Wild Animals in Captivity: An Analysis of Parasite Biodiversity and Transmission among Animals at Two Zoological Institutions with Different Typologies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and Host Species
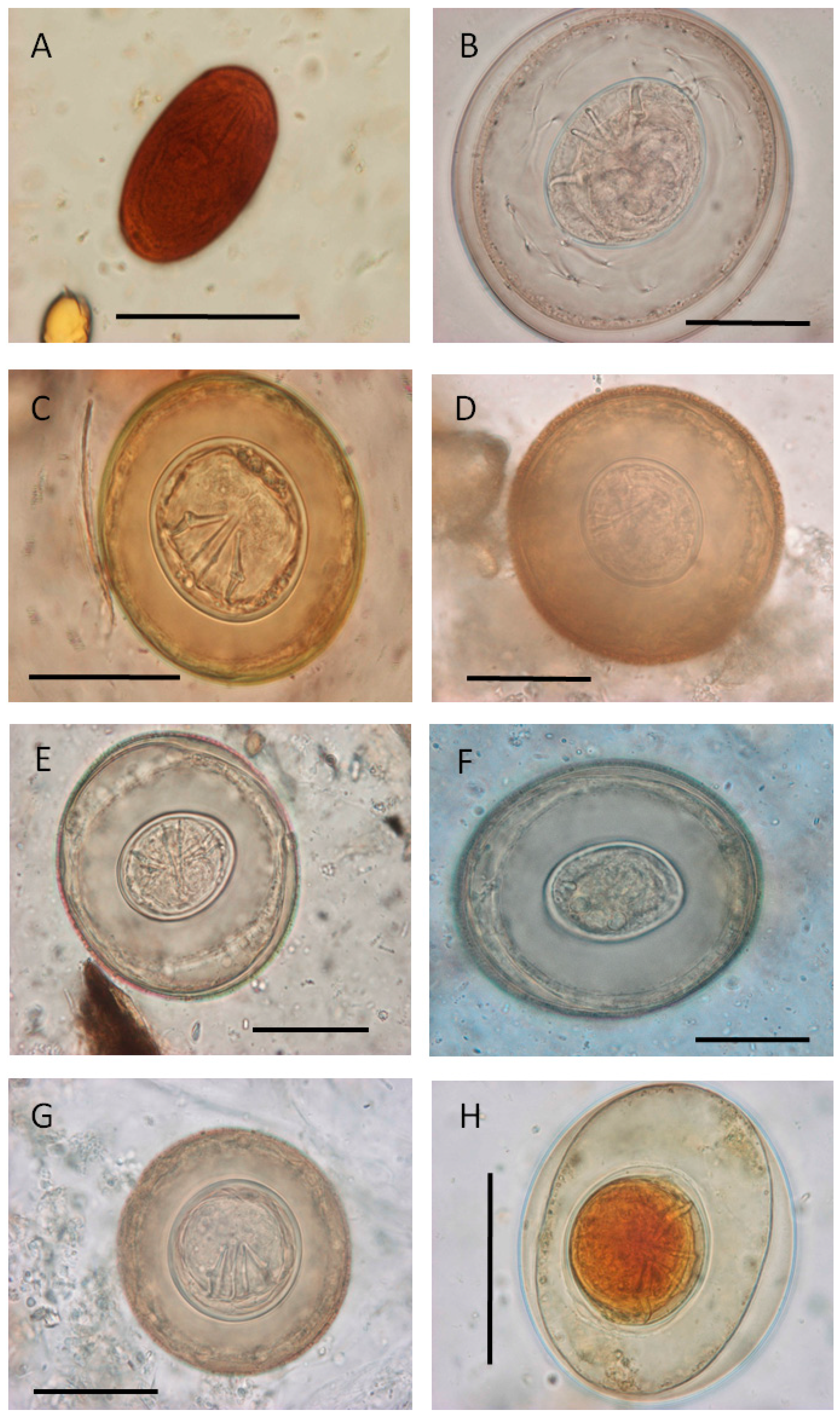
2.2. Sample Collection and Processing
2.3. Parasite Biodiversity, Housing Conditions, and Feeding Habits
3. Results
3.1. Overall Parasite Biodiversity and Prevalence
3.1.1. Avian Hosts
3.1.2. Mammalian Hosts
3.2. Biodiversity and Prevalence in Relation to Feeding Habits and Housing Conditions
4. Discussion
4.1. On the Parasite Epidemiology, Biodiversity, and Species Identification
4.1.1. Avian Hosts
4.1.2. Mammalian Hosts
4.2. Effect of Housing Conditions
4.3. Transmission Risks between Animals and Humans
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Combes, C. Parasites, biodiversity and ecosystem stability. Biodivers. Conserv. 1996, 5, 953–962. [Google Scholar] [CrossRef]
- Scott, M.E. Impact of infection and disease on animal populations: Implications for conservation biology. Conserv. Biol. 1988, 2, 40–56. [Google Scholar] [CrossRef]
- Loye, J.; Carrol, S. Birds, bugs and blood: Avian parasitism and conservation. Trends Ecol. Evol. 1995, 10, 232–235. [Google Scholar] [CrossRef]
- Holmes, J.C. Parasites as threats to biodiversity in shrinking ecosystems. Biodivers. Conserv. 1996, 5, 975–983. [Google Scholar] [CrossRef]
- European Association of Zoos and Aquaria (EAZA), EAZA Ex Situ Programmes (EEPs). Available online: https://www.eaza.net/conservation/programmes/eep-pages/ (accessed on 9 February 2024).
- Lim, Y.A.L.; Ngui, R.; Shukri, J.; Rohela, M.; Mat-Naim, H.R. Intestinal parasites in various animals at a zoo in Malaysia. Vet. Parasitol. 2008, 157, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Da Silva-Barbosa, A.; Pinheiro, J.L.; Dos Santos, C.R.; de Lima, C.S.C.C.; Dib, L.V.; Echarte, G.V.; Augusto, A.M.; Bastos, A.C.M.P.; Antunes-Uchôa, C.M.; Bastos, O.M.P.; et al. Gastrointestinal parasites in captive animals at the Rio de Janeiro Zoo. Acta Parasitol. 2020, 65, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Gracenea, M.; Gómez, M.S.; Torres, J.; Carné, E.; Fernández-Morán, J. Transmission dynamics of Cryptosporidium in primates and herbivores at the Barcelona zoo: A long-term study. Vet. Parasitol. 2002, 104, 19–26. [Google Scholar] [CrossRef]
- Carrera-Jâtiva, P.D.; Morgan, E.R.; Barrows, M.; Wronski, T. Gastrointestinal parasites in captive and free-ranging birds and potential cross-transmission in a zoo environment. J. Zoo Wildl. Med. 2018, 49, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Jâtiva, P.D.; Morgan, E.R.; Barrows, M.; Jiménez-Uzcátegui, G.; Roosvelt, J.; Tituaña, A. Free-ranging avifauna as a source of generalist parasites for captive birds in zoological settings: An overview of parasite records and potential for cross-transmission. J. Adv. Vet. Anim. Res. 2020, 7, 482–500. [Google Scholar] [CrossRef]
- Juncker-Voss, M.; Prosl, H.; Lussy, H.; Enzenberg, U.; Auer, H.; Lassnig, H.; Müller, M.; Nowotny, N. Screening for antiboides against zoonoses among employees of the Zoological Garden of Vienna, Schönbrunn, Austria. Berl. Munch. Tierarztl. Wochenschr. 2004, 117, 404–409. [Google Scholar]
- Parkar, U.; Traub, R.J.; Vitali, S.; Elliot, A.; Levecke, B.; Robertson, I.; Geurden, T.; Steele, J.; Drake, B.; Andrew-Thompson, R.C. Molecular characterization of Blastocystis isolates from zoo animals and their animal-keepers. Vet. Parasitol. 2010, 169, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Arafa, W.M.; Mahrous, L.N.; Aboelhadid, S.M.; Abdel-Ghany, A.E. Investigation of enteric parasites of zoo animals and zookeepers in Beni-Suef Governatore, Egypt. Beni-Suef Vet. Med. J. 2013, 22, 121–125. [Google Scholar] [CrossRef]
- Köster, P.C.; Dashti, A.; Bailo, B.; Muadica, A.S.; Maloney, J.G.; Santín, M.; Chicharro, C.; Migueláñez, S.; Nieto, F.J.; Cano-Terriza, D.; et al. Occurrence and genetic diversity of protest parasites in captive non-human primates, zookeepers, and free-living sympatric rats in the Córdoba Zoo Conservation Centre, Southern Spain. Animals 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Köster, P.; Martínez-Nevado, E.; González, A.; Abelló-Poveda, M.T.; Fernández-Bellón, H.; de la Riva-Fraga, M.; Marquet, B.; Guéry, J.P.; Knauf-Witzens, T.; Weiglod, A.; et al. Intestinal protists in captive non-human primates and their handlers in six European zoological gardens. Molecular evidence of zoonotic transmission. Front. Vet. Sci. 2022, 8, 819887. [Google Scholar] [CrossRef] [PubMed]
- Greigert, V.; Brion, N.; Lang, C.; Regnard, P.; Pfaff, A.W.; Abou-Bacar, A.; Wanert, F.; Dirheimer, M.; Candolfi, E.; Brunet, J. Cestode infections in non-human primates suggest the existence of zoonotic cycles in the area surrounding the Strasbourg primatology center. Parasite 2019, 26, 25. [Google Scholar] [CrossRef]
- Ananias-Lima, T.; Borges-Salgado, P.A.; Fernandes-Chagas, C.R.; Locosque-Ramos, P.; Aparecido-Adriano, E.; Lima-González, I.H. Feral cats: Parasitic reservoirs in our zoos? Open J. Vet. Med. 2020, 10, 126–138. [Google Scholar] [CrossRef]
- Melo, Y.J.O.; Ferraz, H.T.; Saturnino, K.C.; Silva, T.D.P.; Braga, I.A.; Amaral, A.V.C.; Meirelles-Bartoli, R.B.; Ramos, D.G.S. Gastrointestinal parasites in captive and free-lliving wild birds in Goiania Zoo. Braz. J. Biol. 2021, 82, e240386. [Google Scholar] [CrossRef]
- Mammal Diversity Database (Version 1.11) [Data Set]. Zenodo. Available online: https://www.mammaldiversity.org/ (accessed on 9 February 2024). [CrossRef]
- Gill, F.; Donsker, D.; Rasmussen, P. (Eds.) IOC World Bird List (v14.1). 2024. Available online: https://www.worldbirdnames.org (accessed on 9 February 2024).
- Levine, J.A.; Estevez, E.G. Method of concentration of parasites from small amount of feces. J. Clin. Microbiol. 1983, 18, 786–788. [Google Scholar] [CrossRef]
- Levecke, B.; Dorny, P.; Geurden, T.; Vercammen, F.; Vercruysse, J. Gastrointestinal protozoa in non-human primates of four zoological gardens in Belgium. Vet. Parasitol. 2007, 148, 236–246. [Google Scholar] [CrossRef]
- Nath, B.G.; Islam, S.; Chakraborty, A. Prevalence of parasitic infection in captive non human primates of Assam State Zoo, India. Vet. World 2012, 5, 614–616. [Google Scholar] [CrossRef]
- Moreno-Mañas, E.; Gonzálvez-Juan, M.; Ruiz-de Ybáñez Carnero, M.R.; Gilber, T.; Ortiz, J.; Espeso, G.; Benzal, J.; Ibáñez, B.; Valera-Hernández, F. Survey of husbandry practices for bovidae in zoos: The importance of parasite management for reintroduction programmes. Vet. Rec. 2019, 184, 282. [Google Scholar] [CrossRef]
- Adeniyi, I.C.; Morenikeji, O.A.; Emikpe, B.O. The prevalence of gastro-intestinal parasites of carnivores in university zoological gardens in South West Nigeria. J. Vet. Med. Anim. Health 2015, 7, 135–139. [Google Scholar] [CrossRef][Green Version]
- Schieber, M.C.; Štrkolcová, G. Prevalence of endoparasites in carnivores in a zoo and a wolves park in Germany. Folia Vet. 2019, 63, 54–59. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, M.P.; Singla, L.D.; Sharma, S.; Sandhu, B.S.; Sharma, D.R. Parasitic infections in wild herbivores in the Mahendra Choudhury Zoological Park, Chhatbir, Punjab. Zoos’ Print J. 2006, 21, 2459–2461. [Google Scholar] [CrossRef]
- Gurler, A.T.; Beyhan, Y.E.; Acici, M.; Bolukbas, C.S.; Umur, S. Helminths of mammals and birds at the Samsun Zoological Garden, Turkey. J. Zoo Wildl. Med. 2010, 41, 218–223. [Google Scholar] [CrossRef]
- Papini, R.; Girivetto, M.; Marangi, M.; Mancianti, F.; Giangaspero, A. Endoparasite infections in pet and zoo birds in Italy. Sci. World J. 2012, 2012, 253127. [Google Scholar] [CrossRef]
- Fajardo-Sánchez, J.E.; Lasso-Narváez, A.M.; Mera-Eraso, C.M.; Peña-Stadlin, J.; Zapata-Valencia, J.I.; Rojas-Cruz, C. Potential zoonotic enteric parasites in animals in captivity at the Zoo in Cali, Colombia. Neotrop. Helminthol. 2014, 8, 279–290. [Google Scholar] [CrossRef]
- Dashe, D.; Berhanu, A. Study of gastrointestinal parasitism of wild animals in captivity at the Zoological Garden of Haramaya University, Ethiopia. Open J. Vet. Med. 2020, 10, 173–184. [Google Scholar] [CrossRef]
- Dos Santos, I.G.; Vieira-Batista, A.I.; Inacio-da Silva, W.S.; Oliveira-Neto, M.B.; Cerqueira-Schettino, S.; Resende-Oliveira, M.; Nascimento-Ramos, R.A.; Câmara-Alves, L.; Bezerra-Santos, M.; Santana-Lima, V.F. Gastrointestinal parasites in captive wild animals from two Brazilian Zoological Gardens. Res. Soc. Dev. 2022, 10, e28411426637. [Google Scholar] [CrossRef]
- Pérez-Cordón, G.; Hitos-Prados, A.; Romero, D.; Sánchez-Moreno, M.; Pontes, A.; Osuna, A.; Rosales, M.J. Intestinal and haematic parasitism in the birds of the Almuñecar (Granada, Spain) ornithological garden. Vet. Parasitol. 2009, 165, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Fagiolini, M.; Lia, R.P.; Laricchiuta, P.; Cavicchio, P.; Mannella, R.; Cafarchia, C.; Otranto, D.; Finotello, R.; Perrucci, S. Gastrointestinal parasites in mammals of two Italian zoological gardens. J. Zoo Wildl. Med. 2010, 41, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Panayotova-Pencheva, M.S. Parasites in captive animals: A review of studies in some European zoos. Zool. Gart. 2013, 82, 60–71. [Google Scholar] [CrossRef]
- Parsani, H.R.; Momin, R.R.; Bhuva, C.N. Parasitic infections among captive birds at Sakkarbagh Zoo, Junagadh, Gujarat. Zoos’ Print J. 2001, 16, 462–464. [Google Scholar] [CrossRef]
- Pérez-Cordón, G.; Hitos-Prados, A.; Romero, D.; Sánchez-Moreno, M.; Pontes, A.; Osuna, A.; Rosales, M.J. Intestinal parasitism in the animals of the zoological garden “Peña Escrita” (Almuñecar, Spain). Vet. Parasitol. 2008, 156, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Otegbade, A.C.; Morenikeji, O.A. Gastrointestinal parasites of birds in zoological gardens in South-West Nigeria. Trop. Biomed. 2014, 31, 54–62. [Google Scholar] [PubMed]
- Ilić, T.; Becskei, Z.; Gajić, B.; Özvegy, J.; Stepanović, P. Prevalence of endoparasitic infections of birds in zoo gardens in Serbia. Acta Parasitol. 2018, 63, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Borgsteede, F.H.M.; Okulewicz, A.; Zoun, P.E.F.; Okulewicz, J. The helminth fauna of birds of prey (Accipitriformes, Falconiformes and Strigiformes) in the Netherlands. Acta Parasitol. 2003, 48, 200–207. [Google Scholar]
- Parsani, H.R.; Momin, R.R.; Sahu, R.K.; Patel, B.G. Prevalence of gastro-intestinal parasites in captive birds at Kamla Nehru Zoological Garden, Kankaria Zoo, Ahmedabad, Gujarat. Zoos’ Print J. 2003, 18, 987–992. [Google Scholar] [CrossRef]
- Jayathangaraj, M.G.; Gomathinayagam, S.; Bhakyalakshmi, V. Incidence of coccidiosis in captive wild birds. Tamil Nadu J. Vet. Anim. Sci. 2008, 4, 156. [Google Scholar]
- Ponce-Gordo, F.; Herrera, S.; Castro, A.T.; García-Durán, B.; Martínez-Díaz, R.A. Parasites from farmed ostriches (Struthio camelus) and rheas (Rhea americana) in Europe. Vet. Parasitol. 2002, 107, 137–160. [Google Scholar] [CrossRef] [PubMed]
- Kvapil, P.; Kastelic, M.; Dovč, A.; Bártová, E.; Čížek, P.; Lima, N.; Štrus, Š. An eight-year survey of the intestinal parasites of carnivores, hoofed mammals primates, ratites and reptiles in the Ljubljana zoo in Slovenia. Folia Parasitol. 2017, 64, 013. [Google Scholar] [CrossRef]
- Bhowmik, M.K.; Sinha, P.K.; Chakraborty, A.K. Studies on the pathobiology of chicks experimentally infected with Raillietina cesticillus (Cestoda). Indian J. Poult. Sci. 1985, 7, 207–214. [Google Scholar]
- Samad, M.A.; Alam, M.M.; Bari, A.S. Effect of Raillietina echinobothrida infection on blood values and intestinal tissues of domestic fowls of Bangladesh. Vet. Parasitol. 1986, 21, 279–284. [Google Scholar] [CrossRef]
- Yabsley, M.J. Capillarid nematodes. In Parasitic Diseases of Wild Birds; Atkinson, C.T., Thomas, N.J., Hunter, D.B., Eds.; Wiley-Blackwell: Ames, IA, USA, 2008; pp. 463–497. [Google Scholar]
- Fagerholm, H.P.; Overstreet, R.M. Ascaridoid nematodes: Contracaecum, Porrocaecum, and Baylisascaris. In Parasitic Diseases of Wild Birds; Atkinson, C.T., Thomas, N.J., Hunter, D.B., Eds.; Wiley-Blackwell: Ames, IA, USA, 2008; pp. 413–433. [Google Scholar]
- Fedynich, A.M. Heterakis and Ascaridia. In Parasitic Diseases of Wild Birds; Atkinson, C.T., Thomas, N.J., Hunter, D.B., Eds.; Wiley-Blackwell: Ames, IA, USA, 2008; pp. 388–412. [Google Scholar]
- Greiner, E.C. Isospora, Atoxoplasma, and Sarcocystis. In Parasitic Diseases of Wild Birds; Atkinson, C.T., Thomas, N.J., Hunter, D.B., Eds.; Wiley-Blackwell: Ames, IA, USA, 2008; pp. 108–119. [Google Scholar]
- Yabsley, M.J. Eimeria. In Parasitic Diseases of Wild Birds; Atkinson, C.T., Thomas, N.J., Hunter, D.B., Eds.; Wiley-Blackwell: Ames, IA, USA, 2008; pp. 162–180. [Google Scholar]
- Schuster, R.; Krone, O. Infectious diseases—Parasites. In Avian Medicine, 3rd ed.; Samour, J., Ed.; Elsevier Ltd.: St. Louis, MO, USA, 2016; pp. 479–498. [Google Scholar]
- Ponce-Gordo, F.; Martínez-Díaz, R.A.; Herrera, S. Entasmoeba struthionis n.sp. (Sarcomastigophora: Endamoebidae) from ostriches (Struthio camelus). Vet. Parasitol. 2004, 119, 327–335. [Google Scholar] [CrossRef]
- Symeonidou, I.; Diakou, A.; Papadopoulos, E.; Ponce-Gordo, F. Endoparasitism of Greek ostriches: First report of Entamoeba struthionis and Balantioides coli. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100334. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Lebbad, M.; Victory, E.L.; Verweij, J.J.; Tannich, E.; Alfellani, M.; Legarraga, P.; Clark, C.G. Increased sampling reveals novel lineages of Entamoeba: Consequences of genetic diversity and host specificity for taxonomy and molecular detection. Protist 2011, 162, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.S.M.; Ederli, N.B.; Oliveira, F.C.R. Endoparasites and ectoparasites of rheas (Rhea Americana) from South America. Trop. Biomed. 2018, 35, 684–695. [Google Scholar] [PubMed]
- Esteban-Sánchez, L.; García-Rodríguez, J.J.; Ponce-Gordo, F. First Report of Entamoeba Dispar Infecting Captive Rheas (Rhea americana) and Giant Anteater (Myrmecophaga tridactyla); Department of Microbiology and Parasitology, Faculty of Pharmacy, UCM: Madrid, Spain, 2024; manuscript in preparation. [Google Scholar]
- Gallo, S.S.M.; Teixeira, C.S.; Ederli, N.B.; Oliveira, F.C.R. Gastrointestinal parasites of a population of emus (Dromaius novaehollandiae) in Brazil. Braz. J. Biol. 2020, 80, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Silvanose, C.D.; Bailey, T.A.; Samour, J.H.; Naldo, J.L. Intestinal protozoa and associated bacteria in captive houbara bustards (Chlamydotis undulate) in the United Arab Emirates. Avian Pathol. 1999, 28, 94–97. [Google Scholar] [CrossRef][Green Version]
- Ferdous, S.; Chowdhury, J.; Hasan, T.; Dutta, P.; Rahman, M.; Mahmudul-Hassan, M.; Faruque, R.; Abdul-Alim, M. Prevalence of gastrointestinal parasitic infections in wild mammals of a safari park and a zoo in Bangladesh. Vet. Med. Sci. 2023, 9, 1385–1394. [Google Scholar] [CrossRef]
- Maesano, G.; Capasso, M.; Ianniello, D.; Cringoli, G.; Rinaldi, L. Parasitic infections detected by FLOTAC in zoo mammals from Warsaw, Poland. Acta Parasitol. 2014, 59, 343–353. [Google Scholar] [CrossRef]
- Mir, A.Q.; Dua, K.; Singla, L.D.; Sharma, S.; Singh, M.P. Prevalence of parasitic infection in captive wild animals in Bir Moti Bagh mini zoo (Deer Park), Patiala, Punjab. Vet. World 2016, 9, 540–543. [Google Scholar] [CrossRef]
- Singh, P.; Singla, L.D.; Gupta, M.P.; Sharma, S.; Sharma, D.R. Epidemiology and chemotherapy of parasitic infections in wild omnivores in the Mahendra Choudhury Zoological Park, Chhat Bir, Punjab. J. Threat. Taxa 2009, 1, 62–64. [Google Scholar] [CrossRef]
- Thawait, V.K.; Maiti, S.K.; Dixit, A.A. Prevalence of gastro-intestinal parasites in captive wild animals of Nandan Van Zoo, Raipur, Chhattisgarh. Vet. World 2014, 7, 448–451. [Google Scholar] [CrossRef]
- Fernandes-Chagas, C.R.; Lima-Gonzales, I.H.; Borges-Salgado, P.A.; Rodrigues, B.; Locosque-Ramos, P. Giardia spp., ten years of parasitological data in the biggest zoo of Latin America. Ann. Parasitol. 2019, 65, 35–51. [Google Scholar]
- Ponce-Gordo, F.; Martínez-Díaz, R.A. Taxonomía y filogenia del género Entamoeba. Una revisión histórica. Rev. Ibero-Lat-Am. Parasitol. 2010, 69, 5–37. [Google Scholar]
- Tachibana, H.; Cheng, X.J.; Kobayashi, S.; Fujita, Y.; Udono, T. Entamoeba dispar, but not E. histolytica, detected in a colony of chimpanzees in Japan. Parasitol. Res. 2000, 86, 537–541. [Google Scholar] [CrossRef]
- Tachibana, H.; Cheng, X.J.; Kobayashi, S.; Matsubayashi, N.; Gotoh, S.; Matsubayashi, K. High prevalence of infection with Entamoeba dispar, but not E. histolytica, in captive macaques. Parasitol. Res. 2001, 87, 14–17. [Google Scholar] [CrossRef]
- Muehlenbein, M.P. Parasitological analyses of the male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. Am. J. Primatol. 2005, 65, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Yang, B.; Yang, L.; Fu, Y.; Zhuang, Y.; Liang, L.; Xu, Q.; Cheng, X.; Tachibana, H. High prevalence of Entamoeba infections in captive long-tailed macaques in China. Parasitol. Res. 2011, 109, 1093–1097. [Google Scholar] [CrossRef]
- Ravasi, D.F.; O’Riain, M.J.; Adams, V.J.; Appleton, C.C. A coprological survey of protozoan and nematode parasites of free-ranging chacma baboons (Papio ursinus) in the Southwestern Cape, South Africa. S. Afr. J. Wildl. Res. 2012, 42, 35–44. [Google Scholar] [CrossRef]
- Thoisy, B.D.; Vogel, I.; Reynes, J.M.; Pouliquen, J.F.; Carme, B.; Kazanji, M.; Vié, J.C. Health evaluation of translocated free-ranging primates in French Guiana. Am. J. Primatol. 2001, 54, 1–16. [Google Scholar] [CrossRef]
- Villers, L.M.; Jang, S.S.; Lent, C.L.; Lewin-Koh, S.C.; Norosoarinaivo, J.A. Survey and comparison of major intestinal flora in captive and wild ring-tailed lemur (Lemur catta) populations. Am. J. Primatol. 2008, 70, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Regan, C.S.; Yon, L.; Hossain, M.; Elsheikh, H.M. Prevalence of Entamoeba species in captive primates in zoological gardens in the UK. PeerJ 2014, 2, 42. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Ascuña-Durand, K.; Chihi, A.; Belkessa, S.; Kurt, Ö.; El-Badry, A.; van der-Giezen, M.; Clark, C.G. Further insight into the genetic diversity of Entamoeba coli and Entamoeba hartmanni. J. Eukaryot. Microbiol. 2023, 70, e12949. [Google Scholar] [CrossRef]
- Salaki, J.S.; Shirey, J.L.; Strickland, G.T. Successful treatment of symptomatic Entamoeba polecki infection. Am. J. Trop. Med. Hyg. 1979, 28, 190–193. [Google Scholar] [CrossRef]
- Al-shabbani, A.A. Direct detection of Entamoeba bovis in calves infected by diarrhea by using polymerase chain reaction thecnique. Kufa J. Vete Med. Sci. 2016, 7, 132–137. [Google Scholar] [CrossRef]
- Coke, R.L.; Carpenter, J.W.; Aboellail, T.; Armbrust, L.; Isaza, R. Dilated cardiomyopathy and amebic gastritis in a giant anteater (Myrmecophaga tridactyla). J. Zoo Wildl. Med. 2002, 33, 272–279. [Google Scholar] [CrossRef]
- Van Hoven, W.; Gilchrist, F.M.C.; Hamilton-Attwell, V.L. Intestinal ciliated protozoa of African rhinoceros: Two new genera and five new species from the white rhino (Ceratotherium simum Burchell, 1817). J. Protozool. 1987, 34, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Miyazaki, Y.; Imai, S. Description of new Parentodinium ciliates in the family Parentodiniidae n.fam. from Hippopotamus amphibius in comparison with some entodiniomorphs from horses and cattle. Eur. J. Protistol. 2002, 37, 405–426. [Google Scholar] [CrossRef]
- Obanda, V.; Lekolool, I.; Kariuki, J.; Gakuya, F. Composition of intestinal ciliate fauna of free-rangin African elephants in Tsavo West National Park, Kenya. Pachyderm 2007, 42, 92–96. [Google Scholar]
- Cedrola, F.; Bordim, S.; d’Agosto, M.; Pedroso Días, R.J. Intestinal ciliates (Alveolata, Cilioophora) in Brazilian domestic horses (Equus caballus L.) and a review on the ciliate communities associated with horses around the world. Zootaxa 2019, 4585, 478–488. [Google Scholar] [CrossRef]
- Kornilova, O.; Tsushko, K.; Chistyakova, L. The first record of intestinal ciliates from the mountain zebra (Equus zebra) in South Africa. Acta Protozool. 2020, 59, 149–155. [Google Scholar] [CrossRef]
- Kornilova, O.A.; Belokon, M.E.; Skazina, M.A.; Alekseeva, O.S.; Chistyakova, L.V. Ciliates from the intestine of zoo-kept black rhinoceros, with immunofluorescence microscopic and molecular phylogenetic investigation of Rhinozeta rhinozeta (Litostomatea). Eur. J. Protistol. 2023, 90, 126006. [Google Scholar] [CrossRef]
- Tajik, J.; Fard, S.R.N.; Paidar, A.; Anousheh, S.; Dehghani, E. Balantidiasis in a dromedarian camel. Asian Pac. J. Trop. Dis. 2013, 3, 409–412. [Google Scholar] [CrossRef]
- Dianso, J.A.; Garcia, G.G.; Belotindos, L.P.; Mingala, C.N. Molecular identification of Buxtonella sulcata from associated-diarrhea in wáter buffaloes (Bubalus bubalis) in the Philippines. Ann. Parasitol. 2018, 64, 93–100. [Google Scholar]
- Ponce-Gordo, F.; García-Rodriguez, J.J. Balantioides coli. Res. Vet. Sci. 2021, 135, 424–431. [Google Scholar] [CrossRef]
- Esteban-Sanchez, L.; Panayotova-Pencheva, M.; Qablan, M.; Modrý, D.; Hofmannová, L.; Ponce-Gordo, F. Question of agent of camel balantidiosis solved: Molecular identity, taxonomic solution and epidemiological considerations. Vet. Parasitol. 2023, 321, 109984. [Google Scholar] [CrossRef]
- Pomajbíková, K.; Petrželková, K.J.; Profousová, I.; Petrášová, J.; Modrýd, D. Discrepancies in the occurrence of Balantidium coli between wild and captive African great apes. J. Parasitol. 2010, 96, 1139–1144. [Google Scholar] [CrossRef]
- Petrželková, K.J.; Schovancová, K.; Profousová, I.; Kišidayová, S.; Varádyová, Z.; Pekár, S.; Kamler, J.; Modrý, D. The effect of low- and high-fiber diets on the population of entodiniomorphid ciliates Troglodytella abrassarti in captive chimpanzees (Pan troglodytes). Am. J. Primatol. 2012, 74, 669–675. [Google Scholar] [CrossRef]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef]
- Wielinga, C.; Williams, A.; Monis, P.; Thompson, R.C.A. Proposed taxonomic revision of Giardia duodenalis. Infect. Genet. Evol. 2023, 111, 105430. [Google Scholar] [CrossRef]
- Martínez-Díaz, R.A.; Sansano-Maestre, J.; Martínez-Herrero, M.C.; Ponce-Gordo, F.; Gómez-Muñoz, M.T. Occurrence and genetic characterization of Giardia duodenalis from captive nonhuman primates by multi-locus sequence analysis. Parasitol. Res. 2011, 109, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.T.; Raharison, J.L. A review of the endoparasites of the lemurs of Madagascar. Malagasy. Nature 2009, 2, 66–93. [Google Scholar]
- Diniz, L.S.M.; Costa, E.O.; Oliveira, P.M.A. Clinical disorders observed in anteaters (Myrmecophagidae, Edentata) in captivity. Vet. Res. Commun. 1995, 19, 409–415. [Google Scholar] [CrossRef]
- Stevanovic, O.; Nikolic, S.; Nedic, D.; Sladojevic, Z.; Zuko, A. Capillaria bovis (Schnyder, 1906) in farmed falow deer (Dama dama): First record in Bosnia and Herzegovina. In Book of Abstracts of the 20th Symposium of Epizootiologist and Epidemiologist, Vrnjacka Banja, Serbia, 18–20 April 2018; Serbian Veterinary Society: Subotica, Serbia, 2018; pp. 180–181. [Google Scholar]
- Ooi, H.K.; Tenora, F.; Itoh, K.; Kamiya, M. Comparative Study of Trichuris trichiura from non-human primates and from man, and their difference with T. suis. J. Vet. Med. Sci. 1993, 55, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Cutillas, C.; de Rojas, M.; Zurita, A.; Oliveros, R.; Callejón, R. Trichuris colobae n.sp. (Nematoda: Trichuridae), a new species of Trichuris from Colobus guereza kikuyensis. Parasitol. Res. 2014, 113, 2725–2732. [Google Scholar] [CrossRef]
- Callejón, R.; Halajian, A.; Cutillas, C. Description of a new species, Trichuris ursinus n. sp. (Nematoda: Trichuridae) from Papio ursinus Keer, 1792 from South Africa. Infect. Genet. Evol. 2017, 51, 182–193. [Google Scholar] [CrossRef]
- Chabaud, A.G.; Brygoo, E.R. L’endémisme chez les Helminthes de Madagascar. C. R. Geogr. Soc. 1964, 356, 3–13. [Google Scholar]
- Levecke, B.; Dreesen, L.; Dorny, P.; Verweij, J.J.; Vercammen, F.; Casaert, S.; Vercruysse, J.; Geldhof, P. Molecular identification of Entamoeba spp. in captive nonhuman primates. J. Clin. Microbiol. 2010, 48, 2988–2990. [Google Scholar] [CrossRef]
- Kyung-Yeon, E.; Seo, M.-G.; Leung, Y.-M.; Kwak, D.; Kwon, O.-D. Severe whipworm (Trichuris spp.) infection in the hamadryas baboon (Papio hamadryas). J. Vet. Med. Sci. 2019, 81, 53–56. [Google Scholar] [CrossRef]
- Kolapo, T.U.; Jegede, O.H. A survey of gastrointestinal parasites of captive animals at the University of Ilorin Zoological Garden Vom. J. Vet. Sci. 2017, 12, 17–27. [Google Scholar]
- Goossens, E.; Dorny, P.; Boomker, J.; Vercammen, F.; Vercruysse, J. A 12-month survey of the gastro-intestinal helminths of antelopes, gazelles and giraffids kept at two zoos in Belgium. Vet. Parasitol. 2005, 127, 303–312. [Google Scholar] [CrossRef]
- Cai, W.; Zhu, Y.; Wang, F.; Feng, Q.; Zhang, Z.; Xue, N.; Xu, X.; Hou, Z.; Liu, D.; Xu, J.; et al. Prevalence of gastrointestinal parasites in zoo animals and phylogenetic characterization of Toxascaris leonine (Linstow, 1902) and Baylisascaris transfuga (Rudolphi, 1819) in Jiangsu Province3, Eastern China. Animals 2024, 14, 375. [Google Scholar] [CrossRef] [PubMed]
- VanHoy, G. Common gastrointestinal parasites of small ruminants. In Merck Manual Professional Version; Merck & Co., Inc.: Rahway, NJ, USA, 2023; Available online: https://www.msdvetmanual.com/digestive-system/gastrointestinal-parasites-of-ruminants/overview-of-gastrointestinal-parasites-of-ruminants (accessed on 9 February 2024).
- De Ambrogi, M.; Aghazadeh, M.; Hermosilla, C.; Huber, D.; Majnaric, D.; Reljic, S.; Elson-Riggins, J. Occurrence of Baylisascaris transfuga in wild populations of European brown bears (Ursus arctos) as identified by a new PCR method. Vet. Parasitol. 2011, 179, 272–276. [Google Scholar] [CrossRef]
- d’Ovidio, D.; Pantchev, N.; Noviello, E.; del Prete, L.; Maurelli, M.P.; Cringli, G.; Rinaldi, L. Survey of Baylisascaris spp. in captive striped skunks (Mephitis mephitis) in some European áreas. Parasitol. Res. 2017, 116, 483–486. [Google Scholar] [CrossRef]
- Okulewicz, A.; Lonc, E.; Borgsteede, F.H. Ascarid nematodes in domestic and wild terrestrial mammals. Pol. J. Vet. Sci. 2002, 5, 277–281. [Google Scholar] [PubMed]
- Capasso, M.; Maurelli, M.P.; Ianniello, D.; Camara-Alves, L.; Amadesi, A.; Laricchiuta, P.; Silvestre, P.; Campolo, M.; Cringoli, G.; Rinaldi, L. Use of Mini-FLOTAC and Fill-FLOTAC for rapidly diagnosing parasitic infections in zoo animals. Rev. Bras. Parasitol. Vet. 2019, 28, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Zheng, W.; Guo, L.; Sun, Y.; Li, J.; Kang, M. First description of Blastocystis sp. and Entamoeba sp. infecting zoo animals in the Quinghai-Tibetan plateau área, China. Front. Cell. Infect. Microbiol. 2023, 13, 1212617. [Google Scholar] [CrossRef]
- Rahman, R.; Nyema, J.; Imranuzzaman, M.D.; Banik, B.; Pranto, P.S.; Talukder, K.; Sarkar, S.R.; Nath, S.D.; Islam, K.M.; Nath, T.C.; et al. An update on gastrointestinal parasitic infection in captive wild animals in Bangladesh. J. Parasitol. Res. 2023, 2023, 3692471. [Google Scholar] [CrossRef]
- Hossain, M.N.; Dey, A.R.; Begum, N.; Farjan, T. Parasitic infection in captive wild mammals and birds in Bangabandhu Sheikh Mujib Safari Park, Cox’s Bazar, Bangladesh. J. Threat. Taxa 2021, 13, 17889–17894. [Google Scholar] [CrossRef]
- Dhakal, P.; Sharma, H.P.; Shah, R.; Thapa, P.J.; Pokhera, C.P. Copromicroscopic study of gastrointestinal parasites in captive mammals at Central Zoo, Lalitpur, Nepal. Vet. Med. Sci. 2023, 9, 457–464. [Google Scholar] [CrossRef]
- Gonzálvez, M.; Moreno, E.; Pérez-Cutillas, P.; Gilbert, T.; Ortiz, J.; Valera, F.; Espeso, G.; Benzal, J.; Ibáñez, B.; Ruiz-de Ybáñez, M.R. Zoological institutions as hotspots of gastrointestinal parasites that may affect the success of ungulate reintroduction programmes. Vet. Rec. 2021, 189, e506. [Google Scholar] [CrossRef]
- Karshima, S.N. Parasites of importance for human health on edible fruits and vegetables in Nigeria: A systematic review and meta-analysis of published data. Pathog. Glob. Health 2018, 112, 47–55. [Google Scholar] [CrossRef]
- Cardoso-Rodrigues, A.; Castro-da Silva, M.D.; Seixas-Pereira, R.A.; Pinto, L.C. Prevalence of contamination by intestinal parasites in vegetables (Lactuca sativa L. and Coriandrum sativum L.) sold in markets in Belém, northern Brazil. J. Sci. Food Agric. 2020, 100, 2859–2865. [Google Scholar] [CrossRef]
- Alemu, G.; Nega, M.; Alemu, M. Parasitic contamination of fruits and vegetables collected from local markerts of Bahir Dar City, Northwest Ethiopia. Res. Rep. Trop. Med. 2020, 11, 17–25. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Karim, M.R.; Zhang, L. Detection of human intestinal protozoan parasites in vegetables and fruits: A review. Parasit Vectors 2020, 13, 380. [Google Scholar] [CrossRef]
- Federer, K.; Armua-Fernandez, M.T.; Gori, F.; Hoby, S.; Wenker, C.; Deplazes, P. Detection of taeniid (Taenia spp., Echinococcus spp.) eggs contaminating vegetables and fruits sold in European markets and the risk for metacestode infectiions in captive primates. Int. J. Parasitol. Parasites Wildl. 2016, 5, 249–253. [Google Scholar] [CrossRef]
- European Food Safety Authority Panel on Biological Hazards. Scientific Opinion on the public health risks associated with food-borne parasites. EFSA J. 2018, 16, 5495.
- Bais, B.; Tak, L.; Mahla, S. Study of preventive health measures for wildlife in captivity: A review of management approaches. Int. J. Avian Wildl. Biol. 2017, 2, 73–75. [Google Scholar] [CrossRef][Green Version]
- Luzón, M.; de la Fuente-López, C.; Martínez-Nevado, E.; Fernández-Morán, J.; Ponce-Gordo, F. Taenia crassiceps cysticercosis in a ring-tailed lemur (Lemur catta). J. Zoo Wildl. Med. 2010, 41, 327–330. [Google Scholar] [CrossRef]
- Martínez-Nevado, E.; (Veterinary Department, ZooAquarium, Madrid, Spain); García-García, J.; (Veterinary Department, ZooAquarium, Madrid, Spain). ZooAquarium internal records, 2023.
- Faria, P.J.; van Oosterhout, C.; Cable, J. Optimal release strategies for captive-bred animals in reintroduction programs: Experimental infections using the guppy as a model organism. Biol. Conserv. 2010, 143, 35–41. [Google Scholar] [CrossRef]
- Kołodziej-Sobocińska, M.; Demiaszkiewicz, A.W.; Pyziel, A.M.; Kowalczyk, R. Increased parasitic load in captive-released European bison (Bison bonasus) has important implications for reintroduction programs. EcoHealth 2018, 15, 467–471. [Google Scholar] [CrossRef]
- Kock, R.A.; Woodford, M.H.; Rossiter, P.B. Disease risks associated with the translocation of wildlife. Rev. Sci. Tech. 2010, 29, 329–350. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, D. Conservation implications of parasite co-reintroduction. Conserv. Biol. 2015, 29, 602–604. [Google Scholar] [CrossRef]
- Ewen, J.G.; Armstrong, D.P.; Empson, R.; Makan, S.J.T.; McInnes, K.; Parker, K.A.; Richardson, K.; Alley, M. Parasite management in translocations: Lessons from a threatened New Zealand bird. Oryx 2012, 46, 446–456. [Google Scholar] [CrossRef]
- Figueiredo, A.M.; Madeira-de Carvalho, L.; González, M.J.P.; Torres, R.T.; Pla, S.; Núñez-Arjona, J.C.; Rueda, C.; Vallverdú-Coll, N.; Silvestre, F.; Peña, J.; et al. Parasites of the reintroduced Iberian Lynx (Lynx pardinus) and sympatric mesocarnivores in Extremadura, Spain. Pathogens 2021, 10, 274. [Google Scholar] [CrossRef]
- Akinboye, D.O.; Ogunfeitimi, A.A.; Fawole, O.; Agbodale, O.; Ayinde, O.O.; Atulomah, N.O.S.; Amosu, A.M.; Livingstone, R. Control of parasitic infections among workers and inmates in a Nigerian zoo. Niger. J. Parasitol. 2010, 31, 35–38. [Google Scholar] [CrossRef]
- Cian, A.; El Safadi, D.; Osman, M.; Moriniere, R.; Gantois, N.; Benamrouz-Vanneste, S.; Delgado-Viscogliosi, P.; Guyot, K.; Monchy, S.; Noël, C.; et al. Molecular epidemiology of Blastocystis sp. in various animal groups from two French zoos and evaluation of potential zoonotic risk. PLoS ONE 2017, 12, e0169659. [Google Scholar] [CrossRef]
| Zoo | Hosts | Diet Type | Animal Species Studied | Hosts Infected | Samples Analysed | Positive Samples |
|---|---|---|---|---|---|---|
| ZooAquarium | Mammals | Herbivores | 55 | 49 (89.1%) | 1891 | 956 (50.6%) |
| Omnivores | 17 | 9 (53.0%) | 455 | 164 (36.0%) | ||
| Carnivores | 11 | 2 (18.2%) | 254 | 27 (10.6%) | ||
| Total | 83 | 60 (72.3%) | 2600 | 1147 (44.1%) | ||
| Birds | Herbivores | 17 | 0 (0.0%) | 127 | 0 (0.0%) | |
| Omnivores | 15 | 3 (20.0%) | 127 | 8 (6.3%) | ||
| Carnivores | 32 | 4 (12.5%) | 285 | 26 (9.1%) | ||
| Total | 64 | 7 (11.0%) | 539 | 34 (6.3%) | ||
| Faunia | Mammals | Herbivores | 29 | 17 (58.6%) | 783 | 136 (17.4%) |
| Omnivores | 23 | 7 (30.4%) | 671 | 46 (6.9%) | ||
| Carnivores | 16 | 1 (6.3%) | 422 | 2 (0.5%) | ||
| Total | 68 | 25 (36.8%) | 1876 | 184 (9.8%) | ||
| Birds | Herbivores | 14 | 0 (0.0%) | 130 | 0 (0.0%) | |
| Omnivores | 12 | 5 (41.7%) | 188 | 29 (15.4%) | ||
| Carnivores | 14 | 0 (0.0%) | 94 | 0 (0.0%) | ||
| Total | 40 | 5 (12.5%) | 412 | 29 (7.0%) | ||
| Total results * | Mammals | Herbivores | 73 | 62 (84.9%) | 2674 | 1092 (40.8%) |
| Omnivores | 34 | 16 (47.1%) | 1126 | 211 (18.7%) | ||
| Carnivores | 25 | 4 (16.0%) | 676 | 30 (4.4%) | ||
| Total | 132 | 82 (62.1%) | 4476 | 1333 (29.8%) | ||
| Birds | Herbivores | 24 | 0 (0.0%) | 257 | 0 (0.0%) | |
| Omnivores | 22 | 7 (31.8%) | 315 | 37 (11.8%) | ||
| Carnivores | 40 | 4 (10.0%) | 379 | 26 (6.9%) | ||
| Total | 86 | 11 (12.8%) | 951 | 63 (6.6%) |
| ZooAquarium | Faunia | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infected Host Species | Infected Host Species | |||||||||||||
| Mammals | Birds | Mammals | Birds | |||||||||||
| C (2) | O (9) | H (49) | C (4) | O (3) | H (0) | C (1) | O (7) | H (17) | C (0) | O (5) | H (0) | |||
| Amoebae | ||||||||||||||
| Entamoeba Casagrandi and Barbagallo 1897 | 1 | 6 | 34 | 3 | 3 | 9 | 1 | |||||||
| Endolimax Kuenen and Swellengrebel 1913 | 2 | 6 | 2 | |||||||||||
| Flagellates | ||||||||||||||
| Giardia Künstler 1882 | 1 | 4 | 1 | 3 | ||||||||||
| Chilomastix Aléxéieff 1910 | 3 | 8 | 2 | 2 | ||||||||||
| Trichomonads | 1 | 1 | ||||||||||||
| Coccidia | ||||||||||||||
| Eimeria Schneider 1875 | 5 | 2 | ||||||||||||
| Toxoplasma Nicolle and Manceaux 1909/Neospora Dubey et al. 1988 | 1 | |||||||||||||
| Ciliates | ||||||||||||||
| Balantioides Alexeieff 1931 | 5 | 8 | 1 | 2 | 1 | |||||||||
| Buxtonella Jameson 1926 | 5 | |||||||||||||
| Troglodytella (Brumpt and Joyeux 1912) | 1 | |||||||||||||
| Endosymbiotic ciliates | 6 | 2 | ||||||||||||
| Trematodes | ||||||||||||||
| Unidentified eggs | 1 | |||||||||||||
| Cestodes | ||||||||||||||
| Unidentified eggs | 1 | 5 | 1 | |||||||||||
| Nematodes | ||||||||||||||
| Trichuris Roederer 1761 | 3 | 10 | 1 | 1 | 1 | 6 | ||||||||
| Capillaria Zeder 1800/capillariids | 1 | 1 | 2 | 3 | 1 | 3 | ||||||||
| Nematodirus Ransom 1907 | 2 | |||||||||||||
| Trichostrongylids | 1 | |||||||||||||
| Baylisascaris Sprent 1968 | 1 | 1 | ||||||||||||
| Parascaris Yorke and Maplestone 1926 | 2 | |||||||||||||
| Porrocaecum Railliet and Henry 1912 | 1 | |||||||||||||
| Ascaridia Dujardin 1845/Heterakis Schrank 1790 | 2 | |||||||||||||
| Ascarid (unidentified) | 1 | |||||||||||||
| Samples | ||||
|---|---|---|---|---|
| Order | Family | Species | (Total/Positives) | Parasites Found (% of Total Samples) |
| Artiodactyla | Bovidae | Ammotragus lervia (Pallas 1777) | 21/14 | Entamoeba bovis-like (66.7%) |
| Antilope cervicapra (Linnaeus 1758) | 18/9 | Entamoeba bovis-like (50.0%) | ||
| Bison bison (Linnaeus 1758) | 37/15 | Entamoeba bovis-like (40.5%) | ||
| Bison bonasus (Linnaeus 1758) | 27/19 | Entamoeba bovis-like (70.4%), Buxtonella sulcata Jameson 1926 (3.7%) | ||
| Bos grunniens (Linnaeus 1766) | 17/12 | Entamoeba bovis-like (52.9%), Buxtonella sulcata (41.2%), unidentified cestode eggs (5.9%) | ||
| Bos taurus (Linnaeus 1758) | 4/1 | Entamoeba bovis-like (25.0%) | ||
| Boselaphus tragocamelus (Pallas 1766) | 13/12 | Entamoeba bovis-like (92.3%) | ||
| Budorcas taxicolor Hodgson 1850 | 39/26 | Entamoeba bovis-like (66.7%) | ||
| Capra hircus Linnaeus 1758 | 37/31 | Entamoeba bovis-like (81.1%), Eimeria spp. (5.4%), Trichuris spp. (5.4%) | ||
| Capra pyrenaica Schinz 1838 | 30/20 | Entamoeba bovis-like (66.7%) | ||
| Connochaetes gnou (Zimmermann 1780) | 23/14 | Entamoeba bovis-like (60.9%) | ||
| Gazella dorcas osiris Blaine 1913 | 127/106 | Entamoeba bovis-like (70.1%), Eimeria spp. (1.6%), Trichuris spp. (16.5%), Nematodirus spp. (21.3%) | ||
| Nanger dama mhorr (Bennett 1833) | 99/85 | Entamoeba bovis-like (74.7%), Entamoeba spp. (8-nucleated) (2.0%), Giardia spp. (2.0%), Chilomastix spp. (1.0%), Eimeria spp. (2.0%), Trichuris spp. (15.2%), Nematodirus spp. (8.1%), Trichostrongylids (10.1%) | ||
| Ovis aries Linnaeus 1758 | 39/13 | Entamoeba bovis-like (25.6%), Chilomastix spp. (2.6%), Eimeria spp. (5.1%) | ||
| Ovis gmelinii Blyth 1841 | 11/6 | Entamoeba bovis-like (54.6%) | ||
| Syncerus caffer nanus Boddaert 1785 | 59/54 | Entamoeba bovis-like (89.8%), trichomonads (3.4%), Buxtonella sulcata (44.1%) | ||
| Tragelaphus eurycerus (Ogilby 1837) | 17/15 | Entamoeba bovis-like (88.2%) | ||
| Tragelaphus spekii gratus Sclater 1880 | 40/26 | Entamoeba bovis-like (60.0%), Chilomastix spp. (2.5%), Balantioides coli-like (15.0%) | ||
| Camelidae | Camelus bactrianus Linnaeus 1758 | 40/19 | Entamoeba bovis-like (20.0%), Buxtonella cameli (Boschenko 1925) (25.0%), Trichuris spp. (5.0%) | |
| Camelus dromedarius Linnaeus 1758 | 34/7 | Entamoeba bovis-like (5.9%), Buxtonella cameli (20.6%) | ||
| Lama guanicoe (Müller 1776) | 19/8 | Entamoeba bovis-like (42.1%) | ||
| Cervidae | Alces alces (Linnaeus 1758) | 16/11 | Balantioides coli-like (43.8%), Trichuris spp. (56.3%) | |
| Capreolus capreolus (Linnaeus 1758) | 6/5 | Entamoeba bovis-like (83.3%) | ||
| Cervus elaphus Linnaeus 1758 | 34/30 | Entamoeba bovis-like (85.3%), unidentified cestode eggs (2.9%) | ||
| Dama dama (Linnaeus 1758) | 40/35 | Entamoeba bovis-like (87.5%), Capillaria spp. (5.0%) | ||
| Elaphurus davidianus Milne-Edwards 1866 | 29/20 | Entamoeba bovis-like (69.0%) | ||
| Muntiacus reevesi (Ogilby 1839) | 21/18 | Entamoeba bovis-like (85.7%), Chilomastix spp. (4.8%) | ||
| Rangifer tarandus (Linnaeus 1758) | 23/4 | Entamoeba bovis-like (4.4%), Trichuris spp. (13.0%) | ||
| Giraffidae | Giraffa camelopardalis (Linnaeus 1758) | 40/14 | Entamoeba bovis-like (35.0%), Trichuris spp. (2.5%) | |
| Hippopotamidae | Hippopotamus amphibius Linnaeus 1758 | 11/1 | Unidentified cestode eggs (9.1%) | |
| Suidae | Potamochoerus porcus (Linnaeus 1758) | 37/14 | Entamoeba polecki-like (29.7%), Giardia spp. (2.7%), Chilomastix spp. (8.1%), Balantioides coli (24.3%) | |
| Sus scrofa Linnaeus 1758 | 59/43 | Entamoeba polecki-like (52.5%), Chilomastix spp. (8.5%), Balantioides coli (42.4%) | ||
| Tayassuidae | Dicotyles tajacu (Linnaeus 1758) | 1/0 | ||
| Carnivora | Ailuridae | Ailurus fulgens Cuvier 1825 | 33/2 | Capillaria spp. (6.1%) |
| Canidae | Canis lupus occidentalis Linnaeus 1758 | 15/0 | ||
| Speothos venaticus (Lund 1842) | 45/1 | Toxoplasma/Neospora (2.2%) | ||
| Felidae | Lynx lynx (Linnaeus 1758) | 13/0 | ||
| Lynx pardinus (Temminck 1827) | 20/0 | |||
| Panthera leo (Linnaeus 1758) | 16/0 | |||
| Panthera pardus saxicolor (Linnaeus 1758) | 22/0 | |||
| Panthera tigris (Linnaeus 1758) | 16/0 | |||
| Herpestidae | Suricata suricatta (Schreber 1776) | 10/0 | ||
| Mustelidae | Mustela lutreola (Linnaeus 1761) | 30/0 | ||
| Pteronura brasiliensis (Zimmermann 1780) | 20/0 | |||
| Procyonidae | Nasua nasua (Linnaeus 1766) | 9/0 | ||
| Procyon lotor (Linnaeus 1758) | 18/2 | Capillaria spp. (11.1%) | ||
| Ursidae | Ailuropoda melanoleuca (David 1869) | 36/0 | ||
| Helarctos malayanus (Raffles 1822) | 35/1 | Trematoda (2.9%) | ||
| Tremarctos ornatus (Cuvier 1825) | 1/0 | |||
| Ursus americanus Pallas 1780 | 16/0 | |||
| Ursus arctos Linnaeus 1758 | 63/7 | Baylisascaris spp. (11.1%) | ||
| Ursus thibetanus Cuvier 1823 | 37/0 | |||
| Viverridae | Arctictis binturong (Raffles 1822) | 32/0 | ||
| Diprotodontia | Macropodidae | Notamacropus rufogriseus (Desmarest 1817) | 6/0 | |
| Petrogale xanthopus Gray 1855 | 20/5 | Entamoeba bovis-like (25.0%) | ||
| Phascolarctidae | Phascolarctos cinereus (Goldfuss 1817) | 35/0 | ||
| Lagomorpha | Leporidae | Oryctolagus cuniculus (Linnaeus 1758) | 65/4 | Eimeria spp. (6.2%) |
| Perissodactyla | Equidae | Equus quaga Boddaert 1785 | 159/9 | Endosymbiotic ciliates (4.4%), Parascaris equorum (Goeze 1782) (1.3%) |
| Equus asinus Linnaeus 1758 | 25/21 | Endosymbiotic ciliates (84.0%), Parascaris equorum (8.0%) | ||
| Equus caballus Linnaeus 1758 | 44/28 | Endosymbiotic ciliates (63.6%) | ||
| Rhinocerotidae | Ceratotherium simum (Burchell 1817) | 35/18 | Endosymbiotic ciliates (51.4%) | |
| Rhinoceros unicornis Linnaeus 1758 | 32/27 | Endosymbiotic ciliates (84.4%) | ||
| Tapiridae | Tapirus indicus (Desmarest 1819) | 31/1 | Chilomastix spp. (3.2%) | |
| Tapirus terrestris (Linnaeus 1758) | 12/2 | Balantioides coli (8.3%), unidentified cestode eggs (8.3%) | ||
| Pilosa | Myrmecophagidae | Myrmecophaga tridactyla Linnaeus 1758 | 47/26 | Entamoeba spp. (4-nucleated) (2.1%), Tetratrichomonas spp. Parisi 1910 (25.5%), Capillaria-like eggs (36.2%) |
| Primates | Cebidae | Sapajus apella (Linnaeus 1758) | 26/0 | |
| Cercopithecidae | Colobus guereza Rüppell 1835 | 45/40 | Entamoeba coli-like (24.4%), Entamoeba polecki-like (22.2%), Balantioides coli-like (2.2%), Trichuris spp. (84.4%) | |
| Macaca spp. Lacepede 1799 | 1/0 | |||
| Mandrillus sphinx (Linnaeus 1758) | 47/47 | Entamoeba polecki-like (76.6%), Entamoeba coli-like (10.6%), Chilomastix spp. (10.6%), Balantioides coli-like (66.0%), Trichuris spp. (2.1%), unidentified cestode eggs (2.1%) | ||
| Papio spp. Erxleben 1777 | 17/17 | Entamoeba coli-like (94.1%), Endolimax spp. (5.9%), Trichuris spp. (82.4%) | ||
| Hominidae | Pongo pygmaeus (Linnaeus 1760) | 103/79 | Balantioides coli-like (76.7%) | |
| Gorilla gorilla (Savage 1847) | 29/17 | Entamoeba coli-like (3.5%), Balantioides coli-like (51.7%), Troglodytella abrassarti (Brumpt and Joyeux 1912) (10.3%) | ||
| Pan troglodytes (Blumenbach 1775) | 28/21 | Entamoeba coli-like (39.3%), Entamoeba polecki-like (14.3%), Endolimax spp. (3.6%), Balantioides coli-like (39.3%) | ||
| Hylobatidae | Hylobates lar (Linnaeus 1771) | 25/9 | Entamoeba coli-like (4.0%), Entamoeba polecki-like (4.0%), Balantioides coli-like (28.0%) | |
| Hylobates muelleri (Martin 1841) | 28/12 | Entamoeba coli-like (28.6%), Balantioides coli-like (14.3%), Trichuris spp. (3.6%) | ||
| Lemuridae | Eulemur fulvus (Geoffroy 1796) | 14/2 | Giardia spp. (7.1%), unidentified cestode eggs (7.1%) | |
| Lemur catta Linnaeus 1758 | 16/2 | Entamoeba polecki-like (6.3%), Giardia spp. (6.3%), Trichuris spp. (6.3%) | ||
| Varecia variegata (Kerr 1792) | 40/0 | |||
| Proboscidea | Elephantidae | Elephas maximus Linnaeus 1758 | 55/30 | Chilomastix spp. (1.8%), endosymbiotic ciliates (54.5%) |
| Rodentia | Caviidae | Cavia porcellus (Linnaeus 1758) | 17/0 | |
| Dolichotis patagonum (Zimmermann 1780) | 27/8 | Entamoeba muris-like (3.7%), Giardia spp. (14.8%), Chilomastix spp. (7.4%), Trichuris spp. (3.7%) | ||
| Hydrochoerus hydrochaeris (Linnaeus 1766) | 15/2 | Chilomastix spp. (6.7%), Balantioides coli-like (6.7%) | ||
| Chinchillidae | Chinchilla spp. Bennett 1829 | 1/0 |
| Samples | ||||
|---|---|---|---|---|
| Order | Family | Species | (Total/Positives) | Parasites Found (% of Total Samples) |
| Accipitriformes | Accipitridae | Aegypius monachus (Linnaeus 1766) | 7/0 | |
| Aquila adalberti Brehm 1861 | 13/0 | |||
| Aquila verreauxii Lesson 1831 | 3/0 | |||
| Buteo buteo (Linnaeus 1758) | 13/0 | |||
| Geranoaetus melanoleucus (Vieillot 1819) | 3/0 | |||
| Gypohierax angolensis (Gmelin 1788) | 2/0 | |||
| Gyps fulvus (Hablizl 1783) | 23/0 | |||
| Haliaeetus albicilla (Linnaeus 1758) | 3/0 | |||
| Haliaeetus leucocephalus (Linnaeus 1766) | 3/0 | |||
| Haliaeetus pelagicus (Pallas 1811) | 10/3 | Capillariids (34.3%) | ||
| Ichtyophaga vocifer (Daudin 1800) | 3/0 | |||
| Milvus migrans (Boddaert 1783) | 36/21 | Capillariids (38.9%), Porrocaecum spp. (33.3%) | ||
| Neophron percnopterus (Linneaus 1758) | 3/0 | |||
| Parabuteo unicinctus (Temminck 1824) | 28/1 | Trichuris spp. (3.6%) | ||
| Anseriformes | Anatidae | Alopochen aegyptiaca (Linnaeus 1766) | 5/0 | |
| Aythya nyroca (Güldenstädt 1770) | 1/0 | |||
| Cairina moschata (Linnaeus 1758) | 8/0 | |||
| Cygnus atratus (Latham 1790) | 1/0 | |||
| Tadorna ferruginea (Pallas 1764) | 4/0 | |||
| Tadorna tadorna (Linnaeus 1758) | 3/0 | |||
| Bucerotiformes | Bucerotidae | Bycanistes brevis Friedmann 1929 | 5/0 | |
| Bycanistes bucinator (Temminck 1824) | 8/0 | |||
| Bucorvidae | Bucorvus leadbeateri (Vigors 1825) | 5/0 | ||
| Cathartiformes | Cathartidae | Sarcoramphus papa (Linnaeus 1758) | 10/0 | |
| Vultur gryphus Linnaeus 1758 | 5/0 | |||
| Ciconiiformes | Ciconiidae | Ciconia ciconia (Linnaeus 1758) | 10/0 | |
| Leptoptilos crumenifer (Lesson 1831) | 3/0 | |||
| Columbiformes | Columbidae | Columba livia Gmelin 1789 | 11/0 | |
| Coraciiformes | Alcedinidae | Dacelo novaeguineae Hermann 1783 | 2/0 | |
| Falconiformes | Falconidae | Falco naumanni Fleischer 1818 | 13/0 | |
| Caracara plancus (Miller 1777) | 1/0 | |||
| Galliformes | Numididae | Numida meleagris (Linnaeus 1758) | 4/0 | |
| Phasianidae | Gallus gallus (Linnaeus 1758) | 39/1 | Entamoeba gallinarum Tyzzer 1920 (2.6%) | |
| Gruiformes | Gruidae | Balearica regulorum (Bennett 1834) | 4/0 | |
| Musophagiformes | Musophagidae | Tauraco erythrolophus (Vieillot 1819) | 8/0 | |
| Menelikornis leucotis (Rüppell 1835) | 5/0 | |||
| Passeriformes | Corvidae | Corvus corax Linnaeus 1758 | 3/0 | |
| Pelecaniformes | Pelecanidae | Pelecanus rufescens Gmelin 1789 | 8/0 | |
| Threskiornithidae | Eudocimus ruber (Linnaeus 1758) | 11/0 | ||
| Threskiornis aethiopicus (Latham 1790) | 23/0 | |||
| Phoenicopteriformes | Phoenicopteridae | Phoenicopterus ruber Linnaeus 1758 | 7/0 | |
| Piciformes | Ramphastidae | Ramphastos toco Müller 1776 | 5/0 | |
| Psittaciformes | Cacatuidae | Cacatua alba (Müller 1776) | 6/0 | |
| Cacatua galerita (Latham 1790) | 11/0 | |||
| Cacatua goffiniana Roselaar and Michels 2004 | 5/0 | |||
| Cacatua pastinator (Gould 1841) | 8/0 | |||
| Cacatua sulphurea (Gmelin 1788) | 5/0 | |||
| Psittacidae | Amazona aestiva (Linnaeus 1758) | 8/0 | ||
| Ara ararauna (Linnaeus 1758) | 22/0 | |||
| Ara chloropterus Gray 1859 | 8/0 | |||
| Ara rubrogenys Lafresnaye 1847 | 1/0 | |||
| Aratinga solstitialis (Linnaeus 1758) | 15/0 | |||
| Myiopsitta monachus Boddaert 1783 | 1/0 | |||
| Psittacus erithacus Linnaeus 1758 | 7/0 | |||
| Trichoglossus haematodus (Linnaeus 1771) | 4/0 | |||
| Psittaculidae | Eclectus roratus (Müller 1776) | 2/0 | ||
| Strigiformes | Strigidae | Bubo bubo hispanus Rothschild and Hartert 1910 | 15/1 | Capillariids (6.7%) |
| Bubo bubo sibiricus Gloger 1833 | 5/0 | |||
| Bubo scandiacus (Linnaeus 1758) | 2/0 | |||
| Strix nebulosa Forster 1772 | 1/0 | |||
| Casuariformes | Casuariidae | Casuarius casuarius (Linnaeus 1758) | 12/0 | |
| Struthioniformes | Dromaiidae | Dromaius novaehollandiae (Latham 1790) | 20/0 | |
| Rheiformes | Rheidae | Rhea americana (Linnaeus 1758) | 7/2 | Entamoeba spp. (4-nucleated) (28.6%) |
| Struthioniformes | Struthionidae | Struthio camelus Linnaeus 1758 | 12/5 | Entamoeba polecki-like (33.3%), Balantioides coli (8.3%) |
| Samples | ||||
|---|---|---|---|---|
| Order | Family | Species | (Total/Positives) | Parasites Found (% of Total Samples) |
| Afrosoricida | Tenrecidae | Echinops telfairi Martin 1838 | 2/0 | |
| Artiodactyla | Bovidae | Capra hircus | 40/22 | Entamoeba bovis-like (55.0%), Eimeria spp. (2.5%) |
| Madoqua kirkii (Günther 1880) | 43/15 | Entamoeba bovis-like (11.6%), Entamoeba spp. (8-nucleated) (27.9%), Trichuris spp. (2.3%) | ||
| Ovis aries | 26/10 | Entamoeba bovis-like (34.6%), Eimeria spp. (3.9%) | ||
| Cervidae | Subulo gouazoubira (Fischer 1814) | 12/11 | Entamoeba bovis-like (91.7%), Trichuris spp. (8.3%) | |
| Muntiacus muntjack Zimmermann 1780 | 47/18 | Entamoeba bovis-like (36.2%), Entamoeba spp. (8-nucleated) (2.1%), Giardia spp. (2.1%), Trichuris spp. (4.3%) | ||
| Suidae | Sus scrofa | 39/22 | Entamoeba polecki-like (46.2%), Chilomastix spp. (7.7%), Balantioides coli (28.2%) | |
| Tayassuidae | Dicotyles tajacu | 16/1 | Balantioides coli (6.3%) | |
| Carnivora | Ailuridae | Ailurus fulgens | 36/0 | |
| Canidae | Vulpes zerda (Zimmermann 1780) | 52/2 | Trichuris spp. (1.9%), unidentified ascarid (1.9%) | |
| Felidae | Leopardus pardalis (Linnaeus 1758) | 48/0 | ||
| Herpestidae | Helogale parvula (Sundevall 1847) | 32/0 | ||
| Suricata suricatta | 15/0 | |||
| Mephitidae | Mephitis mephitis (Schreber 1776) | 50/3 | Baylisascaris spp. (6.0%) | |
| Mustelidae | Mustela lutreola | 19/0 | ||
| Mustela putorius furo Linnaeus 1758 | 5/0 | |||
| Procyonidae | Nasua nasua | 45/0 | ||
| Potos flavus (Schreber 1774) | 49/0 | |||
| Procyon lotor | 30/0 | |||
| Viverridae | Arctictis binturong | 25/0 | ||
| Genetta genetta (Linnaeus 1758) | 45/0 | |||
| Chiroptera | Phyllostomidae | Carollina perspicillata (Linnaeus 1758) | 13/0 | |
| Pteropodidae | Rousettus aegyptiacus (Saint-Hilaire 1810) | 26/0 | ||
| Cingulata | Chlamyphoridae | Euphractus sexcinctus (Linnaeus 1758) | 16/0 | |
| Chaetophractus villosus (Desmarest 1804) | 16/0 | |||
| Tolypeutes tricinctus (Linnaeus 1758) | 2/0 | |||
| Dasyurimorpha | Dasyuridae | Dasyurus viverrinus (Shaw 1800) | 24/0 | |
| Diprotodontia | Macropodidae | Notamacropus rufogriseus | 46/4 | Entamoeba spp. (one nucleated) (6.5%), Entamoeba spp. (8-nucleated) (2.2%) |
| Osphranter rufus (Desmarest 1822) | 42/0 | |||
| Eulipotyphla | Erinaceidae | Atelerix albiventris (Wagner 1841) | 35/0 | |
| Lagomorpha | Leporidae | Oryctolagus cuniculus | 1/0 | |
| Perissodactyla | Equidae | Equus africanus (Heuglin and Fitzinger 1866) | 27/9 | Endosymbiotic ciliates (33.3%) |
| Equus caballus | 35/8 | Endosymbiotic ciliates (22.9%) | ||
| Pilosa | Choloepodidae | Choloepus didactilus (Linnaeus 1758) | 46/13 | Entamoeba spp. (8-nucleated) (28.3%) |
| Myrmecophagidae | Tamandua tetradactyla (Linnaeus 1758) | 36/0 | ||
| Primates | Aotidae | Aotus nancymaae Hershkovitz 1983 | 7/1 | Entamoeba coli-like (14.3%) |
| Aotus trivirgatus (Humboldt 1812) | 9/0 | |||
| Callitrichidae | Callimico goeldii (Thomas 1904) | 47/0 | ||
| Callithrix jacchus (Linnaeus 1758) | 38/0 | |||
| Cebuella pygmaea (Spix 1823) | 11/0 | |||
| Leontopithecus rosalia (Linnaeus 1766) | 31/0 | |||
| Saguinus geoffroyi (Pucheran 1845) | 30/0 | |||
| Saguinus imperator (Goeldi 1907) | 34/0 | |||
| Saguinus oedipus (Linnaeus 1758) | 26/0 | |||
| Cebidae | Sapajus apella | 30/0 | ||
| Saimiri sciureus (Linnaeus 1758) | 53/0 | |||
| Galagidae | Galago moholi Smith 1836 | 19/0 | ||
| Lemuridae | Eulemur albifrons (Geoffroy 1796) | 17/0 | ||
| Lemur catta | 26/0 | |||
| Varecia variegata | 3/0 | |||
| Varecia rubra (Geoffroy 1812) | 12/1 | Capillaria spp. (8.3%) | ||
| Lorisidae | Xanthonycticebus pygmaeus (Bonhote 1907) | 5/0 | ||
| Perodicticus potto (Müller 1766) | 26/0 | |||
| Pitheciidae | Pithecia pithecia (Linnaeus 1766) | 33/2 | Entamoeba coli-like (6.1%) | |
| Rodentia | Heterocephalidae | Heterocephalus glaber Rüppell 1842 | 29/0 | |
| Caviidae | Cavia porcellus | 54/2 | Balantioides coli (3.7%) | |
| Dolichotis patagonum | 24/7 | Giardia spp. (8.3%), Trichuris spp. (20.8%) | ||
| Hydrochoerus hydrochaeris | 1/0 | |||
| Dasyproctidae | Dasyprocta azarae Lichtenstein 1823 | 1/0 | ||
| Dasyprocta fuliginosa Wagler 1832 | 23/3 | Trichuris spp. (13.0%) | ||
| Dasyprocta punctata Gray 1842 | 8/0 | |||
| Dipodidae | Jaculus orientalis Erxleben 1777 | 12/3 | Entamoeba muris (25.0%), Chilomastix spp. (16.7%) | |
| Echimyidae | Capromys pilorides (Say 1822) | 94/15 | Trichuris spp. (16.0%) | |
| Erethizontidae | Coendou prehensilis (Linnaeus 1758) | 43/5 | Chilomastix spp. (9.3%), Trichuris spp. (2.3%) | |
| Hystricidae | Hystrix cristata Linnaeus 1758 | 47/5 | Entamoeba spp. (8-nucleated) (2.1%), Giardia spp. (8.5%) | |
| Pedetidae | Pedetes capensis (Forster 1778) | 31/0 | ||
| Sciuridae | Cynomys ludovicianus (Ord 1815) | 4/1 | Chilomastix spp. (25.0%) | |
| Tubulidentata | Orycteropodidae | Orycteropus afer (Pallas 1766) | 7/1 | Giardia spp. (14.3%) |
| Samples | ||||
|---|---|---|---|---|
| Order | Family | Species | (Total/Positives) | Parasites Found (% of Total Samples) |
| Accipitriformes | Accipitridae | Necrosyrtes monachus | 8/0 | |
| Aquila nipalensis Hodgson 1833 | 5/0 | |||
| Buteo jamaicensis (Gmelin 1788) | 6/0 | |||
| Buteo regalis (Gray 1844) | 5/0 | |||
| Geranoaetus melanoleucus | 13/0 | |||
| Gyps fulvus | 7/0 | |||
| Parabuteo unicinctus | 14/0 | |||
| Anseriformes | Anatidae | Cygnus atratus | 5/0 | |
| Anhimidae | Chauna torquata (Oken 1816) | 11/0 | ||
| Charadriiformes | Recurvirostridae | Recurvirostra avosetta Linnaeus 1758 | 1/0 | |
| Falconiformes | Falconidae | Phalcoboenus australis (Gmelin 1788) | 2/0 | |
| Galliformes | Numididae | Numida meleagris | 7/3 | Entamoeba gallinarum (14.3%), capillariids (14.3%), Ascaridia spp./Heterakis spp. (28.6%), Raillietina-like eggs (14.3%) |
| Phasianidae | Gallus gallus | 14/1 | Ascaridia spp./Heterakis spp. (7.1%) | |
| Meleagris gallopavo Linnaeus 1758 | 39/0 | |||
| Gruiformes | Gruidae | Grus grus (Linnaeus 1758) | 6/1 | Capillariids (16.7%) |
| Grus virgo (Linnaeus 1758) | 8/0 | |||
| Musophagiformes | Musophagidae | Menelikornis leucotis | 11/0 | |
| Passeriformes | Corvidae | Calocitta formosa (Swainson 1827) | 3/0 | |
| Cotingidae | Rupicola peruvianus (Latham 1790) | 22/0 | ||
| Sturnidae | Lamprotornis purpureus (Müller 1776) | 2/0 | ||
| Pelecaniformes | Ardeidae | Bubulcus ibis (Linnaeus 1758) | 1/0 | |
| Pelecanidae | Pelecanus onocrotalus Linnaeus 1758 | 2/0 | ||
| Phoenicopteriformes | Phoenicopteridae | Phoenicopterus ruber | 5/0 | |
| Piciformes | Ramphastidae | Ramphastos swainsonii Gould, 1833 | 21/17 | Capillaria spp. (81.0%) |
| Ramphastos toco | 16/7 | Capillaria spp. (43.8%) | ||
| Psittaciformes | Cacatuidae | Eolophus roseicapilla (Vieillot 1817) | 5/0 | |
| Psittacidae | Amazona aestiva | 27/0 | ||
| Ara ararauna | 17/0 | |||
| Ara chloropterus | 6/0 | |||
| Ara macao (Linnaeus 1758) | 3/0 | |||
| Ara militaris (Linnaeus 1766) | 1/0 | |||
| Ara rubrogenys | 2/0 | |||
| Aratinga solstitialis | 9/0 | |||
| Psittaculidae | Eclectus rotarus | 8/0 | ||
| Trichoglossus haematodus (Linnaeus 1771) | 3/0 | |||
| Strigiformes | Strigidae | Bubo bubo hispanus | 9/0 | |
| Bubo bubo sibiricus | 6/0 | |||
| Tytonidae | Tyto alba Scopoli 1769 | 11/0 | ||
| Casuariiformes | Casuariidae | Dromaius novaehollandiae | 48/0 | |
| Rheiformes | Rheidae | Rhea americana | 23/0 |
| Zoological Park | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZooAquarium | Faunia | ||||||||||||||
| Feeding Habits | Feeding Habits | ||||||||||||||
| Host Class | Isolation | Soil | Carnivorous | Omnivorous | Herbivorous | Carnivorous | Omnivorous | Herbivorous | |||||||
| Mammal | accessible | Natural | 2 | (10) | 8 | (13) | 48 | (53) | 0 | (1) | 2 | (3) | 12 | (19) | |
| Artificial | 0 | (0) | 0 | (0) | 1 | (1) | 0 | (0) | 0 | (0) | 0 | (0) | |||
| Mixed | 1 | (1) | 1 | (2) | 0 | (1) | 0 | (0) | 0 | (0) | 0 | (0) | |||
| isolated | Natural | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | ||
| Artificial | 0 | (0) | 1 | (2) | 0 | (0) | 1 | (14) | 5 | (20) | 5 | (10) | |||
| Mixed | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (1) | 0 | (0) | 0 | (0) | |||
| Bird | accesible | Natural | 0 | (8) | 3 | (12) | 0 | (2) | 0 | (3) | 3 | (10) | 0 | (3) | |
| Artificial | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (1) | |||
| Mixed | 4 | (24) | 0 | (3) | 0 | (15) | 0 | (11) | 2 | (2) | 0 | (10) | |||
| isolated | Natural | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | ||
| Artificial | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | |||
| Mixed | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | |||
| Function Coefficients | Wald’s X2 Test | ||||||
|---|---|---|---|---|---|---|---|
| Standard Error | Degrees of Freedom | ||||||
| Parameter | B | Score | Significance | ||||
| Feeding type | 14.733 | 2 | <0.001 | ||||
| Omnivorous vs. carnivorous | 1.581 | 0.532 | 8.824 | 1 | 0.003 | ||
| Herbivorous vs. carnivorous | 1.911 | 0.501 | 14.555 | 1 | <0.001 | ||
| Soil type | 2.401 | 2 | 0.301 | ||||
| artificial vs. natural | 0.063 | 1.710 | 0.001 | 1 | 0.971 | ||
| mixed vs. natural | −0.878 | 0.572 | 2.352 | 1 | 0.125 | ||
| Host Class (bird vs. mammal) | −2.103 | 0.480 | 19.150 | 1 | <0.001 | ||
| Zoological institution (Faunia vs. ZooAquarium) | −0.649 | 0.405 | 2.567 | 1 | 0.109 | ||
| Isolation type (isolated vs. accessible) | −1.326 | 1.777 | 0.577 | 1 | 0.456 | ||
| Constant | −1.639 | 0.389 | 17.703 | 1 | <0.001 | ||
| Function Coefficients | Wald’s X2 Test | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | B | Standard Error | Score | Degrees of Freedom | Significance | ||
| Feeding type | 21.061 | 2 | <0.001 | ||||
| Omnivorous vs. carnivorous | 2.057 | 0.780 | 7.571 | 1 | 0.006 | ||
| Herbivorous vs. carnivorous | 3.187 | 0.716 | 19.799 | 1 | <0.001 | ||
| Soil type | 2.453 | 2 | 0.293 | ||||
| artificial vs. natural | 18.336 | 25,170.708 | 0.000 | 1 | 0.999 | ||
| mixed vs. natural | −2.016 | 1.287 | 2.453 | 1 | 0.117 | ||
| Zoological institution (Faunia vs. ZooAquarium) | −1.245 | 0.531 | 5.496 | 1 | 0.019 | ||
| Isolation type (isolated vs. accessible) | −18.995 | 25,170.708 | 0.000 | 1 | 0.999 | ||
| Constant | −4.146 | 4195.118 | 0.000 | 1 | 0.999 | ||
| Function Coefficients | Wald’s X2 Test | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | B | Standard Error | Score | Degrees of Freedom | Significance | ||
| Feeding type | 5.613 | 2 | 0.060 | ||||
| Omnivorous vs. carnivorous | 2.107 | 0.889 | 5.613 | 1 | 0.018 | ||
| Herbivorous vs. carnivorous | −18.913 | 7283.654 | 0.000 | 1 | 0.998 | ||
| Soil type | 1.403 | 2 | 0.496 | ||||
| artificial vs. natural | 0.833 | 40,847.685 | 0.000 | 1 | 1.000 | ||
| mixed vs. natural | 1.030 | 0.870 | 1.403 | 1 | 0.236 | ||
| Zoological institution (Faunia vs. ZooAquarium) | 0.121 | 0.684 | 0.031 | 1 | 0.860 | ||
| Constant | −8.164 | 13,397.685 | 0.000 | 1 | 1.000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteban-Sánchez, L.; García-Rodríguez, J.J.; García-García, J.; Martínez-Nevado, E.; de la Riva-Fraga, M.A.; Ponce-Gordo, F. Wild Animals in Captivity: An Analysis of Parasite Biodiversity and Transmission among Animals at Two Zoological Institutions with Different Typologies. Animals 2024, 14, 813. https://doi.org/10.3390/ani14050813
Esteban-Sánchez L, García-Rodríguez JJ, García-García J, Martínez-Nevado E, de la Riva-Fraga MA, Ponce-Gordo F. Wild Animals in Captivity: An Analysis of Parasite Biodiversity and Transmission among Animals at Two Zoological Institutions with Different Typologies. Animals. 2024; 14(5):813. https://doi.org/10.3390/ani14050813
Chicago/Turabian StyleEsteban-Sánchez, Lorena, Juan José García-Rodríguez, Juncal García-García, Eva Martínez-Nevado, Manuel Antonio de la Riva-Fraga, and Francisco Ponce-Gordo. 2024. "Wild Animals in Captivity: An Analysis of Parasite Biodiversity and Transmission among Animals at Two Zoological Institutions with Different Typologies" Animals 14, no. 5: 813. https://doi.org/10.3390/ani14050813
APA StyleEsteban-Sánchez, L., García-Rodríguez, J. J., García-García, J., Martínez-Nevado, E., de la Riva-Fraga, M. A., & Ponce-Gordo, F. (2024). Wild Animals in Captivity: An Analysis of Parasite Biodiversity and Transmission among Animals at Two Zoological Institutions with Different Typologies. Animals, 14(5), 813. https://doi.org/10.3390/ani14050813








