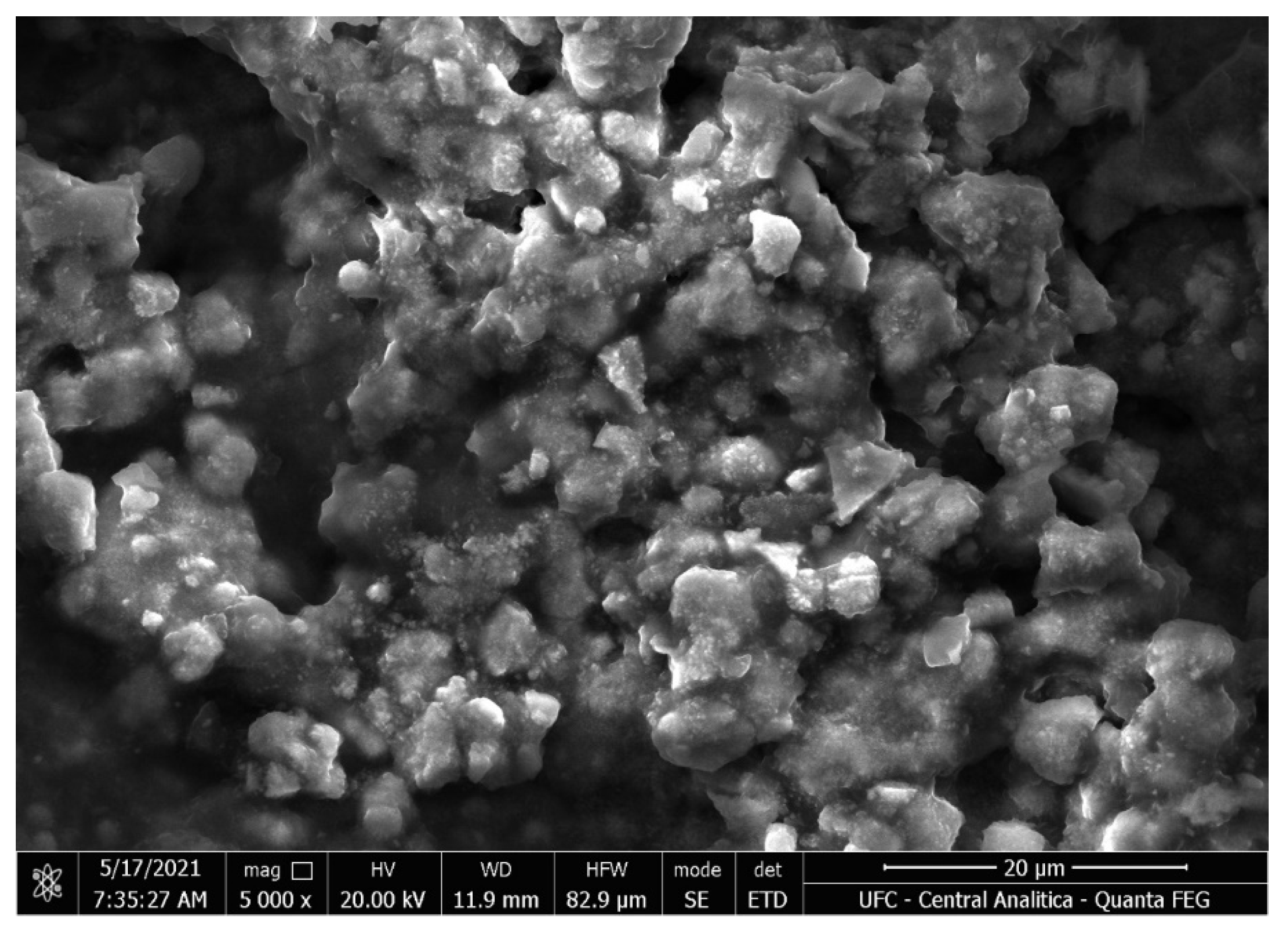
2.9. Scanning Electron Micrography
2.10. Haemonchus contortus Isolate
2.11. Recovery of Eggs and Larvae of Haemonchus contortus
2.12. Proportions of Substances Used in the Combinations
2.13. Egg Hatch Test (EHT)
2.14. Larval Development Test (LDT)
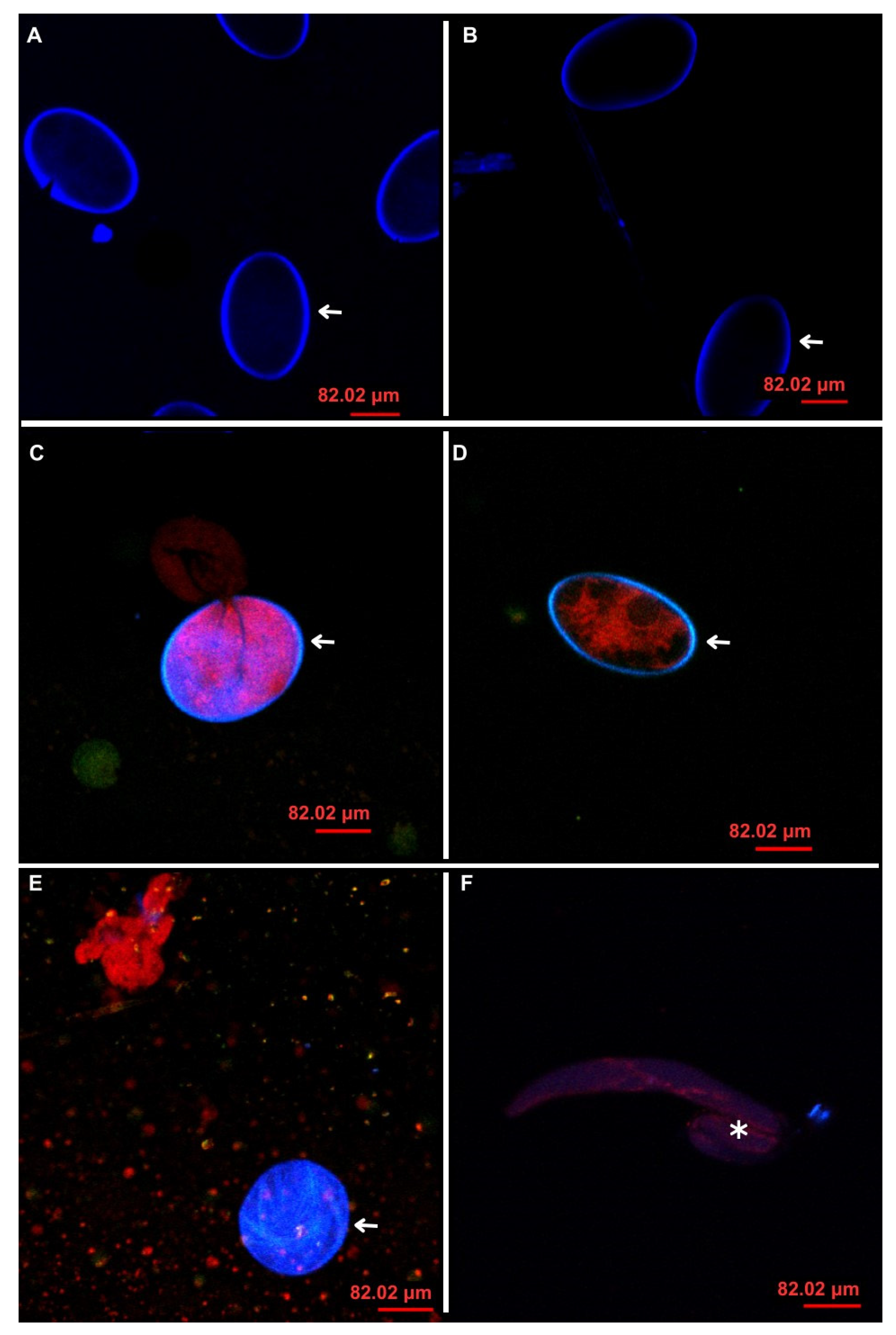
2.15. Confocal Laser Scanning Microscopy (CLSM)
3. Data Analysis
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selzer, P.M.; Epe, C. Antiparasitics in animal health: Quo Vadis? Trends Parasitol. 2020, 37, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Bartram, D.J.; Leathwick, D.M.; Taylor, M.A.; Geurden, T.; Maeder, S.J. The role of combination anthelmintic formulations in the sustainable control of sheep nematodes. Vet. Parasitol. 2012, 186, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Geary, T.G.; Hosking, B.C.; Skuce, P.J.; Von Samson-Himmelstjerna, G.; Maeder, S.; Holdsworth, P.; Pomroy, W.; Vercruysse, J. World Association for the Advancement of Veterinary Parasitology (WAAVP) Guideline: Anthelmintic combination products targeting nematode infections of ruminants and horses. Vet. Parasitol. 2012, 190, 306–316. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y. Combination therapy based on nano codelivery for overcoming cancer drug resistance. Med. Drug Discov. 2020, 6, 100024. [Google Scholar] [CrossRef]
- Anthony, J.P.; Fyfe, L.; Smith, H. Plant active components–a resource for antiparasitic agents? Trends Parasitol. 2005, 21, 462–468. [Google Scholar] [CrossRef]
- Lanusse, C.; Canton, C.; Virkel, G.; Alvarez, L.; Costa-Junior, L.; Lifschitz, A. Strategies to optimize the efficacy of anthelmintic drugs in ruminants. Trends Parasitol. 2018, 34, P664–P682. [Google Scholar] [CrossRef]
- Costa-Junior, L.; Silva, C.R.; Macedo, S.R.D.; Campos, N.R.C.L.; Lifsctiz, A. Association of synthetic anthelmintics and natural monoterpenes against Haemonchus contortus. In Proceedings of the 26th International Conference of the World Association for the Advancement of Veterinary Parasitology, Kuala Lumpur, Malaysia, 16 August 2017; p. 40. [Google Scholar]
- Andre, W.P.P.; Ribeiro, W.L.C.; Cavalcante, G.S.; Santos, J.M.L.; Macedo, I.T.F.; De Paula, H.C.B.; De Freitas, R.M.; De Morais, S.M.; De Melo, J.V.; Bevilaqua, C.M.L. Comparative efficacy and toxic effects of carvacryl acetate and carvacrol on sheep gastrointestinal nematodes and mice. Vet. Parasitol. 2016, 218, 52–58. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. Biotech 2004, 5, 123–127. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nanoemulsions. Adv. Colloid. Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef]
- Bouchemal, K.; Briançon, S.; Perrier, E.; Fessi, H. Nano-emulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimisation. Int. J. Pharm. 2004, 280, 241–251. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Laigle, M.; Michel, T.; Palmeri, V. Essential oil-based nano-emulsions: Effect of different surfactants, sonication and plant species on physicochemical characteristics. Ind. Crop. Prod. 2020, 157, 112935. [Google Scholar] [CrossRef]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate nanoparticles for drug delivery and targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef]
- Matos, F.J.A. Introdução a Fitoquímica Experimental; Edições UFC: Fortaleza, Brazil, 2009; p. 141. [Google Scholar]
- Abreu, F.O.M.S.; Costa, E.F.; Cardial, M.R.L.; André, W.P.P. Polymeric nanoemulsions enriched with Eucalyptus citriodora essential oil. Polímeros 2020, 30, e2020024. [Google Scholar] [CrossRef]
- Park, S.J.; Jeong, U.H.; Lee, J.W.; Park, J.S. Preparation and characterization of bovine serum albumin-loaded cationic liposomes: Effect of hydration phase. J. Pharm. Investig. 2010, 40, 353–356. [Google Scholar] [CrossRef][Green Version]
- Neveu, C.; Charvet, C.; Fauvin, A.; Cortet, J.; Castagnone-Sereno, P.; Cabaret, J. Identification of levamisole resistance markers in the parasitic nematode Haemonchus contortus using a cDNA-AFLP approach. Parasitology 2007, 134, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Fauvin, A.; Charvet, C.; Issouf, M.; Cortet, J.; Neveu, C. cDNA-AFLP analysis in levamisole-resistant Haemonchus contortus reveals alternative splicing in a nicotinic acetylcholine receptor subunit. Mol. Biochem. Parasitol. 2010, 170, 105–107. [Google Scholar] [CrossRef]
- Hubert, J.; Kerboeuf, D.A. Microlarval development assay for the detection of anthelmintic resistance in sheep nematodes. Vet. Rec. 1992, 130, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Coles, G.C.; Jackson, F.; Pomroy, W.E.; Prichard, R.K.; Von Samson-Himmelstjerna, G.; Silvestre, A.; Taylor, M.A.; Vercruysse, J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006, 136, 167–185. [Google Scholar] [CrossRef]
- Camurça-Vasconcelos, A.L.F.; Bevilaqua, C.M.L.; Morais, S.M.; Maciel, M.V.; Costa, C.T.C.; Macedo, I.T.F.; Oliveira, M.L.B.; Braga, R.R.; Silva, R.A.; Vieira, L.S. Anthelmintic activity of Croton zehntneri and Lippia sidoides essential oils. Vet. Parasitol. 2007, 148, 288–294. [Google Scholar] [CrossRef]
- Roberts, F.H.S.; O’Sullivan, P.J. Methods for egg counts and larval cultures for strongyles infesting the gastro-intestinal tract of cattle. Aust. J. Agric. Res. 1950, 1, 99–102. [Google Scholar] [CrossRef]
- Castelo-Branco, D.S.C.M.; Riello, G.B.; Vasconcelos, D.C.; Guedes, G.M.M.; Serpa, R.; Bandeira, T.J.P.G.; Monteiro, A.J.; Cordeiro, R.A.; Rocha, M.F.G.; Sidrim, J.J.C.; et al. Farnesol increases the susceptibility of Burkholderia pseudomallei biofilm to antimicrobials used to treat melioidosis. J. Appl. Microbiol. 2016, 120, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Martin, N. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values; ComboSyn: Paramus, NJ, USA, 2005. [Google Scholar]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Bielza, P.; Espinosa, P.J.; Quinto, V.; Abellan, J.; Contreras, J. Synergism s tudies with binary mixtures of pyrethroid, carbamate and organophosphate insecticides on Frankliniella occidentalis (Pergande). Pest. Manag. Sci. 2007, 63, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, T.; Alaseem, A.M.; Khan, M.M.; Mukhtar, B.; Kamal, M.; Anwer, R.; Ahmed, S.; Alam, A. Preparation and evaluation of nanoemulsion of Citronella essential oil with improved antimicrobial and anti-Cancer properties. Antibiotics 2023, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Bouriche, S.; Alonso García, Á.; Cárceles-Rodríguez, C.; Rezgui, F.; Fernández-Varón, E. An in vivo pharmacokinetic study of metformin microparticles as an oral sustained release formulation in rabbits. BMC Vet. Res. 2021, 17, 315. [Google Scholar] [CrossRef] [PubMed]
- Nixon, S.A.; Saez, N.J.; Herzig, V.; King, G.F.; Kotze, A.C. The antitrypanosomal diarylamidines, diminazene and pentamidine, show anthelmintic activity against Haemonchus contortus in vitro. Vet. Parasitol. 2019, 270, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Křížová-Forstová, V.; Lamka, J.; Cvilink, V.; Hanušová, V.; Skálová, L. Factors affecting pharmacokinetics of benzimidazole anthelmintics in food-producing animals: The consequences and potential risks. Res. Vet. Sci. 2011, 91, 333–341. [Google Scholar] [CrossRef]
- Lambert, S.M.; Nishi, S.M.; Mendonça, L.R.; Souza, B.M.P.; Julião, F.; Gusmão, P.; Almeida, M.A.O. Genotypic profile of benzimidazole resistance associated with SNP F167Y and F200Y beta-tubulin gene in Brazilian populations of Haemonchus contortus of goats. Vet. Parasitol. Reg. Stud. Rep. 2017, 8, 28–34. [Google Scholar] [CrossRef]
- Santos, J.M.L.; Monteiro, J.P.; Ribeiro, W.L.C.; Macedo, I.T.F.; Araújo Filho, J.V.; Andre, W.P.P.; Araùjo, P.R.M.; Vasconcelos, J.F.; Freitas, E.P.; Camurça-Vasconcelos, A.L.F.; et al. High levels of benzimidazole resistance and β-tubulin isotype 1 SNP F167Y in Haemonchus contortus populations from Ceará State, Brazil. Small Rum. Res. 2017, 146, 48–52. [Google Scholar] [CrossRef]
- Santos, J.M.L.; Vasconcelos, J.F.; Frota, G.A.; Freitas, E.P.; Teixeira, M.; Silva, L.V.; Bevilaqua, C.M.L.; Monteiro, J.P. Quantitative molecular diagnosis of levamisole resistance in populations of Haemonchus contortus. Exp. Parasitol. 2019, 205, 107734. [Google Scholar] [CrossRef]
- Moraes, J.; Carvalho, A.A.L.; Nakano, E.; Almeida, A.A.C.; Marques, T.H.C.; Andrade, L.N.; Freitas, M.; Sousa, D.P. Anthelmintic activity of carvacryl acetate against Schistosoma mansoni. Parasitol. Res. 2013, 112, 603–610. [Google Scholar] [CrossRef]
- Calderón-Quintal, J.A.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Alonso, M.A.; Hoste, H.; Aguilar-Caballero, A. Adaptation of Haemonchus contortus to condensed tannins: Can it be possible? Arch. Med. Vet. 2010, 42, 165–171. [Google Scholar] [CrossRef]
- Mansfield, L.S.; Gamble, H.R.; Fetterer, R.H. Characterization of the eggshell of Haemonchus contortus: I structural components. Comp. Biochem. Physiol. 1992, 103, 681–686. [Google Scholar] [CrossRef]
- Wharton, D.A. Nematode egg-shells. Parasitology 1980, 81, 447–454. [Google Scholar] [CrossRef]
- Silva, C.R.; Lifschitz, A.L.; Macedo, S.R.; Campos, N.R.; Viana-Filho, M.; Alcântara, A.C.; Araujo, J.G.; Alencar, L.M.R.; Costa-Junior, L.M. Combination of synthetic anthelmintics and monoterpenes: Assessment of efficacy, and ultrastructural and biophysical properties of Haemonchus contortus using atomic force microscopy. Vet. Parasitol. 2021, 290, 109345. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.F.; Byrne, O. Plant-insect coevolution and inhibition of acetylcholinesterase. J. Chem. Ecol. 1988, 14, 1965–1975. [Google Scholar] [CrossRef]
- Moreno-Guzmán, M.J.; Coles, G.C.; Jiménez-González, A.; Criado-Fornelio, A.; Ros-Moreno, R.M.; Rodríguez-Caabeiro, F. Levamisole binding sites in Haemonchus contortus. Int. J. Parasitol. 1998, 28, 413–418. [Google Scholar] [CrossRef]
- Mottier, L.; Lanusse, C. Bases moleculares de la resistência a fármacos. Rev. Med. Vet. 2001, 82, 74–85. [Google Scholar]
- Marjanovic, D.S.; Zdravković, N.; Milovanović, M.; Trailović, J.N.; Robertson, A.P.; Todorović, Z.; Trailović, S.M. Carvacrol acts as a potent selective antagonist of different types of nicotinic acetylcholine receptors and enhances the effect of monepantel in the parasitic nematode Ascaris Suum. Vet. Parasitol. 2020, 278, 109031. [Google Scholar] [CrossRef]
- Zhang, P.; Wan, Y.; Zhang, C.; Zhao, R.; Sha, J.; Li, Y.; Li, T.; Ren, B. Solubility and mixing thermodynamic properties of levamisole hydrochloride in twelve pure solvents at various temperatures. J. Chem. Thermodyn. 2019, 139, 105882. [Google Scholar] [CrossRef]
- Santana, R.C.; Perrechil, F.A.; Cunha, R.L. High-and low-energy emulsifications for food applications: A focus on process parameters. Food Eng. Rev. 2013, 5, 107–122. [Google Scholar] [CrossRef]
- Lacey, E. The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. Int. J. Parasitol. 1988, 18, 885–936. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]

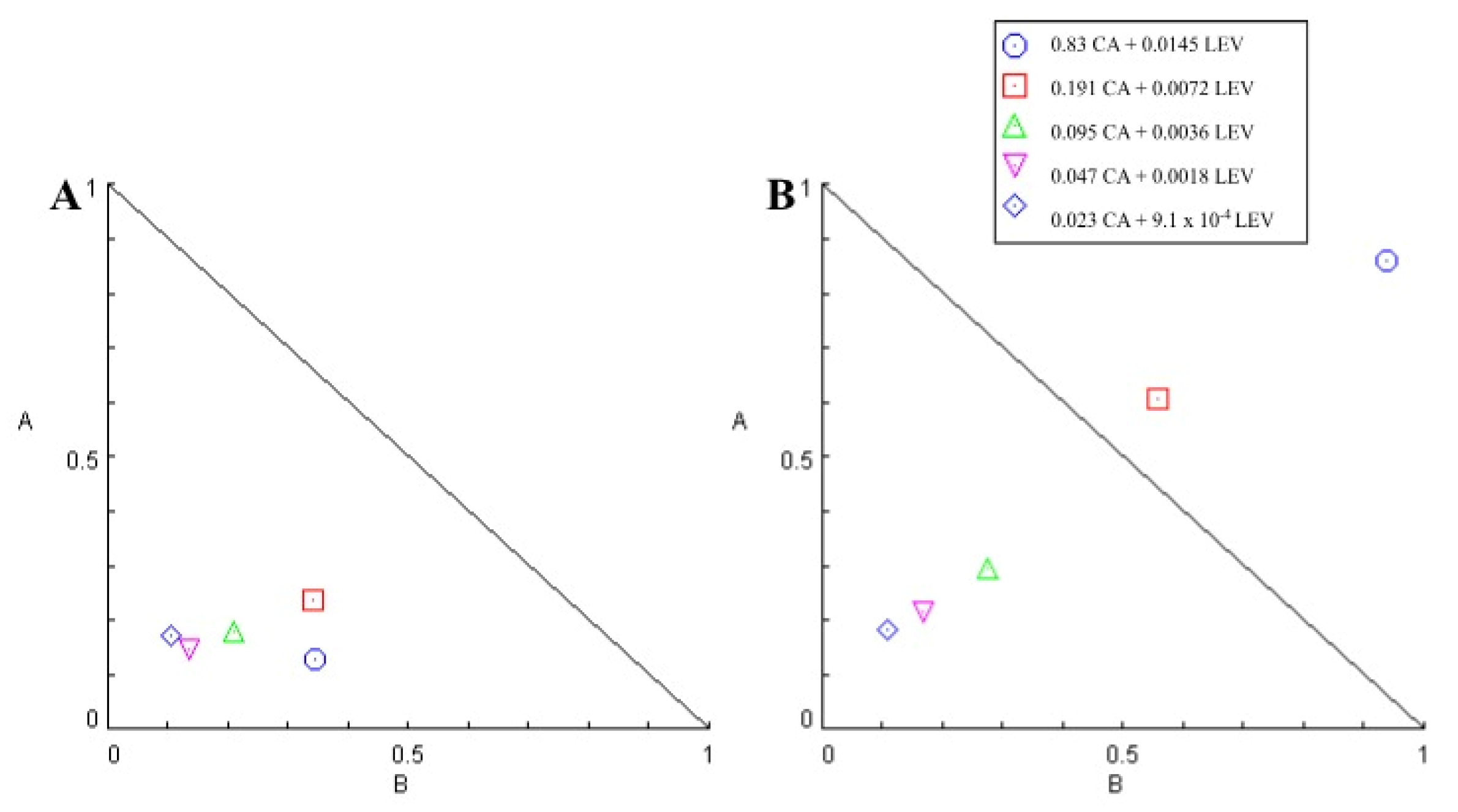
| Sample Name | Oil Phase | Aqueous Phase |
|---|---|---|
| CA/TBZ | Tween 80/CA/TBZ | Distilled water |
| CA/TBZ/ALG | Tween 80/CA/TBZ | ALG 1%wt solution |
| CA/IVM | Tween 80/CA/IVM | Distilled water |
| CA/LEV | Tween 80/CA/LEV | Distilled water |
| CA/LEV/ALG | Tween 80/CA/LEV | ALG 1%wt solution |
| Control | Tween 80 | Water |
| Zeta Potential (ZP) ± Standard Deviation | |
|---|---|
| CA/TBZ/ALG | −57.97mV ± 8.73 |
| CA/TBZ | −26.20 mV ± 4.59 |
| CA/LEV/ALG | −68.80 mV ± 2.36 |
| CA/LEV | −21.60 mV ± 8.20 |
| Thiabendazole (mg/mL) | Mean ± Standard Deviation | Carvacryl Acetate (mg/mL) | Mean ± Standard Deviation |
|---|---|---|---|
| 0.05 | 96.07 ± 3.24 A | 8 | 94.88 ± 2.09 A |
| 2.5 × 10−2 | 76.61 ± 3.20 B | 4 | 90.76 ± 2.41 A |
| 1.25 × 10−2 | 52.77 ± 2.08 C | 2 | 83.42 ± 5.25 B |
| 6 × 10−3 | 28.80 ± 6.20 D | 1 | 60.04 ± 9.94 C |
| 3 × 10−3 | 14.18 ± 4.99 E | 0.5 | 37.68 ± 10.46 D |
| Tween 3% | 4.93 ± 1.18 | Tween 3% | 4.93 ± 1.18 |
| CA (mg/mL) | TBZ (mg/mL) | CA/TBZ | CA/TBZ/ALG | ||
|---|---|---|---|---|---|
| Mean ± Standard Deviation | CI | Mean ± Standard Deviation | CI | ||
| 0.65 | 1.1 × 10−2 | 86.47 ± 4.41 Aa | 0.59 | 87.53 ± 2.81 Aa | 0.55 |
| 0.33 | 5 × 10−3 | 65.82 ± 6.30 Ba | 0.65 | 77.93 ± 6.22 Bb | 0.43 |
| 0.16 | 2.7 × 10−3 | 41.66 ± 4.78 Ca | 0.63 | 67.33 ± 3.23 Cb | 0.31 |
| 0.08 | 1.3 × 10−3 | 28.42 ± 8.79 Da | 0.46 | 46.45 ± 6.09 Db | 0.27 |
| 0.04 | 6.8 × 10−4 | 12.71 ± 4.97 E | 0.45 | 24.08 ± 4.73 Eb | 0.26 |
| Tween 3% | 3.40 ± 2.01 | 4.59 ± 2.57 | |||
| Tween 3% | 3.40 ± 2.01 | 4.59 ± 2.57 | |||
| Ivermectin (mg/mL) | Mean ± Standard Deviation | Levamisole (mg/mL) | Mean ± Standard Deviation | Carvacryl Acetate (mg/mL) | Mean ± Standard Deviation |
|---|---|---|---|---|---|
| 0.05 | 100 ± 0.25 A | 0.8 | 99.25 ± 0.61 A | 2 | 100 ± 0 A |
| 2.5 × 10−2 | 97.74 ± 3.87 A | 0.4 | 84.43 ± 6.05 B | 1 | 97.49 ± 3.96 A |
| 1.25 × 10−2 | 68.06 ± 8.07 B | 0.2 | 63.81 ± 6.30 C | 0.5 | 46.97 ± 12.7 B |
| 6 × 10−3 | 44.03 ± 6.85 C | 0.1 | 35.34 ± 7.51 D | 0.25 | 29.27 ± 13.09 C |
| 3 × 10−3 | 0 ± 3.79 D | 0.05 | 5.92 ± 8.98 E | 0.13 | 0 ± 2.09 D |
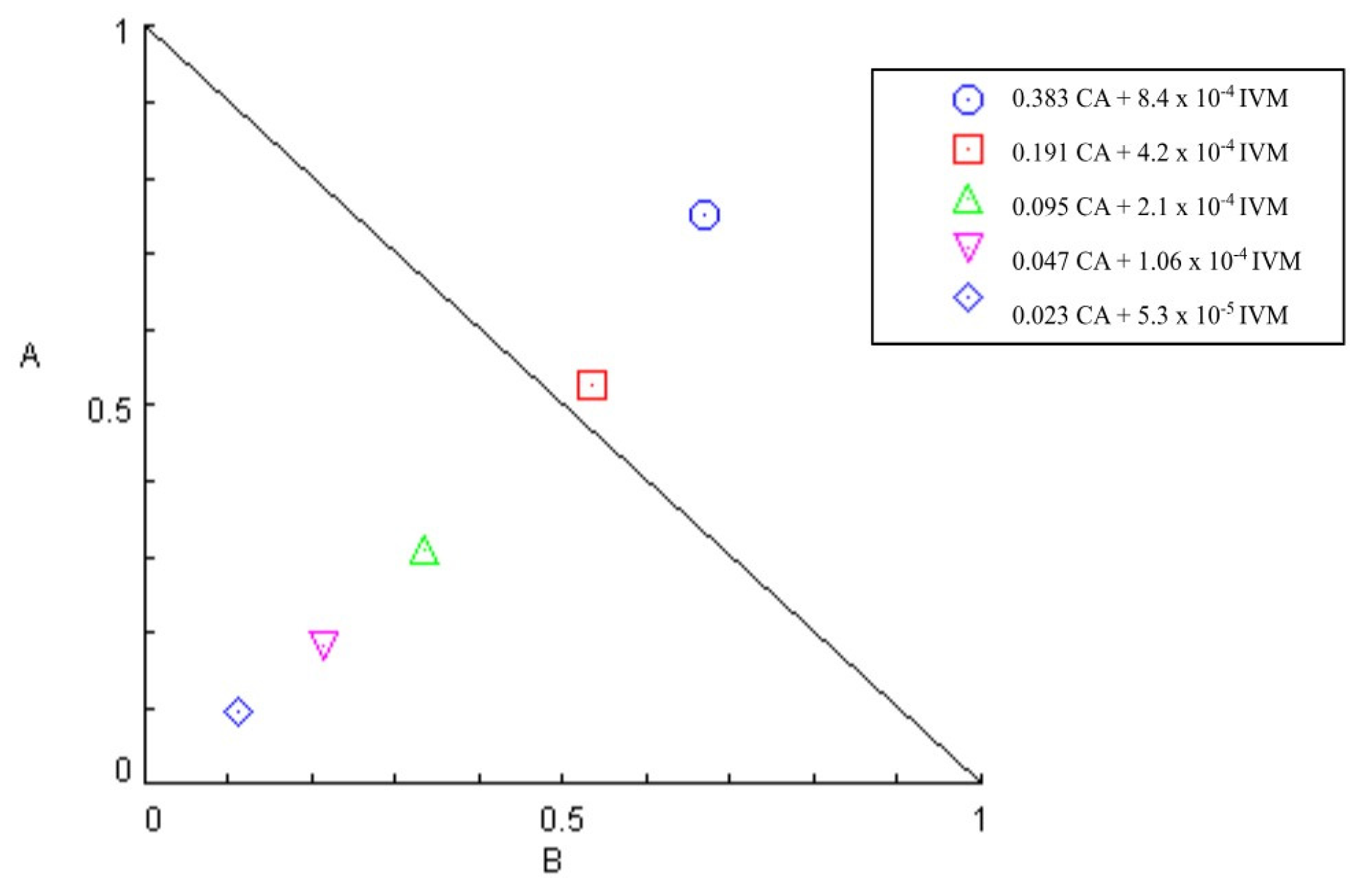
| CA (mg/mL) | IVM (mg/mL) | Mean ± Standard Deviation | CI |
|---|---|---|---|
| 0.38 | 8.4 × 10−4 | 79.32 ± 7.48 A | 1.42 |
| 0.19 | 4.2 × 10−4 | 43.41 ± 7.78 B | 1.06 |
| 0.09 | 2.1 × 10−4 | 26.28 ± 14.9 C | 0.64 |
| 0.05 | 1.06 × 10−4 | 13.24 ± 14.48 C,D | 0.40 |
| 0.02 | 0.53 × 10−4 | 9.62 ± 10.68 D | 0.21 |
| CA (mg/mL) | LEV (mg/mL) | CA/LEV | CA/LEV/ALG | ||
|---|---|---|---|---|---|
| Mean ± Standard Deviation | CI | Mean ± Standard Deviation | CI | ||
| 0.38 | 145 × 10−4 | 99.33 ± 0.54 Aa | 0.47 | 57.0 ± 10.54 Ab | 1.80 |
| 0.19 | 72 × 10−4 | 85.35 ± 5.41 Ba | 0.58 | 36.4 ± 7.56 Bb | 1.16 |
| 0.09 | 36 × 10−4 | 64.77 ± 5.38 Ca | 0.39 | 37.9 ± 4.93 Bb | 0.57 |
| 0.05 | 18 × 10−4 | 37.01 ± 8.61 Da | 0.28 | 18.4 ± 7.47 Cb | 0.38 |
| 0.02 | 9.06 × 10−4 | 5.83 ± 6.31 Ea | 0.28 | 5.05 ± 6.10 C,Da | 0.29 |
| ALG 1% | 0.27 ± 0.55 D | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ximenes, L.F.; Pinheiro, H.N.; Filho, J.V.d.A.; André, W.P.P.; Abreu, F.O.M.d.S.; Cardial, M.R.L.; Castelo-Branco, D.d.S.C.M.; Melo, A.C.F.L.; Lopes, F.F.d.S.; de Morais, S.M.; et al. Effect of the Combination of Synthetic Anthelmintics with Carvacryl Acetate in Emulsions with and without a Sodium Alginate Matrix on Haemonchus contortus. Animals 2024, 14, 1007. https://doi.org/10.3390/ani14071007
Ximenes LF, Pinheiro HN, Filho JVdA, André WPP, Abreu FOMdS, Cardial MRL, Castelo-Branco DdSCM, Melo ACFL, Lopes FFdS, de Morais SM, et al. Effect of the Combination of Synthetic Anthelmintics with Carvacryl Acetate in Emulsions with and without a Sodium Alginate Matrix on Haemonchus contortus. Animals. 2024; 14(7):1007. https://doi.org/10.3390/ani14071007
Chicago/Turabian StyleXimenes, Livia Furtado, Henety Nascimento Pinheiro, José Vilemar de Araújo Filho, Weibson Paz Pinheiro André, Flávia Oliveira Monteiro da Silva Abreu, Mayrla Rocha Lima Cardial, Debora de Souza Colares Maia Castelo-Branco, Ana Carolina Fonseca Lindoso Melo, Francisco Flávio da Silva Lopes, Selene Maia de Morais, and et al. 2024. "Effect of the Combination of Synthetic Anthelmintics with Carvacryl Acetate in Emulsions with and without a Sodium Alginate Matrix on Haemonchus contortus" Animals 14, no. 7: 1007. https://doi.org/10.3390/ani14071007
APA StyleXimenes, L. F., Pinheiro, H. N., Filho, J. V. d. A., André, W. P. P., Abreu, F. O. M. d. S., Cardial, M. R. L., Castelo-Branco, D. d. S. C. M., Melo, A. C. F. L., Lopes, F. F. d. S., de Morais, S. M., de Oliveira, L. M. B., & Bevilaqua, C. M. L. (2024). Effect of the Combination of Synthetic Anthelmintics with Carvacryl Acetate in Emulsions with and without a Sodium Alginate Matrix on Haemonchus contortus. Animals, 14(7), 1007. https://doi.org/10.3390/ani14071007






