The Consumption and Diversity Variation Responses of Agricultural Pests and Their Dietary Niche Differentiation in Insectivorous Bats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Insect Survey
2.2. Passive Acoustic Monitoring of Bats
2.3. Field Investigations and Dietary Sampling of Bats
2.4. DNA Extraction, PCR Amplification, and Sequencing
2.5. Sequence Analysis and Taxonomic Identification
2.6. Sympatric Bat Dietary Analysis and Niche Differentiation Exploration
2.7. Statistical Analyses
3. Results
3.1. Insect Abundance and Bat Activity
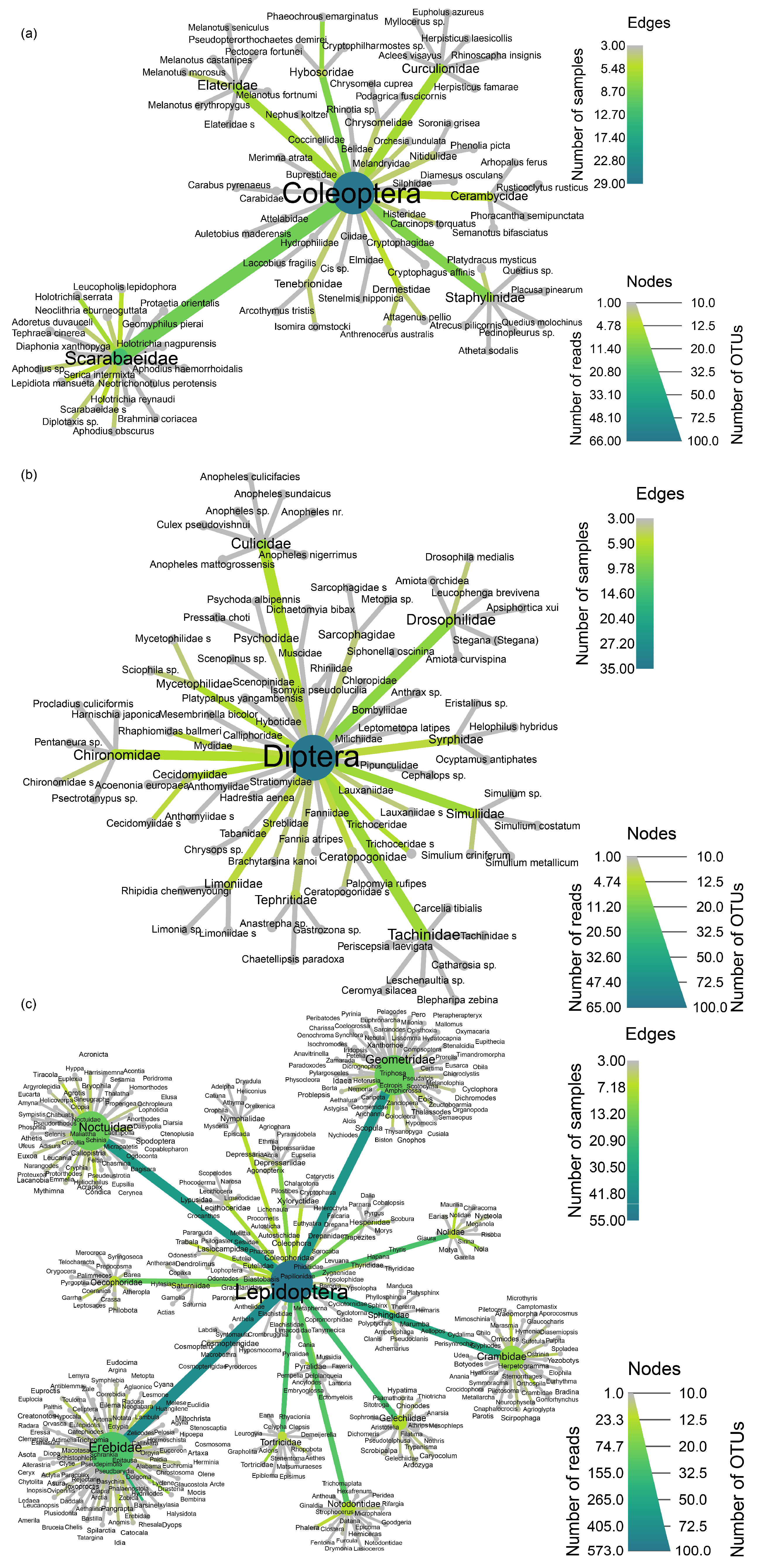
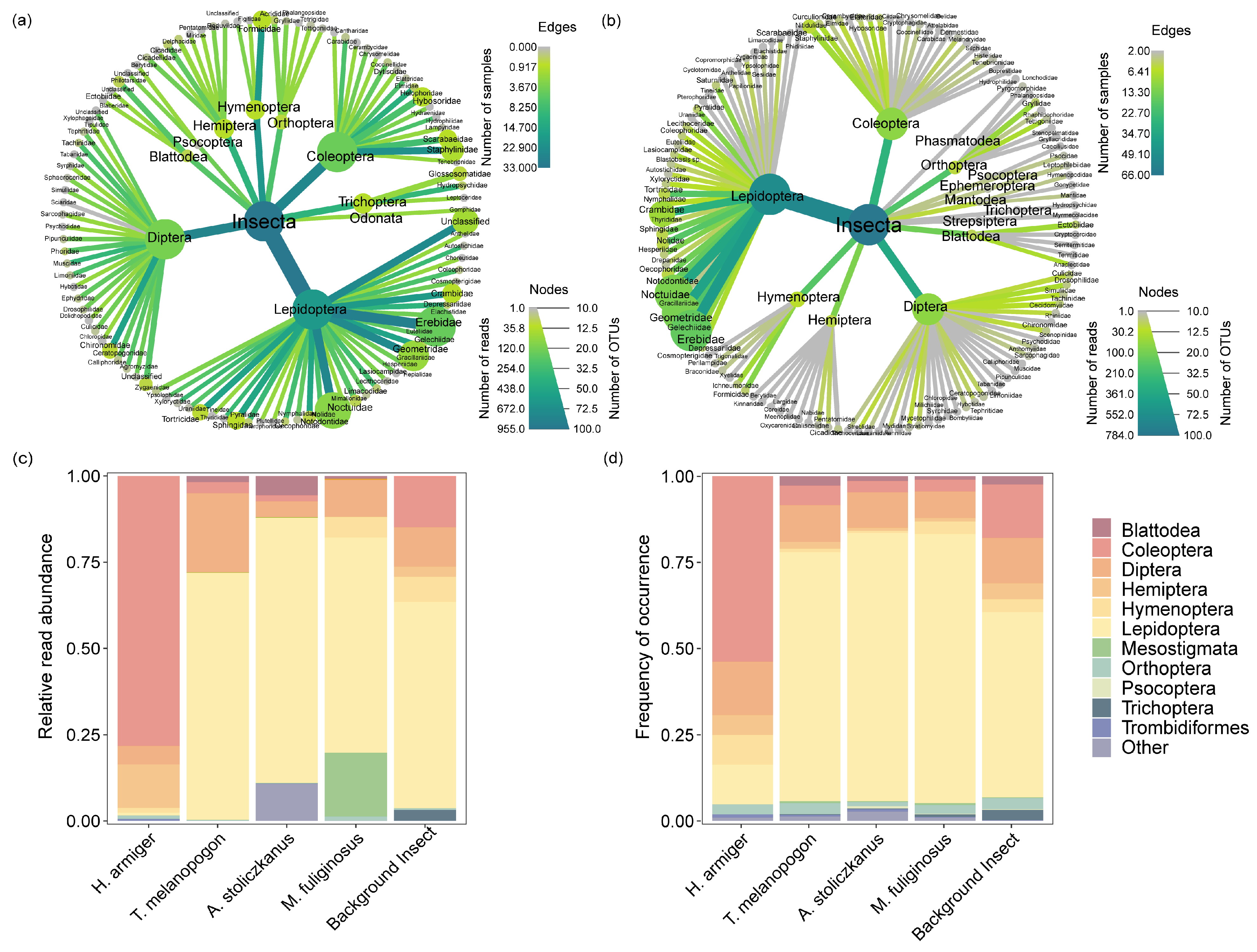
3.2. Prey Composition and Pest Diversity in the Diet of Bats
3.3. Factors Influencing Bat Activity
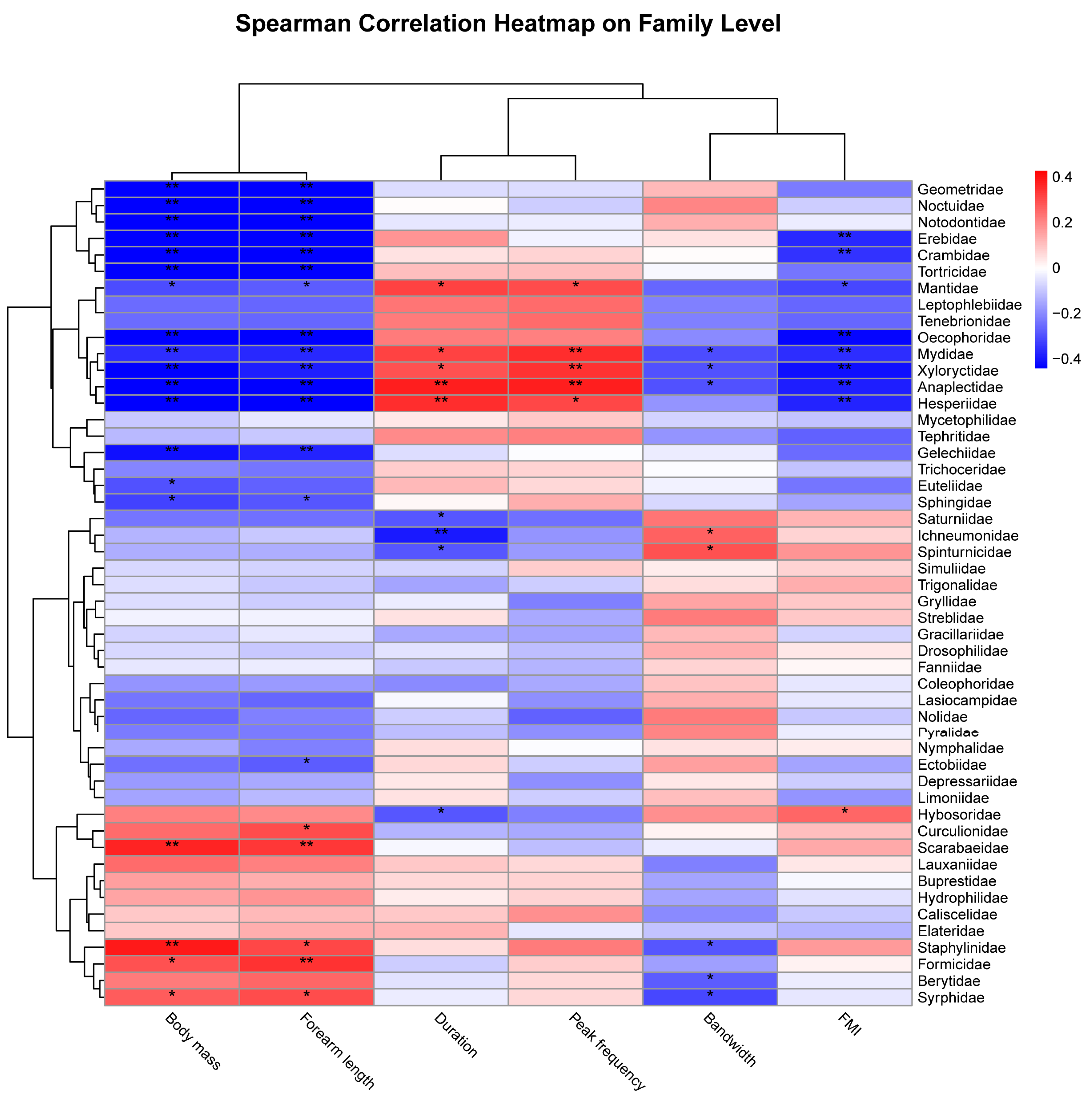
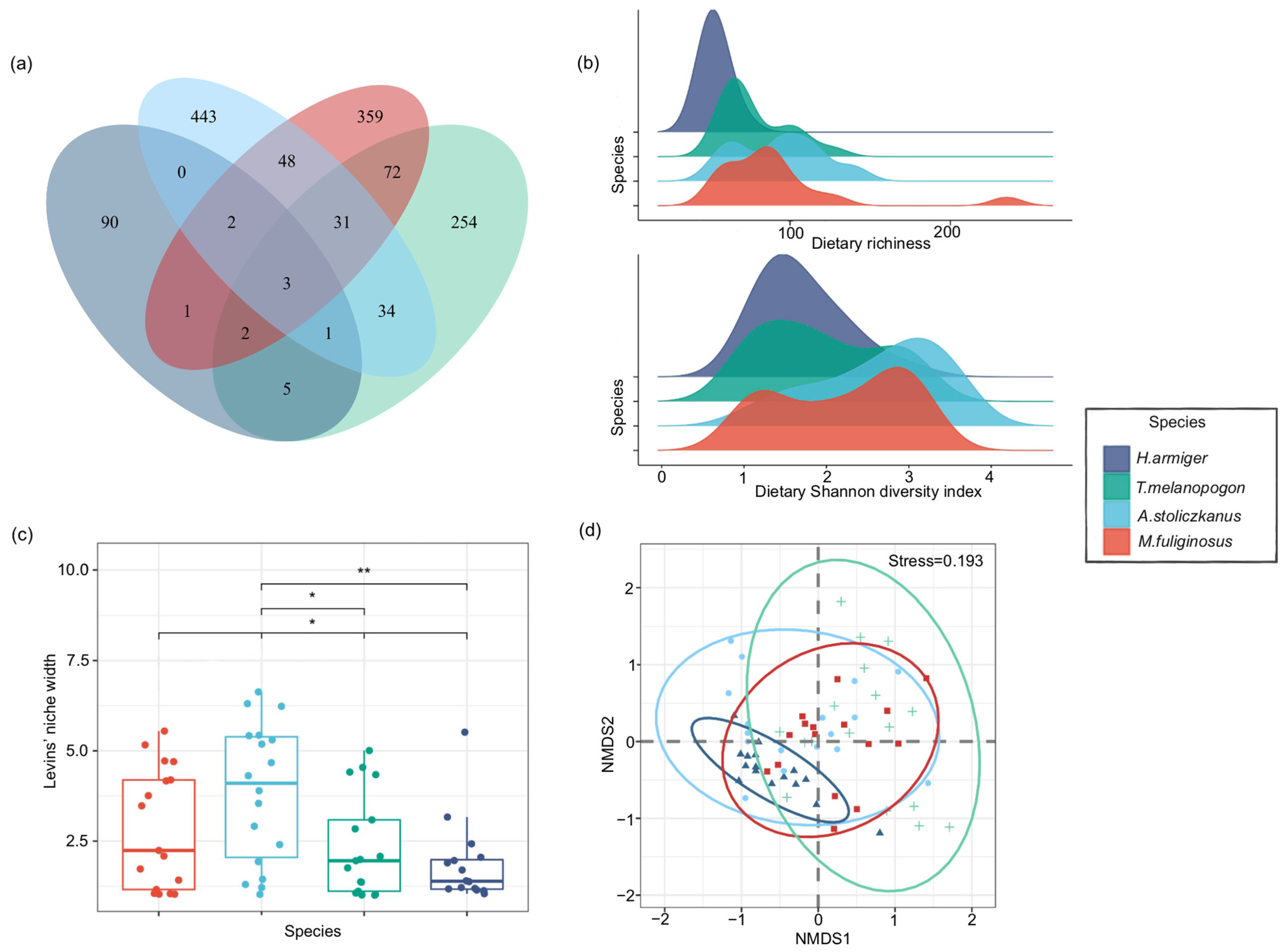
3.4. Dietary Diversity, Overlap, and Niche Width of Bats
3.5. Factors Influencing Prey Consumption by Bats
4. Discussion
4.1. Dietary Composition and Pest Diversity
4.2. The Relationships between Insect Resources and Bat Activity
4.3. Effects of Species Traits on Dietary Niche among Bat Species
4.4. Implications for Pest Control Services and Bat Conservation
- (1)
- Roost conservation
- (2)
- Landscape conservation
- (3)
- Legislation
- (4)
- Pesticide management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B

| Comparison | Sum of Squares | Pseudo-F | R2 | p | Padj |
|---|---|---|---|---|---|
| H. armiger vs. T. melanopogon | 1.408 | 3.421 | 0.099 | 0.001 | 0.006 |
| H. armiger vs. A. stoliczkanus | 0.15 | 2.050 | 0.06 | 0.014 | 0.084 |
| H. armiger vs. M. fuliginosus | 0.729 | 1.816 | 0.055 | 0.023 | 0.138 |
| T. melanopogon vs. A. stoliczkanus | 0.721 | 1.612 | 0.047 | 0.022 | 0.132 |
| T. melanopogon vs. M. fuliginosus | 0.689 | 1.558 | 0.046 | 0.049 | 0.294 |
| A. stoliczkanus vs. M. fuliginosus | 0.465 | 1.062 | 0.031 | 0.327 | 1 |
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Culliney, T.W. Crop Losses to Arthropods. In Integrated Pest Management: Pesticide Problems; Pimentel, D., Peshin, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 3, pp. 201–225. [Google Scholar]
- Hughes, M.J.; Braun de Torrez, E.C.; Ober, H.K. Big bats binge bad bugs: Variation in crop pest consumption by common bat species. Agric. Ecosyst. Environ. 2021, 314, 107414. [Google Scholar] [CrossRef]
- Tabashnik, B.E. Communal benefits of transgenic corn. Science 2010, 330, 189–190. [Google Scholar] [CrossRef]
- Pimentel, D. Pesticides and pest control. In Integrated Pest Management: Innovation-Development Process; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 1, pp. 83–87. [Google Scholar]
- Jia, Y.; Kang, L.; Wu, Y.; Zhou, C.; Li, D.; Li, J.; Pan, C. Review on Pesticide Abiotic Stress over Crop Health and Intervention by Various Biostimulants. J. Agric. Food Chem. 2023, 71, 13595–13611. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; et al. Exposure routes and health risks associated with pesticide application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Moya, N.; Diaz-Siefer, P.; Pozo, R.A.; Fonturbel, F.E.; Lavandero, B.; Celis-Diez, J.L.; Abades, S. The use of cavity-nesting wild birds as agents of biological control in vineyards of Central Chile. Agric. Ecosyst. Environ. 2022, 334, 107975. [Google Scholar] [CrossRef]
- Day, R.; Haggblade, S.; Moephuli, S.; Mwang’ombe, A.; Nouala, S. Institutional and policy bottlenecks to IPM. Curr. Opin. Insect. Sci. 2022, 52, 100946. [Google Scholar] [CrossRef] [PubMed]
- Prischmann, D.A.; James, D.G.; Wright, L.C.; Snyder, W.E. Effects of generalist phytoseiid mites and grapevine canopy structure on spider mite (Acari: Tetranychidae) biocontrol. Environ. Entomol. 2006, 35, 56–67. [Google Scholar] [CrossRef]
- Bhalla, I.S.; Aguirre-Gutiérrez, J.; Whittaker, R.J. Batting for rice: The effect of bat exclusion on rice in North-East India. Agric. Ecosyst. Environ. 2023, 341, 108196. [Google Scholar] [CrossRef]
- Baroja, U.; Garin, I.; Vallejo, N.; Aihartza, J.; Rebelo, H.; Goiti, U. Bats actively track and prey on grape pest populations. Ecol. Indic. 2021, 126, 107718. [Google Scholar] [CrossRef]
- Puig-Montserrat, X.; Torre, I.; López-Baucells, A.; Guerrieri, E.; Monti, M.M.; Ràfols-García, R.; Ferrer, X.; Gisbert, D.; Flaquer, C. Pest control service provided by bats in Mediterranean rice paddies: Linking agroecosystems structure to ecological functions. Mamm. Biol. 2015, 80, 237–245. [Google Scholar] [CrossRef]
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef]
- Ehler, L.E. Invasion biology and biological control. Biol. Control 1998, 13, 127–133. [Google Scholar] [CrossRef]
- Roubinet, E.; Birkhofer, K.; Malsher, G.; Staudacher, K.; Ekbom, B.; Traugott, M.; Jonsson, M. Diet of generalist predators reflects effects of cropping period and farming system on extra- and intraguild prey. Ecol. Appl. 2017, 27, 1167–1177. [Google Scholar] [CrossRef]
- Simmons, N.B.; Cirranello, A.L. Bat Species of the World: A Taxonomic and Geographic Database. 2023. Available online: https://batnames.org (accessed on 15 January 2024).
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef]
- Kunz, T.H.; Stern, A.A. Maternal investment and post-natal growth in bats. Symp. Zool. Soc. Lond. 1995, 57, 123–138. [Google Scholar]
- Linden, V.M.G.; Grass, I.; Joubert, E.; Tscharntke, T.; Weier, S.M.; Taylor, P.J. Ecosystem services and disservices by birds, bats and monkeys change with macadamia landscape heterogeneity. J. Appl. Ecol. 2019, 56, 2069–2078. [Google Scholar] [CrossRef]
- Maslo, B.; Mau, R.L.; Kerwin, K.; McDonough, R.; McHale, E.; Foster, J.T. Bats provide a critical ecosystem service by consuming a large diversity of agricultural pest insects. Agric. Ecosyst. Environ. 2022, 324, 107722. [Google Scholar] [CrossRef]
- Cleveland, C.J.; Betke, M.; Federico, P.; Frank, J.D.; Hallam, T.G.; Horn, J.; López Jr, J.D.; McCracken, G.F.; Medellín, R.A.; Moreno-Valdez, A.; et al. Economic value of the pest control service provided by Brazilian free-tailed bats in south-central Texas. Front. Ecol. Environ. 2006, 4, 238–243. [Google Scholar] [CrossRef]
- Taylor, P.J.; Grass, I.; Alberts, A.J.; Joubert, E.; Tscharntke, T. Economic value of bat predation services—A review and new estimates from macadamia orchards. Ecosyst. Serv. 2018, 30, 372–381. [Google Scholar] [CrossRef]
- Charbonnier, Y.; Barbaro, L.; Theillout, A.; Jactel, H. Numerical and functional responses of forest bats to a major insect pest in pine plantations. PLoS ONE 2014, 9, e109488. [Google Scholar] [CrossRef] [PubMed]
- Kolkert, H.; Smith, R.; Rader, R.; Reid, N. Insectivorous bats foraging in cotton crop interiors is driven by moon illumination and insect abundance, but diversity benefits from woody vegetation cover. Agric. Ecosyst. Environ. 2020, 302, 107068. [Google Scholar] [CrossRef]
- Ramírez-Fráncel, L.A.; García-Herrera, L.V.; Losada-Prado, S.; Reinoso-Flórez, G.; Sánchez-Hernández, A.; Estrada-Villegas, S.; Lim, B.K.; Guevara, G. Bats and their vital ecosystem services: A global review. Integr. Zool. 2022, 17, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Gu, H.; Chang, Y.; Wang, Z.; Shi, B.; Lin, A.; Wu, H.; Feng, J.; Jiang, T. Seasonal variation of population and individual dietary niche in the avivorous bat. Ia Io. Oecologia 2023, 201, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Si, M.; Huang, Z.L.Y.; Feng, J.; Jiang, T.L. Bats are sentinels for invasive pest surveillance based on DNA metabarcoding. Ecol. Indic. 2023, 152, 110354. [Google Scholar] [CrossRef]
- Ma, J.; Liang, B.; Zhang, S.Y.; Metzner, W. Dietary composition and echolocation call design of three sympatric insectivorous bat species from China. Ecol. Res. 2008, 23, 113–119. [Google Scholar] [CrossRef]
- Blanch, E.; López-Baucells, A.; Mata, V.A.; Flaquer, C.; López-Bosch, D. To share or not to share: DNA metabarcoding reveals trophic niche overlap between sympatric trawling bats. Eur. J. Wildl. Res. 2023, 69, 90. [Google Scholar] [CrossRef]
- Pianka, E.R. The Structure of Lizard Communities. Annu. Rev. Ecol. Evol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Sedlock, J.L.; Krüger, F.; Clare, E.L. Island bat diets: Does it matter more who you are or where you live? Mol. Ecol. 2014, 23, 3684–3694. [Google Scholar] [CrossRef]
- McCracken, G.; Brown, V.; Eldridge, M.; Federico, P.; Kunz, T. Bats track and exploit changes in insect pest populations. PLoS ONE 2012, 7, e43839. [Google Scholar] [CrossRef]
- Arrizabalaga-Escudero, A.; Clare, E.L.; Salsamendi, E.; Alberdi, A.; Garin, I.; Aihartza, J.; Goiti, U. Assessing niche partitioning of co-occurring sibling bat species by DNA metabarcoding. Mol. Ecol. 2018, 27, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Pavey, C.R. Comparative echolocation and foraging ecology of horseshoe bats (Rhinolophidae) and old world leaf-nosed bats (Hipposideridae). Aust. J. Zool. 2020, 68, 382–392. [Google Scholar] [CrossRef]
- Pompanon, F.; Deagle, B.E.; Symondson, W.O.C.; Brown, D.S.; Jarman, S.N.; Taberlet, P. Who is eating what: Diet assessment using next generation sequencing. Mol. Ecol. 2012, 21, 1931–1950. [Google Scholar] [CrossRef]
- Pansu, J.; Hutchinson, M.C.; Anderson, T.M.; Te Beest, M.; Begg, C.M.; Begg, K.S.; Bonin, A.; Chama, L.; Chamaillé-Jammes, S.; Coissac, E.; et al. The generality of cryptic dietary niche differences in diverse large-herbivore assemblages. Proc. Natl. Acad. Sci. USA 2022, 119, e2204400119. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, A.; Razgour, O.; Aizpurua, O.; Novella-Fernandez, R.; Aihartza, J.; Budinski, I.; Garin, I.; Ibáñez, C.; Izagirre, E.; Rebelo, H.; et al. DNA metabarcoding and spatial modelling link diet diversification with distribution homogeneity in European bats. Nat. Commun. 2020, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Galan, M.; Pons, J.-B.; Tournayre, O.; Pierre, É.; Leuchtmann, M.; Pontier, D.; Charbonnel, N. Metabarcoding for the parallel identification of several hundred predators and their prey: Application to bat species diet analysis. Mol. Ecol. Resour. 2018, 18, 474–489. [Google Scholar] [CrossRef]
- Hill, A.P.; Prince, P.; Snaddon, J.L.; Doncaster, C.P.; Rogers, A. AudioMoth: A low-cost acoustic device for monitoring biodiversity and the environment. Hardwarex 2019, 6, e00073. [Google Scholar] [CrossRef]
- Hill, A.P.; Prince, P.; Piña Covarrubias, E.; Doncaster, C.P.; Snaddon, J.L.; Rogers, A. AudioMoth: Evaluation of a smart open acoustic device for monitoring biodiversity and the environment. Methods Ecol. Evol. 2018, 9, 1199–1211. [Google Scholar] [CrossRef]
- Wickramasinghe, L.P.; Harris, S.; Jones, G.; Vaughan, N. Bat activity and species richness on organic and conventional farms: Impact of agricultural intensification. J. Appl. Ecol. 2003, 40, 984–993. [Google Scholar] [CrossRef]
- Kerbiriou, C.; Bas, Y.; Le Viol, I.; Lorrilliere, R.; Mougnot, J.; Julien, J.-F. Potential of bat pass duration measures for studies of bat activity. Bioacoustics 2018, 28, 177–192. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, Y.; Huang, Z.; Feng, J.; Jiang, T. Pest suppression services and dietary niche differentiation of bats in Chinese smallholder farming systems: Implications for integrated pest management. J. Pest. Sci. 2023. [Google Scholar] [CrossRef]
- Meng, F.X.; Zhu, L.; Huang, W.J.; Irwin, D.M.; Zhang, S.Y. Bats: Body mass index, forearm mass index, blood glucose levels and SLC2A2 genes for diabetes. Sci. Rep. 2016, 6, 29960. [Google Scholar] [CrossRef]
- Hui, W.; Jiang, T.L.; Liu, S.; Lu, G.J.; Feng, J. Acoustic identification of two morphologically similar bat species, Miniopterus magnater and Miniopterus fuliginosus (Chiroptera, Miniopteridae). Mammalia 2020, 84, 201–206. [Google Scholar]
- Li, Y.Y.; Wang, J.; Metzner, W.; Luo, B.; Jiang, T.L.; Yang, S.L.; Shi, L.M.; Huang, X.B.; Yue, X.K.; Feng, J. Behavioral responses to echolocation calls from sympatric heterospecific bats: Implications for interspecific competition. Behav. Ecol. Sociobiol. 2014, 68, 657–667. [Google Scholar] [CrossRef]
- Sun, C.N.; Zhang, C.M.; Lucas, J.R.; Lin, A.Q.; Feng, J.; Jiang, T.L. Territorial calls of the bat (Hipposideros armiger) may encode multiple types of information: Body mass, dominance rank and individual identity. Anim. Cogn. 2021, 24, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Song, S.; Li, A.; Zhang, Y.; Li, Z.; Xiao, Y.; Jiang, T.; Feng, J.; Lin, A. The roles of morphological traits, resource variation and resource partitioning associated with the dietary niche expansion in the fish-eating bat Myotis pilosus. Mol. Ecol. 2019, 28, 2944–2954. [Google Scholar] [CrossRef] [PubMed]
- Zeale, M.R.K.; Butlin, R.K.; Barker, G.L.A.; Lees, D.C.; Jones, G. Taxon-specific PCR for DNA barcoding arthropod prey in bat faeces. Mol. Ecol. Resour. 2011, 11, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. Taxonomic note: A Place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Microbiol. Soc. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Alberdi, A.; Aizpurua, O.; Gilbert, M.T.P.; Bohmann, K. Scrutinizing key steps for reliable metabarcoding of environmental samples. Methods Ecol. Evol. 2018, 9, 134–147. [Google Scholar] [CrossRef]
- Foster, Z.S.L.; Sharpton, T.J.; Grünwald, N.J. Metacoder: An R package for visualization and manipulation of community taxonomic diversity data. PLoS Comput. Biol. 2017, 13, e1005404. [Google Scholar] [CrossRef]
- Chen, H. VennDiagram: Generate High-Resolution Venn and Euler Plots. R package Version, 1.7.3. 2022. Available online: https://CRAN.R-project.org/package=VennDiagram (accessed on 15 January 2024).
- Gong, Z.; Ma, G.; Wen, H.; Peng, Z. List of alien invasive insects in tropical regions of China. Chin. J. Appl. Entomol. 2021, 58, 27–48. [Google Scholar]
- Ma, Z.; Jiang, C.; Qin, M.; Li, H.; Feng, X.; Zhang, R. Distribution and spread of national quarantine insects of agricultural plants in China. Chinese J. Appl. Entomol. 2018, 55, 1–11. [Google Scholar]
- Peng, P.Y.; Guo, X.G.; Ren, T.G.; Dong, W.G.; Song, W.Y. An updated distribution and hosts: Trombiculid mites (Acari: Trombidiformes) associated with small mammals in Yunnan Province, southwest China. Parasitol. Res. 2016, 115, 1923–1938. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Simpson, G.L. Vegan: Community Ecology Package. R Package Version, 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 January 2024).
- Wallace Jr, R.K. An assessment of diet-overlap indexes. Trans. Am. Fish. Soc. 1981, 110, 72–76. [Google Scholar] [CrossRef]
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations; Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Nakazawa, M. Fmsb: Functions for Medical Statistics Book with Some Demographic Data. R package Version, 0.7.6. 2023. Available online: https://CRAN.R-project.org/package=fmsb (accessed on 15 January 2024).
- Bartoń, K. MuMIn: Multi-Model Inference. 2023. R package Version 1.47.5. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 15 January 2024).
- Hartig, F. Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R package Version 0.4.6. 2022. Available online: http://florianhartig.github.io/DHARMa/ (accessed on 15 January 2024).
- Arbizu, P.M. pairwiseAdonis: Pairwise Multilevel Comparison Using Adonis, R Package Version 0.4. 2020; R Fondation: Vienna, Austria, 2020.
- Novella-Fernandez, R.; Ibañez, C.; Juste, J.; Clare, E.L.; Doncaster, C.P.; Razgour, O. Trophic resource partitioning drives fine-scale coexistence in cryptic bat species. Ecol. Evol. 2020, 10, 14122–14136. [Google Scholar] [CrossRef] [PubMed]
- Best, D.J.; Roberts, D.E. Algorithm AS 89: The Upper Tail Probabilities of Spearman’s Rho. J. R. Stat. Soc. Ser. C Appl. Stat. 1975, 24, 377–379. [Google Scholar] [CrossRef]
- Kolde, R. pheatmap: Pretty Heatmaps. R package Version 1.0.12. 2019. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 15 January 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Bhattacharyya, B.; Gogoi, I.; Das, P.P.G.; Kalita, B. Chapter 7—Management of agricultural insect pests for sustainable agriculture and environment. In Sustainable Agriculture and the Environment; Farooq, M., Gogoi, N., Pisante, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 161–193. [Google Scholar]
- Gao, L.; Yan, R.; He, W.; Wu, K. Use of food attractant to monitor and forecast population dynamics of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae), a long-distance migratory pest. Agronomy 2023, 13, 2141. [Google Scholar] [CrossRef]
- Xiaoxu, S.; Hu, C.; Jia, H.; Wu, Q.; Shen, X.; Jiang, Y.; Wu, K. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar]
- Andriollo, T.; Michaux, J.R.; Ruedi, M. Food for everyone: Differential feeding habits of cryptic bat species inferred from DNA metabarcoding. Mol. Ecol. 2021, 30, 4584–4600. [Google Scholar] [CrossRef]
- Seruyange, E.; Ljungberg, K.; Muvunyi, C.M.; Gahutu, J.B.; Katare, S.; Nyamusore, J.; Gwon, Y.D.; Evander, M.; Norder, H.; Liljeström, P.; et al. Seroreactivity to chikungunya and West Nile Viruses in Rwandan blood donors. Vector Borne Zoonotic Dis. 2019, 19, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Bosso, L.; Ancillotto, L. Novel perspectives on bat insectivory highlight the value of this ecosystem service in farmland: Research frontiers and management implications. Agric. Ecosyst. Environ. 2018, 266, 31–38. [Google Scholar] [CrossRef]
- Burgiell, P. Bats as a potential biological pest control agent in forest. Sylwan. 2018, 162, 707–717. [Google Scholar]
- Weier, S.M.; Moodley, Y.; Fraser, M.F.; Linden, V.M.G.; Grass, I.; Tscharntke, T.; Taylor, P.J. Insect pest consumption by bats in macadamia orchards established by molecular diet analyses. Glob. Ecol. Conserv. 2019, 18, e00626. [Google Scholar] [CrossRef]
- Jiang, T.; Zhao, H.; He, B.; Zhang, L.; Luo, J.; Liu, Y.; Sun, K.; Yu, W.; Wu, Y.; Feng, J. Research progress of bat biology and conservation strategies in China. Acta Theriologica Sinica 2020, 40, 539–559. [Google Scholar]
- Chaperon, P.N.; Rodríguez-San Pedro, A.; Beltrán, C.A.; Allendes, J.L.; Barahona-Segovia, R.M.; Urra, F.; Grez, A.A. Effects of adjacent habitat on nocturnal flying insects in vineyards and implications for bat foraging. Agric. Ecosyst. Environ. 2022, 326, 107780. [Google Scholar] [CrossRef]
- Rodríguez-San Pedro, A.; Allendes, J.L.; Beltrán, C.A.; Chaperon, P.N.; Saldarriaga-Córdoba, M.M.; Silva, A.X.; Grez, A.A. Quantifying ecological and economic value of pest control services provided by bats in a vineyard landscape of central Chile. Agric. Ecosyst. Environ. 2020, 302, 107063. [Google Scholar] [CrossRef]
- Russo, D.; Ancillotto, L. Sensitivity of bats to urbanization: A review. Mamm. Biol. 2015, 80, 205–212. [Google Scholar] [CrossRef]
- Sedlock, J.L.; Stuart, A.M.; Horgan, F.G.; Hadi, B.; Como Jacobson, A.; Alviola, P.A.; Alvarez, J.D.V. Local-scale bat guild activity differs with rice growth stage at ground level in the Philippines. Diversity 2019, 11, 148. [Google Scholar] [CrossRef]
- Toffoli, R.; Rughetti, M. Effect of water management on bat activity in rice paddies. Paddy Water Environ. 2020, 18, 687–695. [Google Scholar] [CrossRef]
- Bailey, A.M.; McCleery, R.A.; Ober, H.K.; Reichert, B.E. Canopy cover shapes bat diversity across an urban and agricultural landscape mosaic. Environ. Conserv. 2019, 46, 193–200. [Google Scholar] [CrossRef]
- Clavel, J.; Morlon, H. Reliable Phylogenetic Regressions for Multivariate Comparative Data: Illustration with the MANOVA and application to the effect of diet on mandible morphology in Phyllostomid bats. Syst. Biol. 2020, 69, 927–943. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Shaner, P.J.; Chang, C.I.; Ke, L.H.; Kao, S.J. Trophic niche width increases with bill-size variation in a generalist passerine: A test of niche variation hypothesis. J. Anim. Ecol. 2014, 83, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Juri, G.L.; Naretto, S.; Mateos, A.C.; Chiaraviglio, M.; Cardozo, G. Influence of life history traits on trophic niche segregation between two similar sympatric. Tupinambis Lizards. S. Am. J. Herpetol. 2015, 10, 132–142. [Google Scholar] [CrossRef]
- Barclay, R.M.R.; Brigham, R.M.J.T.A.N. Prey detection, dietary niche breadth, and body size in bats: Why are aerial insectivorous bats so small? Am. Nat. 1991, 137, 693–703. [Google Scholar]
- Aldridge, H.D.J.N.; Rautenbach, I.L. Morphology, echolocation and resource partitioning in insectivorous bats. J. Anim. Ecol. 1987, 56, 763–778. [Google Scholar] [CrossRef]
- Waters, D.A.; Rydell, J.; Jones, G. Echolocation call design and limits on prey size: A case study using the aerial-hawking bat Nyctalus leisleri. Behav. Ecol. Sociobiol. 1995, 37, 321–328. [Google Scholar] [CrossRef]
- Peixoto, F.P.; Braga, P.H.P.; Mendes, P. A synthesis of ecological and evolutionary determinants of bat diversity across spatial scales. BMC Ecol. 2018, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Emrich, M.; Clare, E.; Symondson, W.; Koenig, S.; Fenton, M. Resource partitioning by insectivorous bats in Jamaica. Mol. Ecol. 2013, 23, 12504. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Mehr, M.; Bassler, C.; Fenton, M.B.; Hothorn, T.; Pretzsch, H.; Klemmt, H.J.; Brandl, R. Aggregative response in bats: Prey abundance versus habitat. Oecologia 2012, 169, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Freeman, P.W. Correspondence of food habits and morphology in insectivorous bats. J. Mammal. 1981, 62, 166–173. [Google Scholar] [CrossRef]
- Gonsalves, L.; Bicknell, B.; Law, B.; Webb, C.; Monamy, V. Mosquito consumption by insectivorous bats: Does size matter? PLoS ONE 2013, 8, e77183. [Google Scholar] [CrossRef] [PubMed]
- Snyder, W.E.; Ives, A.R. Interactions between specialist and generalist natural enemies: Parasitoids, predators, and pea aphid biocontrol. Ecology 2003, 84, 91–107. [Google Scholar] [CrossRef]
- Kolkert, H.; Smith, R.; Rader, R.; Reid, N. Insectivorous bats provide significant economic value to the Australian cotton industry. Ecosyst. Serv. 2021, 49, 101280. [Google Scholar] [CrossRef]
- Lu, M.; Wang, X.; Ye, H.; Wang, H.; Qiu, S.; Zhang, H.; Liu, Y.; Luo, J.; Feng, J. Does public fear that bats spread COVID-19 jeopardize bat conservation? Biol. Conserv. 2021, 254, 108952. [Google Scholar] [CrossRef]
- MacFarlane, D.; Rocha, R. Guidelines for communicating about bats to prevent persecution in the time of COVID-19. Biol. Conserv. 2020, 248, 108650. [Google Scholar] [CrossRef]
- Sasse, B.; Gramza, A. Influence of the COVID-19 pandemic on public attitudes toward bats in Arkansas and implications for bat management. Hum. Dimens. Wildl. 2021, 26, 90–93. [Google Scholar] [CrossRef]
- Long, R.F.; Kiser, W.M.; Kiser, S.B. Well-placed bat houses can attract bats to Central Valley farms. Calif Agric. 2006, 60, 91–94. [Google Scholar] [CrossRef]
- Tuneu-Corral, C.; Puig-Montserrat, X.; Riba-Bertolín, D.; Russo, D.; Rebelo, H.; Cabeza, M.; López-Baucells, A. Pest suppression by bats and management strategies to favour it: A global review. Biol. Rev. 2023, 98, 1564–1582. [Google Scholar] [CrossRef]
- Rodríguez-San Pedro, A.; Rodríguez-Herbach, C.; Allendes, J.L.; Chaperon, P.N.; Beltrán, C.A.; Grez, A.A. Responses of aerial insectivorous bats to landscape composition and heterogeneity in organic vineyards. Agric. Ecosyst. Environ. 2019, 277, 74–82. [Google Scholar] [CrossRef]
- Rodríguez-San Pedro, A.; Chaperon, P.N.; Beltrán, C.A.; Allendes, J.L.; Ávila, F.I.; Grez, A.A. Influence of agricultural management on bat activity and species richness in vineyards of central Chile. J. Mammal. 2018, 99, 1495–1502. [Google Scholar] [CrossRef]
- Froidevaux, J.S.P.; Boughey, K.L.; Hawkins, C.L.; Broyles, M.; Jones, G. Managing hedgerows for nocturnal wildlife: Do bats and their insect prey benefit from targeted agri-environment schemes? J. Appl. Ecol. 2019, 56, 1610–1623. [Google Scholar] [CrossRef]
- Asconcelos, A.G.; Bittencourt, J.S.; Auler, A.S. Mammal tooth traces in a ferruginous cave in southeastern Brazil and their relevance to cave legal protection. An. Acad. Bras. Cienc. 2019, 91, e20180861. [Google Scholar] [CrossRef] [PubMed]
- Niemiller, M.L.; Taylor, S.J.; Bichuette, M.E. Conservation of Cave Fauna, with an Emphasis on Europe and the Americas. In Cave Ecology; Springer Nature: Basel, Switzerland, 2018; pp. 451–478. [Google Scholar]
- Ren, G.P.; Young, S.S.; Wang, L.; Wang, W.; Long, Y.C.; Wu, R.D.; Li, J.S.; Zhu, J.G.; Yu, D.W. Effectiveness of China’s National Forest Protection Program and nature reserves. Conserv. Biol. 2015, 29, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Charbonnier, Y.M.; Barbaro, L.; Barnagaud, J.Y.; Ampoorter, E.; Nezan, J.; Verheyen, K.; Jactel, H. Bat and bird diversity along independent gradients of latitude and tree composition in European forests. Oecologia 2016, 182, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Bommarco, R.; Miranda, F.; Bylund, H.; Björkman, C. Insecticides suppress natural enemies and increase pest damage in cabbage. J. Econ. Entomol. 2011, 104, 782–791. [Google Scholar] [CrossRef] [PubMed]



| Scientific Name | Order | Family | FOO | Pest | Damage Stage |
|---|---|---|---|---|---|
| Hylesia pauper | Lepidoptera | Saturniidae | 0.482 | ** | Fruit-bearing, ornamental and forest plants |
| Bembina albinotata | Coleoptera | Scarabaeidae | 0.434 | * | Trees (Terminalia…) |
| Mythimna separata | Lepidoptera | Noctuidae | 0.385 | ** | Agricultural plants (maize…) |
| Spodoptera frugiperda | Lepidoptera | Noctuidae | 0.337 | ** | Grasses and grain crops |
| Spodoptera mauritia | Lepidoptera | Noctuidae | 0.337 | ** | Various grasses (rice, wheat…) |
| Dendrolimus punctatu | Lepidoptera | Lasiocampidae | 0.337 | * | Larix, Picea and Pinus species |
| Hypomecis lioptilaria | Lepidoptera | Geometridae | 0.337 | * | Trees (oak, birch…) |
| Inopsis funerea | Lepidoptera | Erebidae | 0.337 | / | / |
| Eilema plana | Lepidoptera | Erebidae | 0.337 | / | / |
| Bradina diagonalis | Lepidoptera | Crambidae | 0.337 | ** | Various plants (cabbage, eggplant…) |
| Leucophenga brevivena | Diptera | Drosophilidae | 0.337 | ** | Mushrooms and fruit |
| Holotrichia serrata | Coleoptera | Scarabaeidae | 0.337 | ** | Sugarcane, vegetables, groundnut and coconut |
| Spodoptera exigua | Lepidoptera | Noctuidae | 0.289 | ** | Cereals (rice, wheat…) |
| Scobura cephaloides | Lepidoptera | Hesperiidae | 0.289 | ** | Gramineae species (Indocalamus…) |
| Phaeochrous emarginatus | Coleoptera | Hybosoridae | 0.289 | / | / |
| Estimate | SE | t | p | |
|---|---|---|---|---|
| (Intercept) | −15,219.45 | 3984.94 | −3.819 | 0.0316 * |
| AI | 2867.75 | 632.89 | 4.531 | 0.0201 * |
| DipR | −41.83 | 54.56 | −0.767 | 0.4991 |
| Estimate | SE | t | p | |
|---|---|---|---|---|
| (Intercept) | −74,437.467 | 2968.773 | −25.07 | 0.0016 ** |
| HI | 4576.271 | 292.078 | 15.67 | 0.0041 ** |
| LepA | 13.113 | 1.063 | 12.34 | 0.0065 ** |
| AI | 9282.988 | 424.587 | 21.86 | 0.0021 ** |
| H. armiger | T. melanopogon | A. stoliczkanus | M. fuliginosus | |
|---|---|---|---|---|
| H. armiger | 0 | 0.158 | 0.627 | 0.667 |
| T. melanopogon | 0.158 | 0 | 0.302 | 0.351 |
| A. stoliczkanus | 0.627 | 0.302 | 0 | 0.677 |
| M. fuliginosus | 0.667 | 0.351 | 0.677 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Liu, Y.; Gong, L.; Si, M.; Wang, Q.; Feng, J.; Jiang, T. The Consumption and Diversity Variation Responses of Agricultural Pests and Their Dietary Niche Differentiation in Insectivorous Bats. Animals 2024, 14, 815. https://doi.org/10.3390/ani14050815
Zhu D, Liu Y, Gong L, Si M, Wang Q, Feng J, Jiang T. The Consumption and Diversity Variation Responses of Agricultural Pests and Their Dietary Niche Differentiation in Insectivorous Bats. Animals. 2024; 14(5):815. https://doi.org/10.3390/ani14050815
Chicago/Turabian StyleZhu, Dan, Yingying Liu, Lixin Gong, Man Si, Qiuya Wang, Jiang Feng, and Tinglei Jiang. 2024. "The Consumption and Diversity Variation Responses of Agricultural Pests and Their Dietary Niche Differentiation in Insectivorous Bats" Animals 14, no. 5: 815. https://doi.org/10.3390/ani14050815
APA StyleZhu, D., Liu, Y., Gong, L., Si, M., Wang, Q., Feng, J., & Jiang, T. (2024). The Consumption and Diversity Variation Responses of Agricultural Pests and Their Dietary Niche Differentiation in Insectivorous Bats. Animals, 14(5), 815. https://doi.org/10.3390/ani14050815







