Simple Summary
Organic acids and essential oils have been shown to be effective alternatives to antibiotic growth promoters in pig production. Organic acids and essential oils have antibacterial, antiviral, and antioxidant properties. The article will focus on the effectiveness of organic acids, essential oils, and their blends in pig diets as alternative antibiotic growth promoters. Furthermore, the effects of organic acids, essential oils, and their blends on growth performance, oxidative stress, and meat quality are examined. Organic acids and essential oils, which have antimicrobial properties, can be used in place of antibiotic growth promoters. The use of organic acids and essential oils as growth promoters enhances pig welfare and aids in the fight against antimicrobial resistance.
Abstract
Over the years, the use of management and feeding strategies to enhance pig productivity while minimizing the use of antibiotic growth promoters has grown. Antibiotic growth promoters have been widely used as feed additives to reduce diet-related stress and improve pig performance. However, increasing concern about the consequences of long-term and increased use of antibiotic growth promoters in animal production has led to a paradigm shift towards the use of natural organic alternatives such as plant essential oils and organic acids in pig nutrition to enhance growth. Antibiotic growth promoters endanger human health by allowing multidrug-resistant genes to be transferred horizontally from non-pathogenic to pathogenic bacteria, as well as directly between animals and humans. Scientific research shows that alternative growth promoters such as essential oils and organic acids appear to improve pigs’ ability to prevent pathogenic bacteria from colonizing the intestinal system, stabilizing the gut microflora and promoting eubiosis, as well as improving immunity and antioxidant stability. The purpose of this review was to provide an in-depth review of organic acids and essential oils as growth promoters in pig production, as well as their effects on productivity and meat quality. Organic acids and essential oils in pig diets are a safe way to improve pig performance and welfare while producing antibiotic-free pork.
1. Introduction
Over the years, there has been an increasing global interest in the development of management and feeding strategies that maximize pig productivity while minimizing the use of antibiotic growth promoters [1]. Antibiotic growth promoters were first used as feed additives to prevent the stress resulting from changing feed in the diet [2]. However, growing concern about the long-term and increased use of antibiotic growth promoters in animal production has resulted in a shift toward using natural organic alternatives to boost pig growth, such as plant essential oils and organic acids [3]. Antibiotic growth promoters are harmful to human health because multidrug-resistant genes can be transferred horizontally from non-pathogenic to pathogenic bacteria, resulting in the direct transfer of antibacterial-resistant bacteria from animals to humans [4]. Moreover, antibiotic growth promoters are not eco-friendly as their residues are found in soils and water, negatively impacting the ecosystem and its functions [5]. Alternative growth promoters such as essential oils and organic acids have been reported to improve a pig’s ability to prevent pathogenic bacteria from colonizing the intestinal system [2], stabilize the gut microflora, and promote eubiosis [6]. They also improve mineral utilization, act as an energy source, promote endogenous enzyme secretion, and improve immunity and antioxidant stability [2]. As a result, improved pig performance is comparable to that of antibiotic growth promoters [6,7,8]. The focus of this review is to provide a comprehensive account of organic acids and essential oils as growth promoters in pig production and how they impact productivity and meat quality in pigs. It will provide an overview of the mode of action, performance responses, and potential of essential oils and organic acids in the pig industry.
2. Material and Methods
A systematic literature review was conducted using Web of Science, Google Scholar, Scopus, and PubMed to gather peer-reviewed papers from 1990 to 2023. The search criteria focused on antibiotic growth promoters, alternative growth promoters, essential oils, organic acids, pig nutrition, immune system, pig welfare, and pork quality. The data were analyzed to determine if essential oils and organic acids can be used as alternative antibiotic growth promoters in pig diets and to learn how their inclusion affects pig performance and pork quality. Data were analyzed, synthesized, and presented based on the key questions raised in the development of a review.
3. Mode of Action of Antibiotic Growth Promoters
A large proportion of the pigs produced in the world received antimicrobials in their feeds to counter post-weaning challenges. This equates to 70–80% of all pig starters, 70–80% of grower diets, and 50–60% of finisher feeds in Europe for the last few years [9]. Weaning piglets causes altered stomach pH, post-weaning diarrhea, and performance issues due to a lack of hydrochloric acid, which activates digestive enzymes. Insufficient hydrochloric acid and other environmental stressors disturb intestinal flora balance leading to a proliferation of pathogenic coliforms [10]. Antibiotics enhance feed conversion but do not impact carcass quality [11]. Antibiotics used in swine production can suppress or inhibit the growth of certain microorganisms [9]; however, their chemical composition and bacterial spectrum of antimicrobials vary widely. Antibiotics induce bacterial cell death by inhibiting essential cellular functions. Antibiotics can be classified based on the cellular component or system effect that may induce cell death, or merely inhibit cell growth [11]. Antibiotics can suppress the growth of pathogenic microbes by reducing competition for nutrients, hence reducing microbial metabolites that affect growth rate [11].
However, administering antibiotics to livestock has resulted in the problem of antimicrobial resistance. Antimicrobial resistance compromises the efficacy of preventing and treating a growing number of microbial infections. It arises as a result of natural selection and mutations resulting in antibiotics being ineffective, giving a survival advantage to the mutated strain [12]. Additionally, antibiotics used in livestock production are not fully absorbed and metabolized in animals, resulting in a large dose being highly active when excreted, causing the enrichment of antibiotic-resistant genes and a huge risk to the environment [13].
4. Organic Acids
4.1. Characteristics of Organics Acids
Organic acids are classified as any organic carboxylic acid, with or without keto, hydroxyl, or others from the non-amino functional group, including some short-chain fatty acids, but not all amino acids with the general R-COOH structure [14]. Organic acids are categorized into three main functional classes: short-chain fatty acids, medium-chain fatty acids, and tricarboxylic fatty acids. Short-chain fatty acids (SCFA), or simple mono-carboxylic acids (maximum 5 carbon atoms) such as acetic, formic, propionic, and butyric acids, are organic acids that are synthesized in the lower intestine by the microbial fermentation of indigestible sugars and amino acids. Medium-chain fatty acids (MCFA), or carboxylic acids containing a hydroxyl group (6–12C) such as malic, citric, tartaric, and lactic acids, represent an important energy source with higher antimicrobial activity due to their higher pKa. Lastly, tricarboxylic acids (TCA), or simply carboxylic acids with double bonds such as sorbic and fumaric acids, are intermediates in the Krebs cycle, and are involved in energy metabolism [14,15,16]. Other organic acids, such as sorbic, benzoic, and lactic acids, follow different structures. Organic acids are widely distributed in nature as normal constituents of plants and animal tissues, produced either by chemical synthesis or microbial fermentation of carbohydrates in the large intestine [17]. The dissociation constant (pKa) and carbon chain length (C1–C7) of common organic acids determine their antimicrobial efficacy. The sodium, potassium, and calcium salts of these acids, such as sodium benzoate, calcium formate, and calcium propionate, also have antimicrobial properties. Organic acids can be applied directly to feeds in solid or sprayed form, and are classified as feed preservatives or acidifiers. The efficacy of dietary organic acids depends largely on animal species, chemical composition (acid, salt), molecular weight, MIC value of the acid, targeted microbe species, gastrointestinal tract site, and buffering capacity of the feed [17,18]. Safety, odor, taste, and solubility in water are aspects to consider when applying organic acids to animal nutrition. Organic acids are weak acids that partly dissociate, and upon entering the bacterial cell membrane, they detach themselves in the inner, more alkaline part. The undissociated part then reduces the pH in the cytoplasmic area, disrupting the normal metabolic processes of certain types of bacteria, including E. coli., Listeria spp., Salmonella spp., Clostridia spp., and some coliforms, thereby killing the cell [19]. In European markets, the demand for feed acidifiers grew by 6.6% from 2006–2012. The global market for feed acidifiers is projected to increase, with high demand in developing economies as well as demand for safe meat products from developed economies and an increasing world population [6]. The global market size for animal nutrition organic acids in 2020 was estimated at US$113.3 million, and is expected to expand by a 6.5% compound annual growth rate (CAGR) in 2021 to 187.1 million by 2028 [20]. As stated in this report, growth is driven by demand for low-cost renewable energy sources, as well as an increased need to replace traditional growth promoters. Furthermore, pigs and poultry were in high demand, with lactic acid accounting for 49.0% of total revenue. Table 1 shows the common organic acids used as dietary acidifiers for pigs and poultry.

Table 1.
Chemical Properties of common organic acids used in animal nutrition.
4.2. Mode of Action of Organic Acids
The mode of action of common OAs is not yet fully understood. However, their mode of action may be partially due to factors such as, (a) inhibition of the development of pathogenic microbes in the gastrointestinal tract by reducing gut pH, (b) reduction of gastric emptying rates and maintenance of endogenous enzyme secretion, (c) mineral chelation and stimulation on intermediary metabolism, and (d) facilitation of proper digestion due to lower gastric pH and enhanced pepsin secretion [16].
4.3. Bactericidal Properties
Organic acids are weak acids, and in their undissociated state they can easily diffuse across cell membranes. In the cytoplasm, they dissociate and release hydrogen (H+) ions, which increases intracellular acidity of the cell, influencing cell metabolism and disrupting the normal microbial cell functioning [14]. Bacterial cells are forced to expend energy to expel the protons, leading to intracellular accumulation of RCOO− acid anion. Accumulation of anions will interfere with RNA and DNA synthesis, resulting in impaired cell growth and multiplication as well as osmotic cell pressure, inducing both bactericidal and bacteriostatic effects [15]. The acid regulation properties of OAs allow them to reduce activity of harmful bacteria by altering the ambient pH value in the bacterial cell [6]. Organic acids have a stronger effect on the inhibition of gram-positive bacteria than gram negative bacteria due to structural differences in the cell membrane [19]. The efficiency of organic acids in reducing the microbial count is affected by the type of acid, temperature, buffering capacity, and water activity. Table 2 shows some common organic acids used in pig production.

Table 2.
Common organic acids used in pig production.
4.4. Lowering Stomach pH and Endogenous Enzyme Secretion
Short-chain fatty acids have a stimulating effect on both the endocrine and exocrine pancreatic secretions. Organic acids, when ingested, can create an acidic environment [2]. Low stomach pH alters gut microflora by reducing the non-acid tolerant bacterial species such as E. coli and Salmonella [14]. The acidic environment in the stomach activates the conversion of enzyme precursor pepsinogen to pepsin, which is responsible for protein digestion [1,32]. Organic acids elevate serum secretin content, stimulating pancreatic exocrine secretions and resulting in improved nutrient digestibility in the duodenum [33].
4.5. Energy Source and Mineral Utilization
Organic acids act as an energy source in the gut, as they are intermediary products of the tricarboxylic acid (TCA) cycle [34]. Their inclusion in diets helps in preventing tissue breakdown from gluconeogenesis and lipolysis [6,19]. Organic acid anions can form complexes with minerals like calcium, phosphorous, magnesium, and zinc, enhancing mineral digestion and reducing the excretion of supplemental minerals and nitrogen [2]. Organic acids can improve P solubility and phytate P utilization by competitively chelating Ca2+, reducing the formation of insoluble Ca phytate complexes [14]. Figure 1 illustrates the mode of action for organic acids when they are included in pig diets.
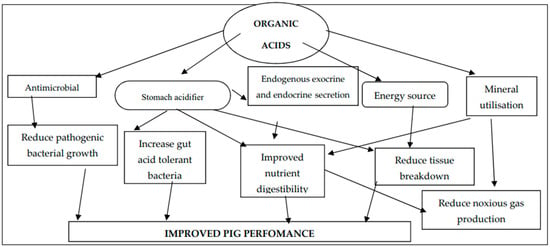
Figure 1.
Summary of organic acidifier mode of action in pigs.
5. Essential Oils
5.1. Characteristics of Essential Oils
Essential oils are a mixture of various compounds, mainly terpenes and terpene derivatives. They are concentrated hydrophobic liquids containing volatile aromatic compounds produced by plants, stored in cavities, secretory cells, and epidermal cells. They are produced as secondary metabolites and they have antibacterial, antifungal, and antiviral properties [35]. These provide essential oils with the ability to replace antibiotic growth promoters and improve animal performance and health [26]. Ecological factors, species, climatic conditions, harvest time, the part of the plant used, and the method of isolation affect the chemical composition of essential oils and their efficacy [36]. Table 3 shows the commercial and non-commercial application of essential oils in pig nutrition and health.

Table 3.
Application of commercial and non-commercial essential oils in pig nutrition and health.
5.2. Antibacterial
Essential oils exhibit a wide spectrum of in vitro antibacterial activities against gram-negative and gram-positive bacteria including E. coli, Salmonella, Staphylococcus, Klebsiella, Proteus, Bacillus, and Clostridium species. Plant extracts kill pathogens due to their hydrophobicity and a high percentage of phenolic compounds. Bioactive compounds in essential oils prevent the development of virulent structures in bacteria, and active compounds disturb the enzyme system of bacteria blocking the virulence of the microbe [2]. Hydrophobicity properties of essential oils enable them to separate lipids present in the cell membrane of bacteria and mitochondria, making it more permeable and disturbing the cell structure [34]. This leads to cell death, due to the leakage of critical molecules and ions from the bacteria [41]. Essential oil containing phenolic groups exhibit antimicrobial properties through their delocalized electrons and the presence of a hydroxyl group on the phenolic ring. The oils initiate damage to bacterial cell membranes by compromising the pH homeostasis of the bacterial cell membranes [41]. Essential oils have a certain degree of selectivity towards gram-negative bacteria than gram-positive bacteria [1].
5.3. Antioxidant and Anti-Inflammatory Ability
The presence of phenolic OH and other pKa in essential oils contributes to their antioxidant properties. They act as hydrogen donors to the peroxy radical produced during lipid oxidation, inhibiting hydroxyl peroxide formation [26]. Essential oils improve redox balance in various organs and protect against oxidative damage caused by psychological stressors [42]. They also improve the oxidative capacity of meat, which influences its quality. Essential oils inhibit the production of pro-inflammatory cytokines and chemokines by endotoxin-stimulated immune and epithelial cells. Anti-inflammatory properties are partially mediated by inhibiting the NF-kB activation pathway [26], which prevents gut morphological changes, mucosa damage, increased mucosal permeability, impaired gut development, and poor nutrient absorption capacity [42].
5.4. Immune Stimulation
Essential oils have immune-stimulating effects on the gastrointestinal tract commonly referred to as the gut-associated lymphoid tissue (GALT) (Figure 2). The gastrointestinal tract possesses the largest mass of lymphoid tissue and plays an important role in antigen defense in the body [32]. Supplementing essential oils improves the immune status of animals by increasing lymphocyte proliferation rate, phagocytosis rate, IgG, IgA, and IgM concentrations, as well as changes in lymphocyte distribution in the gut [9]. Table 4 indicates secondary metabolites found in essential oils.

Table 4.
Secondary metabolites in essential oils.
Table 4.
Secondary metabolites in essential oils.
| Compound Name | Classification | References |
|---|---|---|
| α-amyrin | Pentacyclic triterpene | [43] |
| 1α,4α-dihydroxybishopsolicepolide | Guaianolide sesquiterpene lactone | [44] |
| 12α,4α-dihydroxybishopsolicepolide | Sequiterpene | [43] |
| 3,5-dicaffeoyl quinic acid | Phenylpropanoid | [45] |
| Acacetin | Flavone | [43] |
| Betulinic acid | Pentacyclic triterpenoid | [43] |
| Caffeic acid | Phenylpropanoid | [45] |
| Chlorogenic acid | Phenylpropanoid | [45] |
| Isoalantolactone | Sesquiterpene lactone | [46] |
| Phytol | Diterpene | [43] |
| Scopoletin | Coumarin | [43] |
| Yomogiartemin | Guaianolide sesquiterpene lactone | [44] |
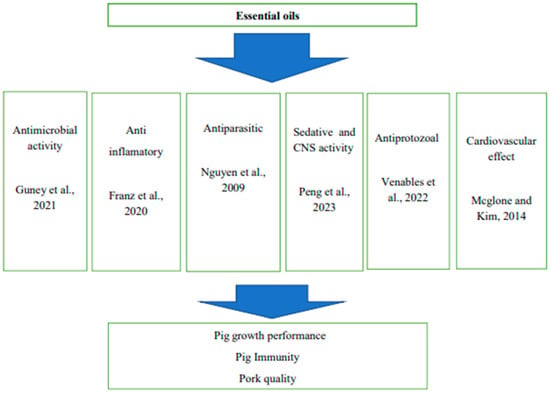
Figure 2.
Summary of biological activities of essential oils [45,46,47,48,49,50].
6. Effect of Organic Acids and Essential Oils on Pig Performance
6.1. Influence on Voluntary Feed Intake (VFI)
Voluntary feed intake in pigs can be influenced by many factors, including dietary characteristics. Essential oils possess an intense smell which makes feed appealing, and pigs tend to consume feed more frequently and/or a larger amount at each meal before gut fill [4]. However, some essential oils result in reduced feed intake which can be attributed to an irritating smell that renders palatability displeasing [45]. Feed intake increased relative to control diets due to supplementation of essential oils ranged from 9% to 12% [49]. Lan, et al. [36] reported a range of 3–19% increase in feed intake. In weaned pigs’ supplementation of organic acids, essential oils and their blend showed to improve average daily feed intake [26]. Essential oils can increase feed palatability and intake due to their flavor-enhancing properties and odor. An increase in palatability associated with the supplementation of essential oils can be attributed to their antioxidative properties that help preserve feed quality and prevent the formation of unpleasant odors [42]. Some organic acids show no effect on feed intake as their performance effect in pigs is mainly on the gastrointestinal tract and nutrient metabolism [6]. Table 5 shows the effect of organic acids and essential oils on voluntary feed intake.

Table 5.
Effects of organic acids and essential oils on voluntary feed intake.
6.2. Influence on Nutrient Digestibility and Growth Efficiency
Studies on organic acids and essential oils have shown that they are beneficial and improve nutrient digestion. Formic acid and its salts lower the pH of the GIT, which increases the activity of digestive enzymes [34]. Organic acids have been shown to improve protein digestion by as much as 4%. Formic acid and its salts increased protein apparent tract digestibility, but did not improve ileal amino acid digestion (this could be due to diet acidification [46]). Citric acid improved the apparent total tract digestibility of protein, calcium, and phosphorous in sows [30]. Dietary benzoic acid improved the apparent digestibility of calcium and phosphorus in growing pigs, as well as crude protein in weanlings. Sows fed benzoic acid diets also had a high digestibility coefficient for organic matter, ether extract, crude protein, and crude fiber [52]. Dietary supplementation with protected acid blends increased the digestibility of dry matter, nitrogen, and energy in lactating sows [53]. Essential oils improved the apparent digestibility of crude protein and dry matter in swine. Studies have shown that phytogenic compounds can regulate ileal mucus gene expression and stimulate digestive secretions, thereby improving nutrient digestibility [42]. Essential oils act as a digestive stimulant by activating three (3) peripheral sensing mechanisms, known as oronasal. Oronasal sensing prepares the GI tract for food reception while also stimulating digestive secretion and gut motility [54].
6.3. Effect on Fecal Characteristics and Noxious Gas Production
Organic acids and essential oils can lower diarrheal incidence due to their ability to alter gut pH and microflora [2,6]. Escherichia coli is a major factor in causing infection and diarrhea in weaned pigs, and it affects growth performance [55]. Organic acids have been shown to increase the number of beneficial bacteria in the GIT in pigs and reduce the concentration of E. coli in feces. OAs penetrate bacterial cells in a non-dissociated form and disrupt the normal physiology of certain bacteria [14]. Organic acids and EO blends work in synergy to suppress growth of pathogenic microbes and promote the growth of beneficial microbes [55]. Formic acid, fumaric acid, and citric acid reduced the incidence and severity of diarrhea, increased microbial diversity in the GIT, and reduced E. coli counts while increasing lactobacilli counts [22,56,57]. Herb and plant extracts reduced the E. coli count and improved energy digestion [58,59]. Improved nutrient digestibility in pigs due to essential oils and organic acid supplementation had an impact on fecal noxious gas production. Kiarie et al. [60] state that protective acids reduced fecal emission of ammonia and hydrogen sulfide in lactating sows. The inclusion of humic substances in pig diets reduced ammonia emission by 3 to 18% in pig manure. Reduced aerial ammonia concentrations have beneficial effects on human health [52].
6.4. Effects on Gut Morphology and Gut Microflora
Low gastric pH due to the addition of acidifiers in diets maximizes the growth of beneficial bacteria in the GIT [61]. Organic acids and medium-chain fatty acids have been demonstrated to reduce pathogenic activity in pigs when fed in combinations rather than individually. They reduce the expression of pro-inflammatory cytokines and increase the proliferation of Lactobacillus bacteria. Formic acid added to diets of weaned pigs showed an increase in intestinal microbial diversity and a change in the concentration of certain microbes. A blend of organic acids also increased fecal Lactobacillus species and decreased E. coli fecal counts [62]. Plant extracts fed to pigs indicated improved gut health by modulating gut microbiota. Supplementation of essential oils decreased ileal total microbial mass and increased the lactobacilli to enterobacterial ratio. In vivo studies showed that essential oils increased the lactobacilli group and decreased E. coli and total coliform in piglets [42].
Organic acid and essential oil blends can increase the villous height of the duodenum. Essential oil supplements increased the villous height of the jejunum and the villous height to crypt ratio [26]. Supplements of essential oils reduced the number of intra epithelial and increased the villus height to crypt depth in the distal small intestines [2]. Essential oils decrease the number of pathogenic bacteria in the gut, favoring an increase in villus length, gut surface area, and crypt depth in the jejunum and colon [32]. Blends of medium-chain fatty acids and short-chain organic acids can be utilized by enterocytes as energy sources and attenuate the negative effect of weaning on villus length and crypt depth in pigs [34]. In weaner pigs, benzoic acid showed an increase in the villus height to crypt depth ratio [63]. However, in a study by Kong et al. [64], butyric acid did not affect the histology of grower-finishing pigs; only their mucosal depth was larger, and this can be attributed to better gut integrity in older animals.
6.5. Effect on Immune Status and Oxidative Stress
Supplementing pig diets with organic acid and essential oil has an impact on the immune system and the regulation of oxidative stress. Studies showed that essential oils reduced the numbers of intra epithelium lymphocytes in the mesenteric lymph nodes. Essential oils improve immunity and reduce the need for immune defense activity in the gut [32]. A mixture of carvacrol, cinnamaldehyde, and capsicum oleoresin decreased the population of intra epithelium lymphocytes in the jejunum and ileum of pigs [26]. Supplementation with butyric acid and essential oil reduced the white blood cell counts in growing pigs [62]. Li et al. [65] also stated that organic acid and essential oil blends can reduce the total white blood cell and neutrophil counts during the post-weaning period. An increase in WBC counts indicates systematic inflammation and the risk of bacterial infection [66].
Oxidative stress is commenced when the amount of ROS produced exceeds the neutralization ability of antioxidants. Excessive ROS leads to oxidative damage of proteins, lipids, and DNA, thereby destroying cell function [67]. Oxidative stress and inflammation are correlated physiological processes [68]. To prevent the accumulation of free radicals, cells develop defense mechanisms including the antioxidant enzymes and non-enzymatic antioxidants. Superoxide dismutase (SOD), glutathione peroxidase (GSH Px), and catalases (CAT) constitute antioxidant enzymes, whereas ascorbic acid, α tocopherol, Glutathione (GSH), carotenoids, and flavonoids are part of non-enzymatic antioxidants [69]. Oxidative stress represents an important chemical mechanism that leads to biological damage. Exposure of pigs to varied stressors leads to the increased production of ROS and the overwhelming of the antioxidant system. Oxidative stress is associated with reduced performance, decreased feed intake, diarrhea, and destruction of liver tissues [70]. In vivo experiments in pigs have shown that the antioxidant effects of essential oils reduce oxidative stress. Frankic et al. [69] state that carvacrol added in drinking water reduced the level of DNA lesions induced in freshly isolated hepatocytes and testicular cells in pigs. Mounir et al. [71] demonstrated that supplementation of plant extracts in pigs reduced DNA damage in lymphocytes which can be a potential benefit to the immune system. Organic acid Na-butyrate supplementation in gestating sow diets and pre-weanling diets had a positive effect on muscle and adipose tissue oxidative genes [70,71]. Improved antioxidant indices can prevent villi from radical-induced damage, which is correlated with better intestinal morphology and nutrient digestibility [72].
6.6. Effect on Growth Performance and Carcass Characteristics
Organic acids and essential oils improve the productivity of pigs to levels comparable to antibiotic growth promoters. According to Lückstädt et al. [6], organic acids improved daily weight gain, feed conversion rate, birth weight, weaning weight, and back fat thickness in pigs. The addition of fulvic acid in pig diets improved average daily gain and growth. Fulvic acid can improve the metabolism of proteins and carbohydrates [73]. A blend of organic acids showed an improvement in growth performance in older pigs and newly weaned pigs [74,75]. In the grower-finisher period, application of different levels and different sources of plant extracts showed positive effects on the growth performance of pigs [76]. [77] reported a higher average daily gain and feed conversion ratio in pigs fed garlic-treated diets. Bedford and Gong [78] observed a significant improvement in average daily gain and feed conversion ratio with the use of an herb mixture in pig diets from 25 to 105 kg.
6.7. Effect on Physicochemical Meat Properties
Meat quality characteristics are generally not affected by changes in diet composition, but can be influenced when diet alters carcass composition. According to Peng et al. [79], supplementing organic acids had no effect on marbling, meat color, cooking losses, drip losses, and water holding capacity in finisher pigs. Organic acids are considered growth promoters. However, there are no scientific studies confirming that they do not alter carcass composition. The addition of an organic acid and essential oil blend in grower-finisher pigs did not affect the pork’s ultimate pH, cooking loss, and shear force. Essential oils showed an effect on meat color by improving the oxidative stability of meat [12]. Rosemary essential oils reduced indicators of lipid oxidation and protein oxidation in pork [8]. Oregano essential oils when supplemented in pig diets prevented lipid oxidation but did not affect the cooking loss, drip loss, shear force, and chemical composition of pork [80]. Supplementing pigs with butyrate showed an effect on boar taint impacting pork sensory attributes. Butyrate has a regulatory effect on cell apoptosis and accumulation of androsterone in pigs, which causes boar taint [81]. However, there is a need for more research on the sensory effects of organic acids and essential oils in pork. Table 6 shows the effects of organic acids and essential oils on pig performance in relation to age.

Table 6.
Effects of organic acid and essential oil blends on pig performance in relation to age.
6.8. Potential of Organic Acids and Essential Oils as Feed Additives in the Pig Industry
Figure 3 summarizes the potential benefits of organic acids, essential oils, and their blend in pig diets. The application of organic acidifiers and essential oils in pig diets has great potential in improving pig performance, pork quality, and reducing environmental pollution. This will, in turn, assist in meeting the ever-increasing demand for animal protein, positively affecting food and nutritional security. The use of organic acid and essential oils has the potential to reduce noxious gas emissions from pig manure, impacting climate change mitigation. The overall adoption of organic acids and essential oils in pig nutrition will lead to a drastic shift in the provision of safe pork that is antibiotic-free [2].
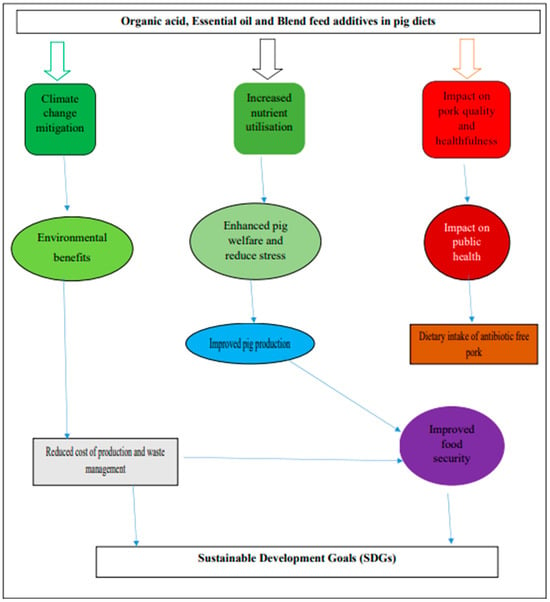
Figure 3.
Summary on potential of organic acids and essential oil feed additives.
7. Conclusions
Organic acid and essential oils can improve nutrient digestibility, growth performance, carcass traits, gut morphology, microflora, meat quality, and chemical composition in pigs. The potential of organic acids and essential oils to improve pig performance and pork quality is comparable to that of antibiotic growth promoters and can be an alternative in smart pig production practices and the production of safe meat. However, information on their specific mode of action in growing pigs is still lacking, and there is a need for further research. Future studies are recommended on the effects of organic acid and essential oils on fermentation indices, immune and enzyme gene expression, fatty acid profile, and lipid quality indices.
Author Contributions
Conceptualization, R.B.N., U.M. and C.W.T.N.; review and editing, R.B.N., U.M. and C.W.T.N.; supervision; U.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Lange, C.F.M.; Pluske, J.; Gong, J.; Nyachoti, C.M. Strategic use of feed ingrédients and feed additives to stimulate gut health and development in young pigs. Livest. Sci. 2010, 134, 124–134. [Google Scholar] [CrossRef]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Stein, H.H. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef]
- Long, S.F.; Xu, Y.T.; Pan, L.; Wang, Q.Q.; Wang, C.L.; Wu, J.Y.; Wu, Y.Y.; Han, Y.M. Mixed organic acids as antibiotic substitutes improve performance, serum immunity, intestinal morphology and microbiota for weaned piglets. J. Anim. Feed Sci. 2018, 235, 23–32. [Google Scholar] [CrossRef]
- Han, Y.S.; Tang, C.H.; Zhao, Q.Y.; Zhan, T.F.; Zhang, K.; Han, Y.M.; Zhang, J.M. Effects of dietary supplementation with combinations of organic and medium chain fatty acids as replacements for chlortetracycline on growth performance, serum immunity, and faecal microbiota of weaned piglets. Livest. Sci. 2018, 216, 210–218. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Kakarlab, D.; Venkateswarluc, K.; Megharajd, M.; Yoone, Y.E.; Lee, Y.B. Agriculture, Ecosystems and Environment Veterinary antibiotics (VAs) contamination as a global agro-ecological issue: A critical view. Agric. Ecosyst. Environ. 2018, 257, 47–59. [Google Scholar] [CrossRef]
- Lückstädt, C.; Wylie, L.; Remmer, R.; De Kok, R.; Costa, H.R.; Brebels, M.; van Heusden, C. Organic Acids in Animal Nutrition; Anitox: Lawrenceville, GA, USA, 2014. [Google Scholar]
- Pateiro, M.; Francisco, B.; Domínguez, J.; Sant, A. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, H.; Wang, S.; Wu, J.; Kluenter, A.M. Potential of essential oils for poultry and pigs. Anim. Nutr. 2018, 4, 179–186. [Google Scholar] [CrossRef]
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002, 13, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Kenworthy, R.; Crabb, W.E. The intestinal flora of young pigs, with reference to early weaning, Escherichia coli and scours. J. Comp. Pathol. Ther. 1963, 73, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Current status and prospects for in-feed antibiotics in the different stages of pork production—A review. Asian-Australas. J. Anim. Sci. 2017, 30, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Liu, X.; Qian, M.; Shen, Y.; Qin, X.; Huang, H.; Yang, H.; He, Y.; Bai, W. An high-through put sequencing approach to the preliminary analysis of bacterial communities associated with changes in amino acid nitrogen, organic acid and reducing sugar contents during soy sauce fermentation. Food Chem. 2021, 349, 129131. [Google Scholar] [CrossRef]
- Khan, S.H.; Iqbal, J. Recent advances in the role of organic acids in poultry nutrition. J. Appl. Anim. Res. 2016, 44, 359–369. [Google Scholar] [CrossRef]
- Tugnoli, B.; Giovagnoni, G.; Piva, A.; Grilli, E. From acidifiers to intestinal health enhancer: How organic acids can improve growth efficiency of pigs. Animals 2020, 10, 134. [Google Scholar] [CrossRef]
- Rathnayake, D.; Mun, H.S.; Dilawar, M.A.; Baek, K.S.; Yang, C.J. Time for a paradigm shift in animal nutrition metabolic pathway: Dietary inclusion of organic acids on the production parameters, nutrient digestibility, and meat quality traits of swine and broilers. Life 2021, 11, 476. [Google Scholar] [CrossRef]
- Hajati, H. Application of organic acids in poultry nutrition. Int. J. Avian Wildl. Biol. 2018, 3, 324–329. [Google Scholar] [CrossRef]
- Kumar, A.; Toghyani, M.; Kheravii, S.K.; Pineda, L.; Han, Y.; Swick, R.A.; Wu, S.-B. Potential of blended organic acids to improve performance and health of broilers infected with necrotic enteritis. Anim. Nutr. 2021, 7, 440–449. [Google Scholar] [CrossRef]
- Suiryanrayna, M.V.A.N.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef]
- Partanen, K.H.; Mroz, Z. Organic acids for performance enhancement in pig diets. Nutr. Res. Rev. 1999, 12, 117–145. [Google Scholar] [CrossRef]
- Luise, D.; Motta, V.; Salvarani, C.; Chiappelli, M.; Fusco, L.; Bertocchi, M.; Mazzoni, M.; Maiorano, G.; Costa, L.N.; Van Milgen, J.; et al. Long-term administration of formic acid to weaners: Influence on intestinal microbiota, immunity parameters and growth performance. Anim. Feed Sci. Technol. 2017, 232, 160–168. [Google Scholar] [CrossRef]
- Duarte, M.E.; Kim, S.W. Phytobiotics from Oregano Extracts Enhance the Intestinal Health and Growth Performance of Pigs. Antioxidants 2022, 11, 2066. [Google Scholar] [CrossRef]
- Gottlob, R.O.; Neill, C.R.; Groesbeck, C.N.; Schneider, J.D.; Frantz, N.Z.; Tokach, M.D.; Goodband, R.D.; DeRouchey, J.M.; Nelssen, J.L.; Dritz, S.S. Effects of water-soluble and in-feed organic acids on the growth performance of weanling pigs. Kans. Agric. Exp. Stn. Res. Rep. 2006, 10, 60–66. [Google Scholar] [CrossRef]
- Kiarie, E.; Voth, C.; Wey, D.; Zhu, C.; Vingerhoeds, P.; Borucki, S.; Squires, E.J. Comparative efficacy of antibiotic growth promoter and benzoic acid on growth performance, nutrient utilization, and indices of gut health in nursery pigs fed corn–soybean meal diet. Can. J. Anim. Sci. 2018, 98, 868–874. [Google Scholar] [CrossRef]
- Xu, Y.T.; Liu, L.I.; Long, S.F.; Pan, L.; Piao, X.S. Effect of organic acids and essential oils on performance, intestinal health and digestive enzyme activities of weaned pigs. Anim. Feed Sci. Technol. 2018, 235, 110–119. [Google Scholar] [CrossRef]
- Kommera, S.K.; Mateo, R.D.; Neher, F.J.; Kim, S.W. Phytobiotics and organic acids as potential alternatives to the use of antibiotics in nursery pig diets. Asian-Australas. J. Anim. Sci. 2006, 19, 1784–1789. [Google Scholar] [CrossRef]
- Loh, T.C.; Low, Y.S.; Chang, Y.K. Effects of Orgacidstm on Growth Performance, Gut Microflora and pH and Meat Cholesterol in Growing Pigs. In Proceedings of the 11th International Conference of the Association of Institutions for Tropical Veterinary Medicine, Petaling Jaya, Malaysia, 23–27 August 2004; p. 392. [Google Scholar]
- Rodrigues, L.M.; Neto, T.O.D.A.L.; Garbossa, C.A.P.; Martins, C.C.D.S.; Garcez, D.; Alves, L.K.S.; de Abreu, M.L.T.; Ferreira, R.A.; Cantarelli, V.D.S. Benzoic acid combined with essential oils can be an alternative to the use of antibiotic growth promoters for piglets challenged with E. coli F4. Animals 2020, 10, 1978. [Google Scholar] [CrossRef]
- Liu, F.J.; Ding, G.S.; Tang, A.N. Simultaneous separation and determination of five organic acids in beverages and fruits by capillary electrophoresis using diamino moiety functionalized silica nanoparticles as pseudostationary phase. Food Chem. 2014, 145, 109–114. [Google Scholar] [CrossRef]
- Choi, J.; Wang, L.; Liu, S.; Lu, P.; Zhao, X.; Liu, H.; Lahaye, L.; Santin, E.; Liu, S.; Nyachoti, M.; et al. Effects of a Microencapsulated Formula of Organic Acids and Essential Oils on Nutrient Absorption, Immunity, Gut Barrier Function, and Abundance of Enterotoxigenic Escherichia coli F4 in Weaned Piglets Challenged with E. coli F4. J. Anim. Sci. 2020, 98, skaa259. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, H.; Zhang, H.; Wang, J.; Wu, S.; Qiu, K.; Qi, G.; Tekeste, A.; Wassie, T.; Chanie, D. Potential Feed Additives as Antibiotic Alternatives in Broiler Production. Front. Vet. Sci. 2022, 9, 916473. [Google Scholar] [CrossRef] [PubMed]
- Papatsiros, V.G.; Christodoulopoulos, G.; Filippopoulos, L.C. The use of organic acids in monogastric animals (swine and rabbits). J. Cell Anim. Biol. 2012, 6, 154–159. [Google Scholar] [CrossRef]
- Li, S.; Zheng, J.; Deng, K.; Chen, L.; Zhao, X.L.; Jiang, X.; Fang, Z.; Che, L.; Xu, S.; Feng, B.; et al. Supplementation with organic acids showing different effects on growth performance, gut morphology, and microbiota of weaned pigs fed with highly or less digestible diets. J. Anim. Sci. 2018, 96, 3302–3318. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.X.; Li, T.S.; Kim, I.H. Effects of essential oils supplementation in different nutrient densities on growth performance, nutrient digestibility, blood characteristics and fecal microbial shedding in weaning pigs. Anim. Feed Sci. Technol. 2016, 214, 77–85. [Google Scholar] [CrossRef]
- Tan, B.F.; Lim, T.; Boontiam, W. Effect of dietary supplementation with essential oils and a Bacillus probiotic on growth performance, diarrhoea and blood metabolites in weaned pigs. Anim. Prod. Sci. 2020, 61, 64–71. [Google Scholar] [CrossRef]
- Montoya, D.; D’angelo, M.; Martín-Orúe, S.M.; Rodríguez-Sorrento, A.; Saladrigas-García, M.; Araujo, C.; Chabrillat, T.; Kerros, S.; Castillejos, L. Effectiveness of two plant-based in-feed additives against an Escherichia coli F4 oral challenge in weaned piglets. Animals 2021, 11, 2024. [Google Scholar] [CrossRef]
- Samanta, A.K.; Gali, J.M.; Dutta, T.K.; Rajkhowa, T.K.; Mandal, G.P.; Patra, A.K. Effect of dietary phytobiotic mixture on growth performance, nutrient utilization, and immunity in weaned piglets. Trop. Anim. Health Prod. 2021, 53, 459. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef] [PubMed]
- More, G.; Lall, N.; Hussein, A.; Tshikalange, T.E. Antimicrobial constituents of Artemisia afra Jacq. ex Willd. against periodontal pathogens. Evid.-Based Complement. Altern. Med. 2012, 2012, 252758. [Google Scholar] [CrossRef] [PubMed]
- Moyo, P.; Kunyane, P.; Selepe, M.A.; Eloff, J.N.; Niemand, J.; Louw, A.I.; Maharaj, V.J.; Birkholtz, L.-M. Bioassay-Guided Isolation and Identification of Gametocytocidal Compounds from Artemisia afra (Asteraceae). Malar. J. 2019, 18, 65. [Google Scholar] [CrossRef]
- Liu, N.Q.; Cao, M.; Frédérich, M.; Choi, Y.H.; Verpoorte, R.; van der Kooy, F. Metabolomic investigation of the ethnopharmacological use of Artemisia afra with NMR spectroscopy and multivariate data analysis. J. Ethnopharmacol. 2010, 128, 230–235. [Google Scholar] [CrossRef]
- Venables, L.; Koekemoer, T.C.; Van de Venter, M.; Goosen, E.D. Isoalantolactone, a sesquiterpene lactone from Artemisia afra Jacq. ex Willd and its in vitro mechanism of induced cell death in HeLa cells. S. Afr. J. Bot. 2016, 103, 216–221. [Google Scholar] [CrossRef]
- Güney, M.; Karaca, S.; Erdogan, S.; Kor, A.; Kale, C.; Onalan, S.; Demirel, M.; Bingol, N.T. Effects of Dietary Supplementation with Rosemary Oil on Methanogenic Bacteria Density, Blood and Rumen Parameters and Meat Quality of Fattening Lambs. Ital. J. Anim. Sci. 2021, 20, 794–805. [Google Scholar] [CrossRef]
- Franz, C.; Baser, K.H.C.; Windisch, W. Essential oils and aromatic plants in animal feeding—A European perspective. A review. Flavour Fragr. J. 2010, 25, 327–340. [Google Scholar] [CrossRef]
- Michiels, J.; Missottenb, J.A.M.; Fremauta, D.; DeSmetb, S.; Diericket, N.A. Animal Feed Science and Technology In vitro characterisation of the antimicrobial activity of selected essential oil components and binary combinations against the pig gut flora. J. Anim. Feed Sci. 2009, 151, 111–127. [Google Scholar] [CrossRef]
- Nguyen, N.P.K.; Tran, K.N.; Nguyen, L.T.H.; Shin, H.M.; Yang, I.J. Effects of Essential Oils and Fragrant Compounds on Appetite: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7962. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, Q.; Wu, C.; Zhao, J.; Tan, Q.; He, Y.; Hu, L.; Fang, Z.; Lin, Y.; Xu, S.; et al. Effects of dietary supplementation with essential oils and protease on growth performance, antioxidation, in fl ammation and intestinal function of weaned pigs. Anim. Nutr. 2022, 9, 39–48. [Google Scholar] [CrossRef]
- Mcglone, J.J.; Kim, S.W. Effects of dietary humic substances on pig growth performance, carcass characteristics, and ammonia emission 1. J. Anim. Sci. 2014, 84, 2482–2490. [Google Scholar] [CrossRef]
- Ma, J.; Piao, X.; Shang, Q.; Long, S.; Liu, S.; Mahfuz, S. Mixed organic acids as an alternative to antibiotics improve serum biochemical parameters and intestinal health of weaned piglets. Anim. Nutr. 2021, 7, 737–749. [Google Scholar] [CrossRef]
- Resende, M.; Chaves, R.F.; Garcia, R.M.; Barbosa, J.A.; Marques, A.S.; Rezende, L.R.; Peconick, A.P.; Garbossa, C.A.P.; Mesa, D.; Silva, C.C.; et al. Benzoic Acid and Essential Oils Modify the Cecum Microbiota Composition in Weaned Piglets and Improve Growth Performance in Finishing Pigs. Livest. Sci. 2020, 242, 104311. [Google Scholar] [CrossRef]
- Pluske, J.R.; Turpin, D.L.; Sahibzada, S.; Pineda, L.; Han, Y.; Collins, A. Impacts of Feeding Organic Acid-Based Feed Additives on Diarrhea, Performance, and Fecal Microbiome Characteristics of Pigs after Weaning Challenged with an Enterotoxigenic Strain of Escherichia coli. Transl. Anim. Sci. 2021, 5, txab212. [Google Scholar] [CrossRef] [PubMed]
- Kluge, H.; Broz, J.; Eder, K. Effects of dietary benzoic acid on urinary pH and nutrient digestibility in lactating sows. Livest. Sci. 2010, 134, 119–121. [Google Scholar] [CrossRef]
- Devi, S.M.; Lee, K.Y.; Kim, I.H. Analysis of the effect of dietary protected organic acid blend on lactating sows and their piglets. Rev. Bras. Zootec. 2016, 45, 39–47. [Google Scholar] [CrossRef]
- Brenes, A.; Roura, E. Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed Sci. Technol. 2010, 158, 1–14. [Google Scholar] [CrossRef]
- Tsiloyiannis, V.K.; Kyriakis, S.C.; Vlemmas, J.; Sarris, K. The effect of organic acids on CTR of porcine postweaning diarrhea. Res. Vet. Sci. 2001, 70, 287–293. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Lee, K.Y.; Kim, I.H. Influence of protected organic acid blends and diets with different nutrient densities on growth performance nutrient digestibility and faecal noxious gas emission in growing pigs. Vet. Med. 2014, 59, 491–497. [Google Scholar] [CrossRef]
- Bontempo, V.; Jiang, X.R.; Cheli, F.; Lo Verso, L.; Mantovani, G.; Vitari, F.; Domeneghini, C.; Agazzi, A. Administration of a novel plant extract product via drinking water to post-weaning piglets: Effects on performance and gut health. Animal 2014, 8, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Radzikowski, D.; Milczarek, A. Efficiency of Herbs and Botanicals in Pig Feeding. Anim. Sci. Genet. 2022, 18, 73–87. [Google Scholar] [CrossRef]
- Kiarie, E.; Walsh, M.C.; Nyachoti, C.M. Performance, digestive function, and mucosal responses to selected feed additives for pigs. J. Anim. Sci. 2016, 94, 169–180. [Google Scholar] [CrossRef]
- Huang, Y.; Yoo, J.S.; Kim, H.J.; Wang, Y.; Chen, Y.J.; Cho, J.H.; Kim, I.H. Effects of Dietary Supplementation with Blended Essential Oils on Growth Performance, Nutrient Digestibility, Blood Profiles and Fecal Characteristics in Weanling Pigs. Asian-Australas. J. Anim. Sci. 2010, 23, 607–613. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.W.; Hall, H.; Nyachoti, C.M. Effect of dietary organic acids supplementation on growth performance, nutrient digestibility, and gut morphology in weaned pigs. Can. J. Anim. Sci. 2021, 102, 255–265. [Google Scholar] [CrossRef]
- Morel, P.C.H.; Chidgey, K.L.; Jenkinson, C.M.C.; Lizarraga, I.; Schreurs, N.M. Effect of benzoic acid, sodium butyrate and sodium butyrate coated with benzoic acid on growth performance, digestibility, intestinal morphology and meat quality in grower- finisher pigs. Livest. Sci. 2019, 226, 107–113. [Google Scholar] [CrossRef]
- Wang, J.P.; Lee, J.H.; Yoo, J.S.; Cho, J.H.; Kim, H.J.; Kim, I.H. Effects of phenyllactic acid on growth performance, intestinal microbiota, relative organ weight, blood characteristics, and meat quality of broiler chicks. Poult. Sci. 2010, 89, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Chen, G.; Wang, H.; Li, J.; Yin, S.; Cao, X.; Wang, T.; Li, X.; Li, Y.; Zhang, H.; et al. Blood leukocyte count as a systemic inflammatory biomarker associated with a more rapid spirometric decline in a large cohort of iron and steel industry workers. Respir. Res. 2021, 22, 254. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, S.; Chen, F.; Guan, W.; Zhang, S. Nutritional strategies to alleviate oxidative stress in sows. Anim. Nutr. 2022, 9, 60–73. [Google Scholar] [CrossRef]
- Ahasan, A.S.M.L.; Invernizzi, G.; Farina, G.; Pilotto, A.; Barbé, F.; Bontempo, V.; Rossi, R.; Bellagamba, F.; Lecchi, C.; Savoini, G.; et al. The effects of superoxide dismutase-rich melon pulp concentrate on inflammation, antioxidant status and growth performance of challenged post-weaning piglets. Animal 2019, 13, 136–143. [Google Scholar] [CrossRef]
- Slamenova, D.; Horvathova, E.; Marsalkova, L.; Wsolova, L. Carvacrol given to rats in drinking water reduces the level of DNA lesions induced in freshly isolated hepatocytes and testicular cells by H2O2. Neoplasma 2008, 55, 394–399. [Google Scholar]
- Frankic, T.; Levart, A.; Salobir, J. The effect of vitamin E and plant extract mixture composed of carvacrol, cinnamaldehyde and capsaicin on oxidative stress induced by high PUFA load in young pigs. Animal 2010, 4, 572–578. [Google Scholar] [CrossRef]
- Le Gall, M.; Gallois, M.; Seve, B.; Louveau, I.; Holst, J.J.; Oswald, I.P.; Lallès, J.-P.; Guilloteau, P. Comparative effect of orally administered sodium butyrate before or after weaning on growth and several indices of gastrointestinal biology of piglets. Br. J. Nutr. 2009, 102, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ren, E.; Xu, R.; Su, Y. Transcriptomic Responses Induced Inmuscle and Adipose Tissues of Growing Pigs by Intravenous Infusion of Sodium Butyrate. Biology 2021, 10, 559. [Google Scholar] [CrossRef]
- Mounir, M.; Ibijbijen, A.; Farih, K.; Rabetafika, H.N.; Razafindralambo, H.L. Synbioticsand Their Antioxidant Properties, Mechanisms, and Benefits on Human and Animal Health: A Narrative Review. Biomolecules 2022, 12, 1443. [Google Scholar] [CrossRef]
- Grilli, E.; Messina, M.R.; Tedeschi, M.; Piva, A. Feeding a microencapsulated blend of organic acids and nature identical compounds to weaning pigs improved growth performance and intestinal metabolism. Livest. Sci. 2010, 133, 173–175. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Hwang, J.A.; Hoon, J.; Mun, H.S.; Yang, C.J. Comparison of Single and Blend Acidifiers as Alternative to Antibiotics on Growth Performance, Faecal Microflora, and Humoral Immunity in Weaned Piglets. Asian-Australas. J. Anim. Sci. 2014, 27, 93–100. [Google Scholar] [CrossRef]
- Cho, J.H.; Liu, S.D.; Kim, I.H. Effects of Dietary Korean Garlic Extract Aged by Leuconostoc Mesenteroides KCCM35046 on Growth Performance, Digestibility, Blood Profiles, Gas Emissions, and Microbiota in Weanling Pigs. Can. J. Anim. Sci. 2020, 100, 462–469. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, H.; Tian, Z.; Deng, D.; Ma, X. Current Trends of Chinese Herbal Medicines on Meat Quality of Pigs. A Review. J. Anim. Feed Sci. 2021, 30, 187–205. [Google Scholar] [CrossRef]
- Gumus, R.; Gelen, S.U. Effects of dietary thyme and rosemary essential oils on performance parameters with lipid oxidation, water activity, pH, colour and microbial quality of breast and drumstick meats in broiler chickens. Arch. Anim. Breed. 2023, 66, 17–29. [Google Scholar] [CrossRef]
- Ranucci, D.; Miraglia, D.; Trabalza-Marinucci, M.; Acuti, G.; Codini, M.; Ceccarini, M.R.; Forte, C.; Branciari1, R. Dietary effects of oregano (Origanum vulgaris L.) plant or sweet chestnut (Castanea sativa Mill.) wood extracts on microbiological, characteristics and lipid oxidation of cooked ham during storage. Int. J. Food Sci. 2015, 4, 5497. [Google Scholar] [CrossRef]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef]
- Peng, S.; Zhu, J.; Liu, Z.; Hu, B.; Wang, M.; Pu, S. Prediction of Ammonia Concentration in a Pig House Based on Machine Learning Models and Environmental Parameters. Animals 2022, 13, 165. [Google Scholar] [CrossRef]
- Sístková, M.; Bartoš, P. Effects of phytogenic feed additives on growth performance and on ammonia and greenhouse gases emissions in growing-finishing pigs. J. Anim. Feed Sci. 2016, 212, 143–148. [Google Scholar] [CrossRef]
- Kutlu, T. The Effects of Feed Additive, Selenium Source, Polyphenol, Lysine Level, and Sow Lactation Feeder Type on Pigs. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2023. [Google Scholar]
- Walia, K.; Argüello, H.; Lynch, H.; Leonard, F.C.; Grant, J.; Yearsley, D.; Kelly, S.; Duffy, G.; Gardiner, G.E.; Lawlor, P.G. Effect of strategic administration of an encapsulated blend of formic acid, citric acid, and essential oils on Salmonella carriage, seroprevalence, and growth of finishing pigs. Prev. Vet. Med. 2017, 137, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, L.; Cao, G.; Feng, J.; Yue, M.; Xu, Y.; Dai, B.; Han, Q.; Guo, X. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J. Anim. Sci. 2019, 97, 133–143. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).